Key Words: Diffuse interstitial lung disease, Interstitial pneumonia, Pulmonary fibrosis, Tomographic patterns
Abstract
Objective
To determine the main clinical and tomographic characteristics of patients with diffuse interstitial lung disease at Trujillo Regional Teaching Hospital.
Methods
Case series. Tomographic examinations and clinical data were obtained from patients with interstitial pulmonary disease who attended the pulmonology service of Trujillo Regional Teaching Hospital. The information collected was recorded and systematized in Excel. For the statistical analysis, SPSS 23.0 program was used.
Results
Data from 103 patients were obtained, of which 60.2% were female, and 39.8% were male. The average age was 72 years for both groups. Main clinical manifestations were cough (82.5%), dyspnea (76.7%), joint pain (43.7%), weight loss (40.8%), velcro crackles (35%) and digital clubbing (28.2%). Exposure to wood smoke was present in 46.6%, exposure to inorganic dust in 12.6% and fowl ownership in 9.7% of cases. Thirty-one (30.1%) patients presented comorbidities. Among these, rheumatic diseases and arterial hypertension were the most frequent. Non-specific interstitial pneumonia pattern was present in 26.2% of the cases; probable usual interstitial pneumonia in 16.5%; organized type in 12.6%; usual interstitial in 10.7%; acute interstitial in 2.9% and 27.1% had no defined tomographic pattern.
Conclusions
In the studied population, clinical and tomographic characteristics of interstitial lung parenchymal diseases are variable in magnitude and forms of presentation. Female sex and exposure to fuels were the most frequent associated factors. Connective tissue diseases could also explain study findings.
Main messages
|
Introduction
Patients with interstitial lung disease usually present respiratory distress due to decreased distensibility, increased interstitium stiffness and alveoli obliteration. These scenarios come with generalized inflammation, fibrosis of alveolar walls, pulmonary vasculature, and interstitium, altering diffusion and decreasing ventilation/perfusion ratio [1].
The main clinical manifestations of interstitial lung disease are cough, dyspnea, joint pain, weight loss, velcro crackles, and clubbing. These occur in varying magnitude and frequency, depending on the underlying type of interstitial lung pathology [2],[3],[4]. There are more than 300 conditions associated with the different causes of interstitial lung diseases [1]. These include connective tissue disease [5],[6],[7], hypersensitivity pneumonitis, pneumoconiosis, sarcoidosis, organizing pneumonia, interstitial lung disease associated with occupational exposures, and unclassifiable interstitial lung disease. The most common interstitial diseases are idiopathic interstitial pneumonia, sarcoidosis, and extrinsic allergic alveolitis [8],[9]. Idiopathic pulmonary fibrosis is the most common subtype of idiopathic interstitial pneumonia with severe and progressive presentation [10],[11].
Epidemiological data on interstitial lung disease vary widely due to differences in data collection methods and classifications. The estimated incidence of interstitial lung disease is 31.5 cases per 100 000 males and 26.1 cases per 100 000 females [12]. On the other hand, throughout Europe and North America, the estimated incidence of idiopathic pulmonary fibrosis varies between 2.8 and 19 cases per 100 000 persons per year [13],[14]. In Peru and Latin America, we do not have epidemiological information that allows us to estimate the full magnitude of the problem. In the region, information is being collected through the Idiopathic Pulmonary Fibrosis Registry, sponsored by the Latin American Thoracic Association (ALAT).
Multiple exposures, such as environmental pollution, accelerates lung function decline. However, there is scarce information on its role in the development and progression of early stages of interstitial lung disease [15]. Exposure to elemental carbon may increase the risk of progressive interstitial lung disease [16]. Associations with other forms of environmental pollution, such as particulate matter and ozone, are not yet clearly defined [15],[16]. On the other hand, hypersensitivity pneumonitis (a type of interstitial lung disease) is most frequently caused by exposure to avian proteins (bird fancier’s lung), fungi and other microorganisms. Among the latter, thermophilic actinomycetes species (farmer's lung) stand out [17].
To unify the nomenclature and definition criteria used and to favor research, in 2015, the American Thoracic Society and the European Respiratory Society formed the ERS/ATS Task Force on Undifferentiated Forms of Connective Tissue Disease-associated Interstitial Lung Disease. This team proposed the terminology for the Interstitial Pneumonia with Autoimmune Features, IPAF [2],[3],[4].
High-resolution computed axial tomography is the standard imaging method used [18] for lung and small airway diseases [19]. Current technical advances in new computed tomography equipment allow simultaneous acquisition of slice data from multiple detector rows. High-resolution imaging for complete chest study during a single apnea phase, volumetric acquisition, and the use of multiplanar reconstructions provide additional information to conventional studies [20],[21]. For the interpretation and determination of tomographic patterns and different types of pneumonia, a multidisciplinary consensus is recommended for a better approach to diagnose interstitial pneumonia [4],[22].
In Peru, the prevalence of interstitial lung disease, the most affected groups and its epidemiological characteristics are unknown. For this reason, this study aimed to determine tomographic patterns, main clinical manifestations, risk factors and comorbidities of patients with interstitial lung disease, treated at the Trujillo Regional Teaching Hospital. This is a third-level hospital center belonging to the Ministry of Health, and attends to a large proportion of public health patients. In addition, it trains undergraduate and graduate students. This information is necessary for proper diagnosis, specific therapeutic management – and most importantly – preventive measures.
Methods
Data sources
We analyzed the clinical history and tomographic information of patients with a diagnostic hypothesis of interstitial lung disease seen in the pulmonology service of the Trujillo Regional Teaching Hospital. Data was obtained between 2 January 2 and 30 December 2019.
Methods and techniques
We did a case series. Patients with clinical history and chest tomographic studies who attended the pulmonology service were selected. We also included patients referred from lower complexity health facilities and patients referred from other hospital services that needed diagnosis and management referring to respiratory symptomatology. Tomographic studies were reviewed in those who had them requested and those who did not.
The determination, interpretation and qualification of the tomographic patterns of interstitial lung disease were performed by pulmonologist investigators trained in reading chest tomography and correlated with the respective radiological report. If there were disagreements, the tomography was reread and reviewed with the radiologists who had reported the studies, based on the consensus of the American Thorax Society, Europe Respiratory Society, Japan Respiratory Society and ALAT. There was a recollection of study’s predefined clinical characteristics for patients that met inclusion criteria. This included demographic data, clinical data, risk factors, and comorbidities noted in the respective medical records and an ad hoc registry.
The information obtained was recorded and systematized by Microsoft Excel spreadsheets. SPSS 23.0 program (Statistical Package for the Social Sciences 23) was used to analyze age, sex and tomographic patterns of interstitial pneumonia. To these, we added the predefined clinical characteristics for interstitial lung disease (i.e., cough, dyspnea, joint pain, weight loss, auscultation of velcro crackles and clubbing).
Inclusion criteria
- Patients older than 15 years of age.
- Patients with high-resolution tomographic chest studies with or without contrast, printed on plates or CD.
- Medical history with demographic data and clinical characteristics of patients with interstitial lung disease.
Exclusion criteria
- Patients with incomplete data.
- Patients without high-resolution tomographic chest studies.
Results
Data were obtained from 112 patients with a presumed interstitial lung disease diagnosis, of which 103 (92%) had clinical history data and tomographic studies of interstitial lung disease that met the inclusion criteria. The majority were females (60.2%), and there were no age differences, as shown in Table 1.
Table 1. Demographic characteristics of patients with interstitial lung disease.
Cough and dyspnea were the most predominant symptoms. Auscultation of velcro crackles was the most frequent clinical sign, as shown in Table 2.
Table 2. Demographic characteristics of patients with interstitial lung disease.
Table 3 shows that exposure to wood smoke was the most frequent risk factor (46.6%). However, in 29.1% of patients with interstitial lung disease, no risk factor was obtained.
Table 3. Determination of risk factors in patients with interstitial lung disease.
Of the total, 31 patients had comorbidities (30.1%), and 72 had none (69.9%). Figure 1 shows that rheumatologic diseases and arterial hypertension were the most frequent comorbidities.
Figure 1. Comorbidities in patients with interstitial lung disease.
In our study, non-specific interstitial pneumonia tomographic pattern was the most frequent (26.2%), and the usual interstitial pneumonia pattern was 10.7% of the cases, as shown in Table 4.
Table 4. Determination of interstitial pneumonia patterns according to tomo-graphic evaluation.
Table 5 shows that 5.8% of patients with usual interstitial pneumonia tomographic pattern and 11.7% with probable usual interstitial pneumonia had comorbidities and risk factors.
Discussion
This study aimed to identify the main demographic, clinical, risk factors, comorbidities and tomographic characteristics associated with interstitial lung disease in patients attending the Trujillo Regional Teaching Hospital. This is one of the first descriptive studies at a regional level to assess this issue. Among 103 patients studied, females were more frequent. In addition, the average age was 72 years, with no differences between genders.
The present study is similar in age but different in gender, as reported by Diaz and Miranda in Huancayo, Peru [23]. In contrast, Dueñas and colleagues [24] found dissimilar results in Colombia, in which lower mean age (41.7 years) and males were predominant.
These differences could be explained because the prevalence of certain interstitial pneumonias (related to age and gender onset, which depends on work activity, environmental factors and connective tissue diseases) can appear at any stage of life and without distinction for race, age, education or sex. However, females and older adults seem to be the most affected as a result of the aging process [13],[25],[26],[27],[28].
Dyspnea and cough are the most frequent symptoms in patients with interstitial lung disease, although they may be asymptomatic and discovered by incidental findings [29]. Common signs include velcro crackles and digital clubbing. In the present work, it was also observed that such manifestations were the most prevalent, in addition to joint pain. The magnitude of these clinical manifestations would be associated with work activity, underlying pathology (such as connective tissue diseases) and environmental risk factors related to female predominance, as shown in the present study [14],[23],[30].
We observed that the main associated risk factors were wood smoke and inorganic dust exposure. Diaz and Miranda [23] report similar results. In Colombia, Restrepo and colleagues – based on histological studies in animal experiments and clinical findings and transbronchial lung biopsies in humans – proved that the smoke cooking produced by firewood causes a pneumoconiosis type lung damage [31]. In Costa Rica, it was shown that wood smoke is a major source of contamination, mainly in rural areas where it is used for household combustion. Therefore, this study suggests wood smoke is an important factor in the genesis of interstitial lung disease [32],[33]. In our work, the association of wood smoke with interstitial lung disease could be explained by female sex predominance; and this may be because they are more likely to be exposed to biomass elements due to domestic activities. In Peru, an average of 23% of women prepare their food with biomass fuels [34].
Bird keeping was present in 9.7% of the cases, which is also important to consider because of its relationship with interstitial lung disease. Exposure by direct contact with birds is a frequent and recognized form of hypersensitivity pneumonitis, which is a type of interstitial lung disease. Prolonged exposure to avian proteins, which are the main antigenic sources, causes constant inflammation leading to irreversible pulmonary fibrosis over time [35]. Typical tomographic findings include fibrosis with reticulation, architecture distortion, bronchiectasis and traction bronchiolectasis. The honeycomb pattern has been reported in 16 to 69% of cases of chronic hypersensitivity pneumonitis. In contrast to patients with idiopathic pulmonary fibrosis, the honeycomb pattern rarely has basal predominance [36]. In our study, domestic bird keeping could be related to our tomographic findings [37],[38],[39].
Smoking is a prevalent risk factor for interstitial lung disease, according to Behr J and colleagues [38] and Rodas and Espinoza in Guayaquil, Ecuador [28]. On the other hand, Xaubet A et al. differ from our findings [40]. This difference may be explained for two reasons. Firstly, because smoking prevalence for people over 15 years old in the northern region of Peru (where we conducted the study) is 15% on average, which is low compared to other areas. Secondly, because smoking is more frequent among young men. In women, the prevalence is between 0.6 and 2% in this age group [34]. Our study population was predominantly women over 70 years of age, and we had only one smoking-related event. Also, other studies have found an important association with drugs such as methotrexate [41]. In our study, we had only one patient related to methotrexate use.
Rheumatologic diseases and essential arterial hypertension were the most prevalent comorbidities of our study. These results are similar to Gaudiano J and colleagues in Uruguay. They mention a high frequency of women with rheumatologic diseases, especially connective tissue diseases as the main disease triggers [42],[43]. This is different from Buitrón et al. in Huancayo, where diabetes mellitus was present in most cases [44]; and also different from other studies, where they found other important comorbidities such as gastroesophageal reflux [45], chronic viral infections [46],[47],[48], hepatitis C [49] or family history of interstitial lung disease [51],[52],[53].
In the present study, the non-specific interstitial tomographic pattern was most prevalent among interstitial pneumonia. Imaging manifestations were followed in prevalence as probable usual interstitial pneumonia, organized pneumonia, usual interstitial pneumonia, and acute interstitial pneumonia. In addition, 27.1% of patients did not have a defined tomographic pattern and had variable forms and causes of the interstitial involvement. This last finding is half of what was found by Gaudiano J and colleagues in a tertiary hospital in Uruguay [43]. The prevalence of interstitial pneumonia tomographic patterns will depend on the frequency of underlying pulmonary pathologies. In our study, tomographic pattern results could be related to the high frequency of females and joint pain, which may correspond to a high prevalence of connective tissue diseases [54].
Our imaging findings of usual interstitial pneumonia and probable usual interstitial pneumonia without comorbidities and risk factors, could correspond to cases of idiopathic pulmonary fibrosis. Some reports say that 50% of usual interstitial pneumonia is idiopathic pulmonary fibrosis [55]. The remaining cases of usual and probable usual interstitial pneumonia could correspond to other pathologies associated with interstitial lung involvement, such as connective tissue diseases [55].
Recommendations
As a result of our work, we suggest conducting new regional studies to assess the prevalence of interstitial lung diseases and their respective associations with risk factors, etiologic agents, and underlying comorbidities.
Limitations
It should be mentioned that the study has limitations, which could affect results interpretation. For example, because it was a descriptive observational study, biomarker studies were not performed (such as autoantibodies) so that the prevalence of connective tissue diseases cannot be assessed with great precision. Also, we did not quantify exposures to risk factors such as biomass fuels, and there were no pathological anatomy studies.
Conclusions
In the studied population, clinical and tomographic characteristics of interstitial lung parenchymal diseases are variable in magnitude and forms of presentation.
Female sex and exposure to biomass fuels (wood smoke) were the most frequent factors associated with interstitial lung diseases. These study findings could also explain the high prevalence in women with joint pain and connective tissue disease characteristics.
This is relevant information for decision-making because there are modifiable factors that can curtail the progression of interstitial lung diseases.
Notes
Authorship contributions
LARH: conceptualization, validation, research, original draft writing, editorial review and editing, visualization, oversight, project management, funding acquisition. LACU: research, resourcing, fund acquisition. JSHV: methodology, formal analysis, resources, writing original draft, review and editing, oversight, acquisition of funds. JLCP, ONAH: research and acquisition of funds.
Competing interests
The authors completed the ICMJE conflict of interest statement and declared that they received no funding for the completion of this article; have no financial relationships with organizations that may have an interest in the published article within the past three years; and have no other relationships or activities that may influence the publication of the article. Forms can be requested by contacting the corresponding author or the Editorial Committee of the Journal.
Funding
Funding was provided by the authors; no external funding was received.
Ethics
This study was authorized by the Ethics Committee of Trujillo Regional Teaching Hospital Office N° 203-2019, dated 9 December 2019, both for the management of information from medical records, tomographic studies, and research development.
Language of submission
Spanish.

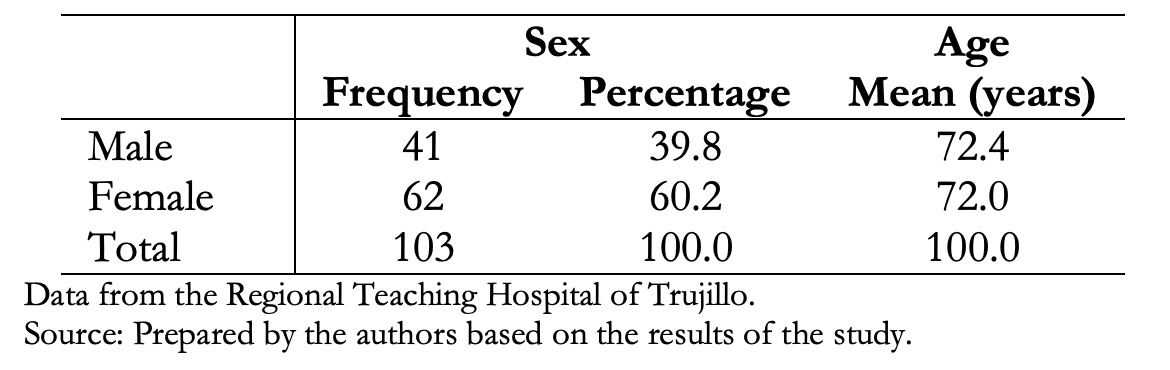 Table 1. Demographic characteristics of patients with interstitial lung disease.
Table 1. Demographic characteristics of patients with interstitial lung disease.

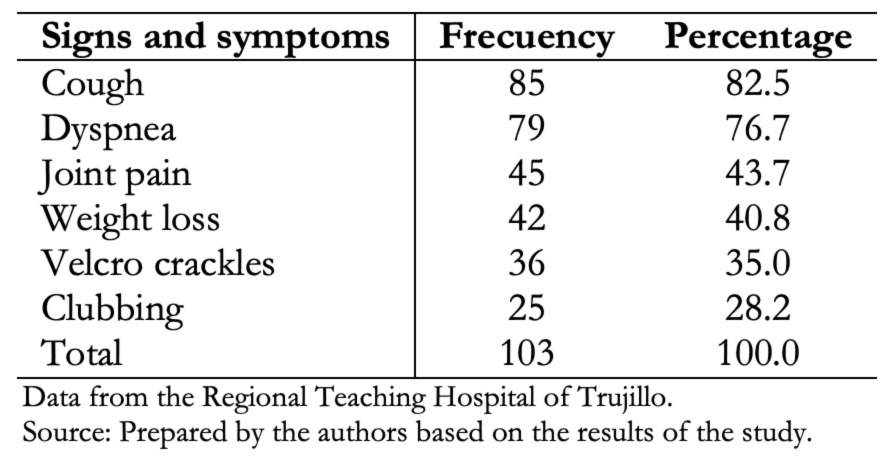 Table 2. Demographic characteristics of patients with interstitial lung disease.
Table 2. Demographic characteristics of patients with interstitial lung disease.

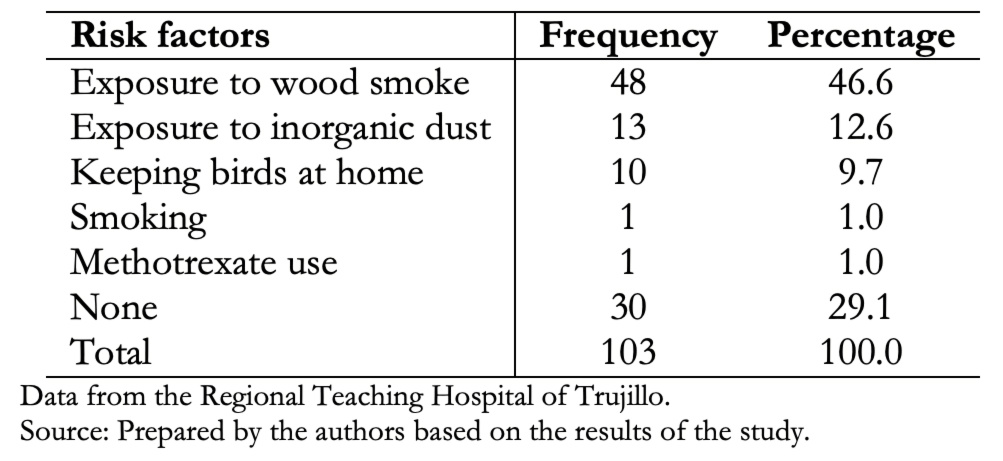 Table 3. Determination of risk factors in patients with interstitial lung disease.
Table 3. Determination of risk factors in patients with interstitial lung disease.

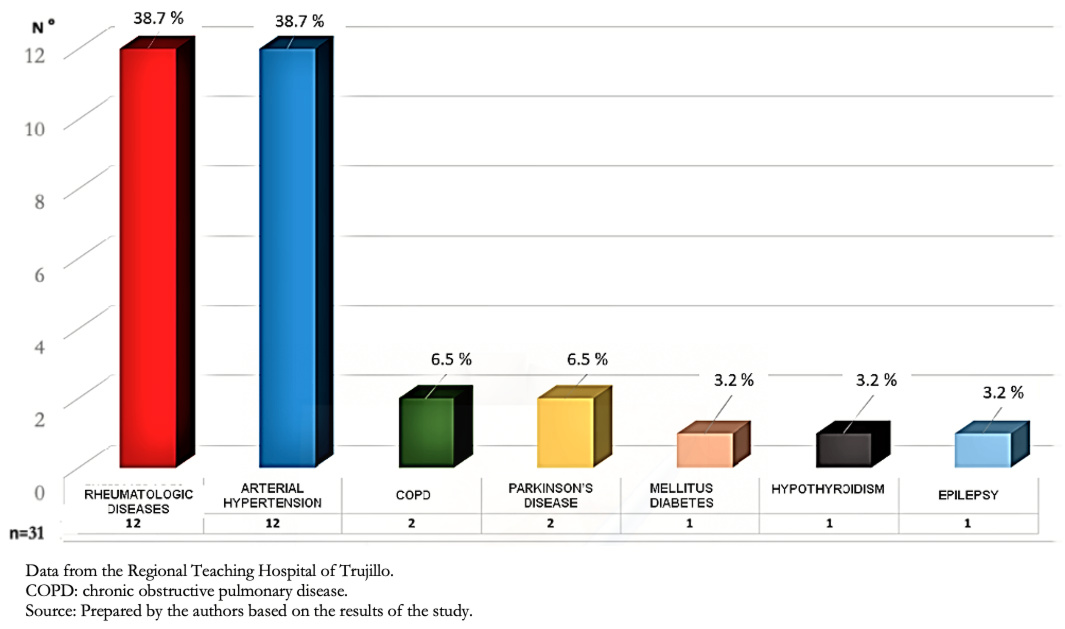 Figure 1. Comorbidities in patients with interstitial lung disease.
Figure 1. Comorbidities in patients with interstitial lung disease.

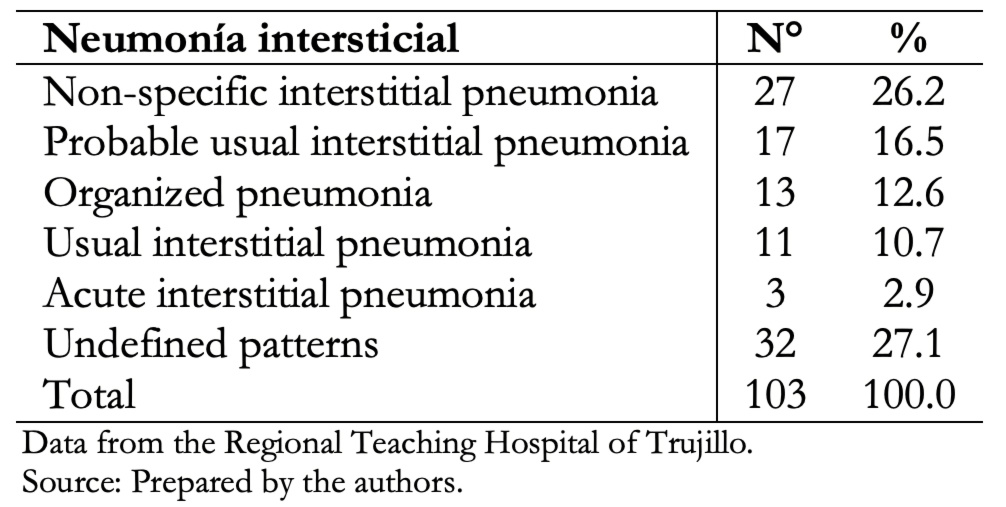 Table 4. Determination of interstitial pneumonia patterns according to tomo-graphic evaluation.
Table 4. Determination of interstitial pneumonia patterns according to tomo-graphic evaluation.

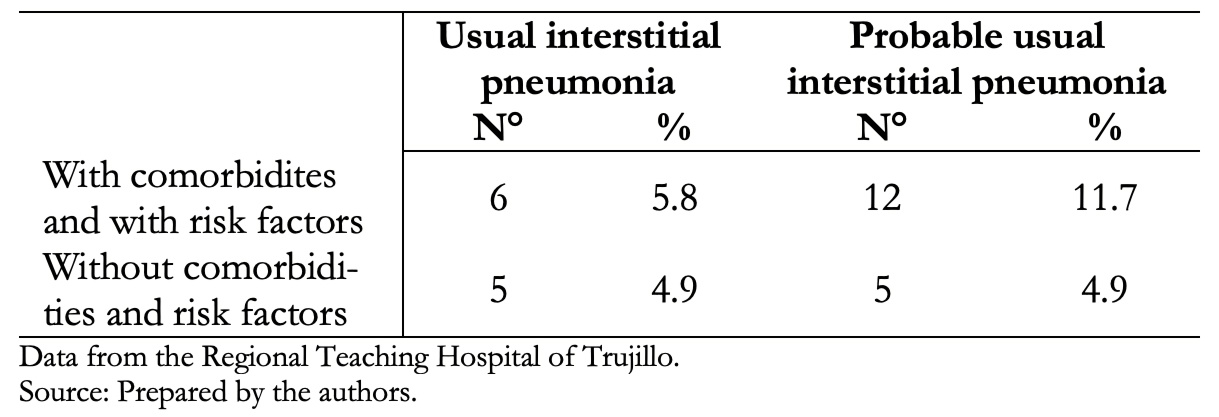 Table 5. The relationship of comorbidities and/or risk factors with tomographic studies compatible with usual interstitial pneumonia and probable usual interstitial pneumonia. N = 103.
Table 5. The relationship of comorbidities and/or risk factors with tomographic studies compatible with usual interstitial pneumonia and probable usual interstitial pneumonia. N = 103.

Objective
To determine the main clinical and tomographic characteristics of patients with diffuse interstitial lung disease at Trujillo Regional Teaching Hospital.
Methods
Case series. Tomographic examinations and clinical data were obtained from patients with interstitial pulmonary disease who attended the pulmonology service of Trujillo Regional Teaching Hospital. The information collected was recorded and systematized in Excel. For the statistical analysis, SPSS 23.0 program was used.
Results
Data from 103 patients were obtained, of which 60.2% were female, and 39.8% were male. The average age was 72 years for both groups. Main clinical manifestations were cough (82.5%), dyspnea (76.7%), joint pain (43.7%), weight loss (40.8%), velcro crackles (35%) and digital clubbing (28.2%). Exposure to wood smoke was present in 46.6%, exposure to inorganic dust in 12.6% and fowl ownership in 9.7% of cases. Thirty-one (30.1%) patients presented comorbidities. Among these, rheumatic diseases and arterial hypertension were the most frequent. Non-specific interstitial pneumonia pattern was present in 26.2% of the cases; probable usual interstitial pneumonia in 16.5%; organized type in 12.6%; usual interstitial in 10.7%; acute interstitial in 2.9% and 27.1% had no defined tomographic pattern.
Conclusions
In the studied population, clinical and tomographic characteristics of interstitial lung parenchymal diseases are variable in magnitude and forms of presentation. Female sex and exposure to fuels were the most frequent associated factors. Connective tissue diseases could also explain study findings.
 Authors:
Luis Alejandro Rodríguez-Hidalgo[1,2], Luis Alberto Concepción-Urteaga[1,2], Julio Santos Hilario-Vargas[3], Jorge Luis Cornejo-Portella[1,2], Oscar Nieri Alquizar-Horna[1,2]
Authors:
Luis Alejandro Rodríguez-Hidalgo[1,2], Luis Alberto Concepción-Urteaga[1,2], Julio Santos Hilario-Vargas[3], Jorge Luis Cornejo-Portella[1,2], Oscar Nieri Alquizar-Horna[1,2]
Affiliation:
[1] Hospital Regional Docente de Trujillo, Trujillo, Perú
[2] Departamento de Medicina, Facultad de Medicina de la Universidad Nacional de Trujillo, Trujillo, Perú
[3] Departamento de Fisiología, Facultad de Medicina Universidad Nacional de Trujillo, Trujillo, Perú
E-mail: alejandrorh2011@gmail.com
Author address:
[1] Manzana “A” Lote 1-M, Urbanización Los Portales del Golf
Víctor Larco, Trujillo
Perú
13009

Citation: Rodríguez-Hidalgo LA, Concepción-Urteaga LA, Hilario-Vargas JS, Cornejo-Portella JL, Alquizar-Horna ON. Clinical and tomographic characterization of patients with interstitial lung disease at the Trujillo Regional Teaching Hospital, Peru. Medwave 2021;21(05):e8221 doi: 10.5867/medwave.2021.05.8221
Submission date: 24/7/2020
Acceptance date: 2/5/2021
Publication date: 29/6/2021
Origin: Not commissioned.
Type of review: Externally peer-reviewed by two reviewers, double-blind.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013 Sep 15;188(6):733-48. | CrossRef | PubMed |
- Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du Bois RM, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015 Oct;46(4):976-87. | CrossRef | PubMed |
- Fischer A, Collard HR, Cottin V; “ERS/ATS Task Force on Undifferentiated Forms of Connective Tissue Disease-associated Interstitial Lung Disease”. Interstitial pneumonia with autoimmune features: the new consensus-based definition for this cohort of patients should be broadened. Eur Respir J. 2016 Apr;47(4):1295-6. | CrossRef | PubMed |
- American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002 Jan 15;165(2):277-304. | CrossRef | PubMed |
- Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL. Progressive Decline of Lung Function in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2017 Mar;69(3):542-549. | CrossRef | PubMed |
- Guler SA, Winstone TA, Murphy D, Hague C, Soon J, Sulaiman N, et al. Does Systemic Sclerosis-associated Interstitial Lung Disease Burn Out? Specific Phenotypes of Disease Progression. Ann Am Thorac Soc. 2018 Dec;15(12):1427-1433. | CrossRef | PubMed |
- Reiseter S, Gunnarsson R, Mogens Aaløkken T, Lund MB, Mynarek G, Corander J, et al. Progression and mortality of interstitial lung disease in mixed connective tissue disease: a long-term observational nationwide cohort study. Rheumatology (Oxford). 2018 Feb 1;57(2):255-262. | CrossRef | PubMed |
- Flaherty KR. High-resolution computed tomography and the many faces of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008 Feb 15;177(4):367-8. | CrossRef | PubMed |
- Johkoh T, Müller NL, Cartier Y, Kavanagh PV, Hartman TE, Akira M, et al. Idiopathic interstitial pneumonias: diagnostic accuracy of thin-section CT in 129 patients. Radiology. 1999 May;211(2):555-60. | CrossRef | PubMed |
- Wong AW, Ryerson CJ, Guler SA. Progression of fibrosing interstitial lung disease. Respir Res. 2020 Jan 29;21(1):32. | CrossRef | PubMed |
- Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011 Feb 15;183(4):431-40. | CrossRef | PubMed |
- Caminati A, Madotto F, Cesana G, Conti S, Harari S. Epidemiological studies in idiopathic pulmonary fibrosis: pitfalls in methodologies and data interpretation. Eur Respir Rev. 2015 Sep;24(137):436-44. | CrossRef | PubMed |
- Rivas-Vargas D. Análisis del índice masa corporal y factores de riesgo de neumopatía intersticial en pacientes con esclerosis sistémica. Rev Colomb Reumatol. 1 de enero de 2020;27(1):9-19. | CrossRef |
- Benítez-Cano M, Hernández J, Sierra LM, El Boutaibi K, Gómez MT, Sanz A, et al. Epidemiología de las enfermedades pulmonares intersticiales difusas idiopáticas. Arch Bronchoneumol. 2018; 54 Supl Congr 1: 115. [citado 7 de mayo de 2021]. [Internet]. | Link |
- Rice MB, Li W, Schwartz J, Di Q, Kloog I, Koutrakis P, et al. Ambient air pollution exposure and risk and progression of interstitial lung abnormalities: the Framingham Heart Study. Thorax. 2019 Nov;74(11):1063-1069. | CrossRef | PubMed |
- Johannson KA, Balmes JR, Collard HR. Air pollution exposure: a novel environmental risk factor for interstitial lung disease? Chest. 2015 Apr;147(4):1161-1167. | CrossRef | PubMed |
- Selman M, Pardo A, King TE Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012 Aug 15;186(4):314-24. | CrossRef | PubMed |
- Fernández JM, María Nieto A, Bustos A. Enfermedades pulmonares intersticiales. Abordaje diagnóstico. Monografías Neumomadrid. volumen XII / 2008. [Internet]. | Link |
- Orens JB, Kazerooni EA, Martinez FJ, Curtis JL, Gross BH, Flint A, et al. The sensitivity of high-resolution CT in detecting idiopathic pulmonary fibrosis proved by open lung biopsy. A prospective study. Chest. 1995 Jul;108(1):109-15. | CrossRef | PubMed |
- Johkoh T, Müller NL, Nakamura H. Multidetector spiral high-resolution computed tomography of the lungs: distribution of findings on coronal image reconstructions. J Thorac Imaging. 2002 Oct;17(4):291-305. | CrossRef | PubMed |
- Johkoh T, Honda O, Yamamoto S, Tomiyama N, Koyama M, Kozuka T, et al. Evaluation of image quality and spatial resolution of low-dose high-pitch multidetector-row helical high-resolution CT in 11 autopsy lungs and a wire phantom. Radiat Med. 2001 Nov-Dec;19(6):279-84. | PubMed |
- Giménez Palleiro A, Franquet T. Patrones radiológicos en la enfermedad pulmonar intersticial. Semin Fund Esp Reumatol. 1 de octubre de 2013;14(4):97-105. | CrossRef |
- Díaz AG, Miranda BC, Características clínicas y prevalencia de la enfermedad pulmonar intersticial difusa en el Hospital Nacional Ramiro Prialé Prialé – EsSalud. Huancayo 2012 [Tesis de grado]. Huancayo: Universidad del Centro del Perú; 2012. [citado 2020 Mar 31]. [Internet]. | Link |
- Dueñas C; Alejandro L; Manzano AC; Ojeda P. Enfermedad pulmonar intersticial difusa (EPID): experiencia clínica, fisiológica y radiopatológica en 60 pacientes. Acta Méd Colomb 1991. [citado 2020 Mar 31]; 16(3):110-7. [Internet]. | Link |
- Chahuán S JM, Fuenzalida L MJ, Cataldo V P, Lagos C M, De La Fuente M I, Pereira R G, et al. Caracterización clínica, serológica y patrón radiológico de una cohorte unicéntrica de pacientes con enfermedad pulmonar difusa. Rev Chil Enfermedades Respir. marzo de 2017;33(1):31-6. | CrossRef |
- Díaz C, Consani S, Torres V, Alonso F, Berez A. Estudio multicéntrico descriptivo de enfermedades pulmonares intersticiales asociadas a enfermedades autoinmunes en centros de salud en Montevideo, Uruguay. Rev. urug. med. Int. 2018 oct. [citado 2020 Abr 01]; 3(3): 12-19. [Internet]. | CrossRef | Link |
- Vargas GR, Canales CF, Bioque RJ. Manual de diagnóstico y terapéutica en neumología. 1era Edición 2005 Madrid. Enfermedad pulmonar difusa I. Fibrosis pulmonar idiopática pág. 363-372. [consultado 2020 Mar 31]. [Internet]. | Link |
- Poalasín Narváez LA, González Benítez SN, Bascó Fuentes EL, González Gavilánez AM, Poalasín Narváez LA, González Benítez SN, et al. Enfermedades del tejido conectivo y sus cambios morfoestructurales. Rev Cuba Reumatol. diciembre de 2018 [citado 7 de mayo de 2021];20(3). [Internet]. | CrossRef | Link |
- Xaubetm A, Ancochea J, Blanquer R, Montero C, Morell F, Rodríguez-Becerra E, et al. Diagnóstico y tratamiento de las enfermedades pulmonares intersticiales difusas. Arch Bronconeumol 2003;39(12):580-600. [citado 2020 Abr 01]. [Internet]. | Link |
- Soriano AS, Rivera JCB, Tejero DP, Osuna LS, González BP. Enfermedades intersticiales difusas del pulmón secundarias o asociadas a procesos no bien definidos.14. [citado 2020 Abr 10]. [Internet]. | Link |
- Restrepo J, Reyes P, De Ochoa P, Patiño E. Neumoconiosis por inhalación de humo de leña. Acta Med. Col. 1983,8:191-204. [citado 2020 Abr 10]. [Internet]. | Link |
- Vélez H., Barrero J., Restrepo J., Rojas W. Corporación para Investigaciones Biológicas, Medellín Colombia. "Fundamentos de Medicina- Neumología". 1989, 3a. edición: 274-275.
- Chacón Chaves R., Alfara Rodríguez C. Neumopatía asociada a la inhalación de humo de leña. Rev. Cost. Cien. Med. 1991:13(3,4): 7-13. [citado 2020 Abr 10]. [Internet]. | Link |
- Instituto Nacional de Estadística e Informática Perú. Hogares en los que cocinan con combustibles contaminantes. Abril 2019. [citado 2020 Abr 10]. [Internet]. | Link |
- Pereira CA, Gimenez A, Kuranishi L, Storrer K. Chronic hypersensitivity pneumonitis. J Asthma Allergy. 2016 Sep 21;9:171-181. | CrossRef | PubMed |
- Churg A, Bilawich A, Wright JL. Pathology of Chronic Hypersensitivity Pneumonitis What Is It? What Are the Diagnostic Criteria? Why Do We Care? Arch Pathol Lab Med. 2018 Jan;142(1):109-119. | CrossRef | PubMed |
- Rodas SM, Espinoza DG. Caracterización clínica y epidemiológica de los pacientes con enfermedades pulmonares intersticiales difusas que acudieron al área de neumología del Hospital Teodoro Maldonado Carbo – IESS del 2006 AL 2016. [Tesis de titulación]. Guayaquil: Universidad de especialidades Espíritu Santo, Facultad de Ciencias Médicas, Escuela de medicina;2018. [citado 2020 Mar 31]. [Internet]. | Link |
- Behr J, Kreuter M, Hoeper MM, Wirtz H, Klotsche J, Koschel D, et al. Management of patients with idiopathic pulmonary fibrosis in clinical practice: the INSIGHTS-IPF registry. Eur Respir J. 2015 Jul;46(1):186-96. | CrossRef | PubMed |
- Villar G A. Neumonitis por hipersensibilidad y fibrosis pulmonar: estudio etiológico y del perfil inflamatorio. Universitat Autónoma de Barcelona. Junio 2017. [Tesis doctoral][ citado en 2020 Mar 31]. [Internet] | Link |
- Xaubet A, Molina-Molina M, Ancochea J. Fibrosis pulmonar relacionada con el tabaco. Medicina respiratoria. 2015 [citado 2020 Mar 31]; 8 (1): 39-46. [citado 2020 Abr 21]. [Internet]. | Link |
- Trinidad GD, Landin LC, Hamdan PN, et al. Interstitial lung disease associated to metrotexate. Neumol Cir Torax. 2009; 68(1):35-40. [Internet] | Link |
- Raghu G, Freudenberger TD, Yang S, Curtis JR, Spada C, Hayes J, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006 Jan;27(1):136-42. | CrossRef | PubMed |
- Gaudiano J, Betolaza S de, Amaral M. Descripción de una población de pacientes portadores de enfermedad pulmonar intersticial asistidos en el Hospital Pasteur de Montevideo, Uruguay. Rev Urug Med Interna. 2020;5(2):9-16. | CrossRef |
- Buitrón UF, Rojas DA. Validez de dos escalas pronosticas de mortalidad por enfermedad pulmonar intersticial difusa en pacientes de la altura - Huancayo del 2014 al 2015. [Tesis de titulación]. Huancayo: Universidad Nacional del centro, Facultad de medicina; 2015. [citado 2020 Abr 21]. [Internet]. | Link |
- Raghu G, Meyer KC. Silent gastro-oesophageal reflux and microaspiration in IPF: mounting evidence for anti-reflux therapy? Eur Respir J. 2012 Feb;39(2):242-5. | CrossRef | PubMed |
- Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002 Aug 15;166(4):510-3. | CrossRef | PubMed |
- Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, et al. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol. 2003 Jun;41(6):2633-40. | CrossRef | PubMed |
- Zamò A, Poletti V, Reghellin D, Montagna L, Pedron S, Piccoli P, et al. HHV-8 and EBV are not commonly found in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005 Jun;22(2):123-8. | CrossRef |
- Arase Y, Ikeda K, Tsubota A, Saitoh S, Suzuki Y, Kobayashi M, et al. Usefulness of serum KL-6 for early diagnosis of idiopathic pulmonary fibrosis in patients with hepatitis C virus. Hepatol Res. 2003 Oct;27(2):89-94. | CrossRef | PubMed |
- Raghu G, Amatto VC, Behr J, Stowasser S. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J. 2015 Oct;46(4):1113-30. | CrossRef | PubMed |
- Margaritopoulos GA, Antoniou KM, Wells AU. Comorbidities in interstitial lung diseases. Eur Respir Rev. 2017 Jan 3;26(143):160027. | CrossRef | PubMed |
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018 Sep 1;198(5):e44-e68. | CrossRef | PubMed |
- Devaraj A. Imaging: how to recognise idiopathic pulmonary fibrosis. Eur Respir Rev. 2014 Jun;23(132):215-9. | CrossRef | PubMed |
- Gómez Carrera L, Bonilla Hernan G. Pulmonary manifestations of collagen diseases. Arch Bronconeumol. 2013 Jun;49(6):249-60. | CrossRef | PubMed |
- Tzelepis GE, Toya SP, Moutsopoulos HM. Occult connective tissue diseases mimicking idiopathic interstitial pneumonias. Eur Respir J. 2008 Jan;31(1):11-20. | CrossRef | PubMed |
 Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013 Sep 15;188(6):733-48. | CrossRef | PubMed |
Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013 Sep 15;188(6):733-48. | CrossRef | PubMed | Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du Bois RM, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015 Oct;46(4):976-87. | CrossRef | PubMed |
Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du Bois RM, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015 Oct;46(4):976-87. | CrossRef | PubMed | Fischer A, Collard HR, Cottin V; “ERS/ATS Task Force on Undifferentiated Forms of Connective Tissue Disease-associated Interstitial Lung Disease”. Interstitial pneumonia with autoimmune features: the new consensus-based definition for this cohort of patients should be broadened. Eur Respir J. 2016 Apr;47(4):1295-6. | CrossRef | PubMed |
Fischer A, Collard HR, Cottin V; “ERS/ATS Task Force on Undifferentiated Forms of Connective Tissue Disease-associated Interstitial Lung Disease”. Interstitial pneumonia with autoimmune features: the new consensus-based definition for this cohort of patients should be broadened. Eur Respir J. 2016 Apr;47(4):1295-6. | CrossRef | PubMed | American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002 Jan 15;165(2):277-304. | CrossRef | PubMed |
American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002 Jan 15;165(2):277-304. | CrossRef | PubMed | Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL. Progressive Decline of Lung Function in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2017 Mar;69(3):542-549. | CrossRef | PubMed |
Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL. Progressive Decline of Lung Function in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2017 Mar;69(3):542-549. | CrossRef | PubMed | Guler SA, Winstone TA, Murphy D, Hague C, Soon J, Sulaiman N, et al. Does Systemic Sclerosis-associated Interstitial Lung Disease Burn Out? Specific Phenotypes of Disease Progression. Ann Am Thorac Soc. 2018 Dec;15(12):1427-1433. | CrossRef | PubMed |
Guler SA, Winstone TA, Murphy D, Hague C, Soon J, Sulaiman N, et al. Does Systemic Sclerosis-associated Interstitial Lung Disease Burn Out? Specific Phenotypes of Disease Progression. Ann Am Thorac Soc. 2018 Dec;15(12):1427-1433. | CrossRef | PubMed | Reiseter S, Gunnarsson R, Mogens Aaløkken T, Lund MB, Mynarek G, Corander J, et al. Progression and mortality of interstitial lung disease in mixed connective tissue disease: a long-term observational nationwide cohort study. Rheumatology (Oxford). 2018 Feb 1;57(2):255-262. | CrossRef | PubMed |
Reiseter S, Gunnarsson R, Mogens Aaløkken T, Lund MB, Mynarek G, Corander J, et al. Progression and mortality of interstitial lung disease in mixed connective tissue disease: a long-term observational nationwide cohort study. Rheumatology (Oxford). 2018 Feb 1;57(2):255-262. | CrossRef | PubMed | Flaherty KR. High-resolution computed tomography and the many faces of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008 Feb 15;177(4):367-8. | CrossRef | PubMed |
Flaherty KR. High-resolution computed tomography and the many faces of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008 Feb 15;177(4):367-8. | CrossRef | PubMed | Johkoh T, Müller NL, Cartier Y, Kavanagh PV, Hartman TE, Akira M, et al. Idiopathic interstitial pneumonias: diagnostic accuracy of thin-section CT in 129 patients. Radiology. 1999 May;211(2):555-60. | CrossRef | PubMed |
Johkoh T, Müller NL, Cartier Y, Kavanagh PV, Hartman TE, Akira M, et al. Idiopathic interstitial pneumonias: diagnostic accuracy of thin-section CT in 129 patients. Radiology. 1999 May;211(2):555-60. | CrossRef | PubMed | Wong AW, Ryerson CJ, Guler SA. Progression of fibrosing interstitial lung disease. Respir Res. 2020 Jan 29;21(1):32. | CrossRef | PubMed |
Wong AW, Ryerson CJ, Guler SA. Progression of fibrosing interstitial lung disease. Respir Res. 2020 Jan 29;21(1):32. | CrossRef | PubMed | Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011 Feb 15;183(4):431-40. | CrossRef | PubMed |
Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011 Feb 15;183(4):431-40. | CrossRef | PubMed | Caminati A, Madotto F, Cesana G, Conti S, Harari S. Epidemiological studies in idiopathic pulmonary fibrosis: pitfalls in methodologies and data interpretation. Eur Respir Rev. 2015 Sep;24(137):436-44. | CrossRef | PubMed |
Caminati A, Madotto F, Cesana G, Conti S, Harari S. Epidemiological studies in idiopathic pulmonary fibrosis: pitfalls in methodologies and data interpretation. Eur Respir Rev. 2015 Sep;24(137):436-44. | CrossRef | PubMed | Rivas-Vargas D. Análisis del índice masa corporal y factores de riesgo de neumopatía intersticial en pacientes con esclerosis sistémica. Rev Colomb Reumatol. 1 de enero de 2020;27(1):9-19. | CrossRef |
Rivas-Vargas D. Análisis del índice masa corporal y factores de riesgo de neumopatía intersticial en pacientes con esclerosis sistémica. Rev Colomb Reumatol. 1 de enero de 2020;27(1):9-19. | CrossRef | Benítez-Cano M, Hernández J, Sierra LM, El Boutaibi K, Gómez MT, Sanz A, et al. Epidemiología de las enfermedades pulmonares intersticiales difusas idiopáticas. Arch Bronchoneumol. 2018; 54 Supl Congr 1: 115. [citado 7 de mayo de 2021]. [Internet]. | Link |
Benítez-Cano M, Hernández J, Sierra LM, El Boutaibi K, Gómez MT, Sanz A, et al. Epidemiología de las enfermedades pulmonares intersticiales difusas idiopáticas. Arch Bronchoneumol. 2018; 54 Supl Congr 1: 115. [citado 7 de mayo de 2021]. [Internet]. | Link | Rice MB, Li W, Schwartz J, Di Q, Kloog I, Koutrakis P, et al. Ambient air pollution exposure and risk and progression of interstitial lung abnormalities: the Framingham Heart Study. Thorax. 2019 Nov;74(11):1063-1069. | CrossRef | PubMed |
Rice MB, Li W, Schwartz J, Di Q, Kloog I, Koutrakis P, et al. Ambient air pollution exposure and risk and progression of interstitial lung abnormalities: the Framingham Heart Study. Thorax. 2019 Nov;74(11):1063-1069. | CrossRef | PubMed | Johannson KA, Balmes JR, Collard HR. Air pollution exposure: a novel environmental risk factor for interstitial lung disease? Chest. 2015 Apr;147(4):1161-1167. | CrossRef | PubMed |
Johannson KA, Balmes JR, Collard HR. Air pollution exposure: a novel environmental risk factor for interstitial lung disease? Chest. 2015 Apr;147(4):1161-1167. | CrossRef | PubMed | Selman M, Pardo A, King TE Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012 Aug 15;186(4):314-24. | CrossRef | PubMed |
Selman M, Pardo A, King TE Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012 Aug 15;186(4):314-24. | CrossRef | PubMed | Fernández JM, María Nieto A, Bustos A. Enfermedades pulmonares intersticiales. Abordaje diagnóstico. Monografías Neumomadrid. volumen XII / 2008. [Internet]. | Link |
Fernández JM, María Nieto A, Bustos A. Enfermedades pulmonares intersticiales. Abordaje diagnóstico. Monografías Neumomadrid. volumen XII / 2008. [Internet]. | Link | Orens JB, Kazerooni EA, Martinez FJ, Curtis JL, Gross BH, Flint A, et al. The sensitivity of high-resolution CT in detecting idiopathic pulmonary fibrosis proved by open lung biopsy. A prospective study. Chest. 1995 Jul;108(1):109-15. | CrossRef | PubMed |
Orens JB, Kazerooni EA, Martinez FJ, Curtis JL, Gross BH, Flint A, et al. The sensitivity of high-resolution CT in detecting idiopathic pulmonary fibrosis proved by open lung biopsy. A prospective study. Chest. 1995 Jul;108(1):109-15. | CrossRef | PubMed | Johkoh T, Müller NL, Nakamura H. Multidetector spiral high-resolution computed tomography of the lungs: distribution of findings on coronal image reconstructions. J Thorac Imaging. 2002 Oct;17(4):291-305. | CrossRef | PubMed |
Johkoh T, Müller NL, Nakamura H. Multidetector spiral high-resolution computed tomography of the lungs: distribution of findings on coronal image reconstructions. J Thorac Imaging. 2002 Oct;17(4):291-305. | CrossRef | PubMed | Johkoh T, Honda O, Yamamoto S, Tomiyama N, Koyama M, Kozuka T, et al. Evaluation of image quality and spatial resolution of low-dose high-pitch multidetector-row helical high-resolution CT in 11 autopsy lungs and a wire phantom. Radiat Med. 2001 Nov-Dec;19(6):279-84. | PubMed |
Johkoh T, Honda O, Yamamoto S, Tomiyama N, Koyama M, Kozuka T, et al. Evaluation of image quality and spatial resolution of low-dose high-pitch multidetector-row helical high-resolution CT in 11 autopsy lungs and a wire phantom. Radiat Med. 2001 Nov-Dec;19(6):279-84. | PubMed | Giménez Palleiro A, Franquet T. Patrones radiológicos en la enfermedad pulmonar intersticial. Semin Fund Esp Reumatol. 1 de octubre de 2013;14(4):97-105. | CrossRef |
Giménez Palleiro A, Franquet T. Patrones radiológicos en la enfermedad pulmonar intersticial. Semin Fund Esp Reumatol. 1 de octubre de 2013;14(4):97-105. | CrossRef | Díaz AG, Miranda BC, Características clínicas y prevalencia de la enfermedad pulmonar intersticial difusa en el Hospital Nacional Ramiro Prialé Prialé – EsSalud. Huancayo 2012 [Tesis de grado]. Huancayo: Universidad del Centro del Perú; 2012. [citado 2020 Mar 31]. [Internet]. | Link |
Díaz AG, Miranda BC, Características clínicas y prevalencia de la enfermedad pulmonar intersticial difusa en el Hospital Nacional Ramiro Prialé Prialé – EsSalud. Huancayo 2012 [Tesis de grado]. Huancayo: Universidad del Centro del Perú; 2012. [citado 2020 Mar 31]. [Internet]. | Link | Dueñas C; Alejandro L; Manzano AC; Ojeda P. Enfermedad pulmonar intersticial difusa (EPID): experiencia clínica, fisiológica y radiopatológica en 60 pacientes. Acta Méd Colomb 1991. [citado 2020 Mar 31]; 16(3):110-7. [Internet]. | Link |
Dueñas C; Alejandro L; Manzano AC; Ojeda P. Enfermedad pulmonar intersticial difusa (EPID): experiencia clínica, fisiológica y radiopatológica en 60 pacientes. Acta Méd Colomb 1991. [citado 2020 Mar 31]; 16(3):110-7. [Internet]. | Link | Chahuán S JM, Fuenzalida L MJ, Cataldo V P, Lagos C M, De La Fuente M I, Pereira R G, et al. Caracterización clínica, serológica y patrón radiológico de una cohorte unicéntrica de pacientes con enfermedad pulmonar difusa. Rev Chil Enfermedades Respir. marzo de 2017;33(1):31-6. | CrossRef |
Chahuán S JM, Fuenzalida L MJ, Cataldo V P, Lagos C M, De La Fuente M I, Pereira R G, et al. Caracterización clínica, serológica y patrón radiológico de una cohorte unicéntrica de pacientes con enfermedad pulmonar difusa. Rev Chil Enfermedades Respir. marzo de 2017;33(1):31-6. | CrossRef | Díaz C, Consani S, Torres V, Alonso F, Berez A. Estudio multicéntrico descriptivo de enfermedades pulmonares intersticiales asociadas a enfermedades autoinmunes en centros de salud en Montevideo, Uruguay. Rev. urug. med. Int. 2018 oct. [citado 2020 Abr 01]; 3(3): 12-19. [Internet]. | CrossRef | Link |
Díaz C, Consani S, Torres V, Alonso F, Berez A. Estudio multicéntrico descriptivo de enfermedades pulmonares intersticiales asociadas a enfermedades autoinmunes en centros de salud en Montevideo, Uruguay. Rev. urug. med. Int. 2018 oct. [citado 2020 Abr 01]; 3(3): 12-19. [Internet]. | CrossRef | Link | Vargas GR, Canales CF, Bioque RJ. Manual de diagnóstico y terapéutica en neumología. 1era Edición 2005 Madrid. Enfermedad pulmonar difusa I. Fibrosis pulmonar idiopática pág. 363-372. [consultado 2020 Mar 31]. [Internet]. | Link |
Vargas GR, Canales CF, Bioque RJ. Manual de diagnóstico y terapéutica en neumología. 1era Edición 2005 Madrid. Enfermedad pulmonar difusa I. Fibrosis pulmonar idiopática pág. 363-372. [consultado 2020 Mar 31]. [Internet]. | Link | Poalasín Narváez LA, González Benítez SN, Bascó Fuentes EL, González Gavilánez AM, Poalasín Narváez LA, González Benítez SN, et al. Enfermedades del tejido conectivo y sus cambios morfoestructurales. Rev Cuba Reumatol. diciembre de 2018 [citado 7 de mayo de 2021];20(3). [Internet]. | CrossRef | Link |
Poalasín Narváez LA, González Benítez SN, Bascó Fuentes EL, González Gavilánez AM, Poalasín Narváez LA, González Benítez SN, et al. Enfermedades del tejido conectivo y sus cambios morfoestructurales. Rev Cuba Reumatol. diciembre de 2018 [citado 7 de mayo de 2021];20(3). [Internet]. | CrossRef | Link | Xaubetm A, Ancochea J, Blanquer R, Montero C, Morell F, Rodríguez-Becerra E, et al. Diagnóstico y tratamiento de las enfermedades pulmonares intersticiales difusas. Arch Bronconeumol 2003;39(12):580-600. [citado 2020 Abr 01]. [Internet]. | Link |
Xaubetm A, Ancochea J, Blanquer R, Montero C, Morell F, Rodríguez-Becerra E, et al. Diagnóstico y tratamiento de las enfermedades pulmonares intersticiales difusas. Arch Bronconeumol 2003;39(12):580-600. [citado 2020 Abr 01]. [Internet]. | Link | Soriano AS, Rivera JCB, Tejero DP, Osuna LS, González BP. Enfermedades intersticiales difusas del pulmón secundarias o asociadas a procesos no bien definidos.14. [citado 2020 Abr 10]. [Internet]. | Link |
Soriano AS, Rivera JCB, Tejero DP, Osuna LS, González BP. Enfermedades intersticiales difusas del pulmón secundarias o asociadas a procesos no bien definidos.14. [citado 2020 Abr 10]. [Internet]. | Link | Restrepo J, Reyes P, De Ochoa P, Patiño E. Neumoconiosis por inhalación de humo de leña. Acta Med. Col. 1983,8:191-204. [citado 2020 Abr 10]. [Internet]. | Link |
Restrepo J, Reyes P, De Ochoa P, Patiño E. Neumoconiosis por inhalación de humo de leña. Acta Med. Col. 1983,8:191-204. [citado 2020 Abr 10]. [Internet]. | Link | Vélez H., Barrero J., Restrepo J., Rojas W. Corporación para Investigaciones Biológicas, Medellín Colombia. "Fundamentos de Medicina- Neumología". 1989, 3a. edición: 274-275.
Vélez H., Barrero J., Restrepo J., Rojas W. Corporación para Investigaciones Biológicas, Medellín Colombia. "Fundamentos de Medicina- Neumología". 1989, 3a. edición: 274-275.  Chacón Chaves R., Alfara Rodríguez C. Neumopatía asociada a la inhalación de humo de leña. Rev. Cost. Cien. Med. 1991:13(3,4): 7-13. [citado 2020 Abr 10]. [Internet]. | Link |
Chacón Chaves R., Alfara Rodríguez C. Neumopatía asociada a la inhalación de humo de leña. Rev. Cost. Cien. Med. 1991:13(3,4): 7-13. [citado 2020 Abr 10]. [Internet]. | Link | Instituto Nacional de Estadística e Informática Perú. Hogares en los que cocinan con combustibles contaminantes. Abril 2019. [citado 2020 Abr 10]. [Internet]. | Link |
Instituto Nacional de Estadística e Informática Perú. Hogares en los que cocinan con combustibles contaminantes. Abril 2019. [citado 2020 Abr 10]. [Internet]. | Link | Pereira CA, Gimenez A, Kuranishi L, Storrer K. Chronic hypersensitivity pneumonitis. J Asthma Allergy. 2016 Sep 21;9:171-181. | CrossRef | PubMed |
Pereira CA, Gimenez A, Kuranishi L, Storrer K. Chronic hypersensitivity pneumonitis. J Asthma Allergy. 2016 Sep 21;9:171-181. | CrossRef | PubMed | Churg A, Bilawich A, Wright JL. Pathology of Chronic Hypersensitivity Pneumonitis What Is It? What Are the Diagnostic Criteria? Why Do We Care? Arch Pathol Lab Med. 2018 Jan;142(1):109-119. | CrossRef | PubMed |
Churg A, Bilawich A, Wright JL. Pathology of Chronic Hypersensitivity Pneumonitis What Is It? What Are the Diagnostic Criteria? Why Do We Care? Arch Pathol Lab Med. 2018 Jan;142(1):109-119. | CrossRef | PubMed | Rodas SM, Espinoza DG. Caracterización clínica y epidemiológica de los pacientes con enfermedades pulmonares intersticiales difusas que acudieron al área de neumología del Hospital Teodoro Maldonado Carbo – IESS del 2006 AL 2016. [Tesis de titulación]. Guayaquil: Universidad de especialidades Espíritu Santo, Facultad de Ciencias Médicas, Escuela de medicina;2018. [citado 2020 Mar 31]. [Internet]. | Link |
Rodas SM, Espinoza DG. Caracterización clínica y epidemiológica de los pacientes con enfermedades pulmonares intersticiales difusas que acudieron al área de neumología del Hospital Teodoro Maldonado Carbo – IESS del 2006 AL 2016. [Tesis de titulación]. Guayaquil: Universidad de especialidades Espíritu Santo, Facultad de Ciencias Médicas, Escuela de medicina;2018. [citado 2020 Mar 31]. [Internet]. | Link | Behr J, Kreuter M, Hoeper MM, Wirtz H, Klotsche J, Koschel D, et al. Management of patients with idiopathic pulmonary fibrosis in clinical practice: the INSIGHTS-IPF registry. Eur Respir J. 2015 Jul;46(1):186-96. | CrossRef | PubMed |
Behr J, Kreuter M, Hoeper MM, Wirtz H, Klotsche J, Koschel D, et al. Management of patients with idiopathic pulmonary fibrosis in clinical practice: the INSIGHTS-IPF registry. Eur Respir J. 2015 Jul;46(1):186-96. | CrossRef | PubMed | Villar G A. Neumonitis por hipersensibilidad y fibrosis pulmonar: estudio etiológico y del perfil inflamatorio. Universitat Autónoma de Barcelona. Junio 2017. [Tesis doctoral][ citado en 2020 Mar 31]. [Internet] | Link |
Villar G A. Neumonitis por hipersensibilidad y fibrosis pulmonar: estudio etiológico y del perfil inflamatorio. Universitat Autónoma de Barcelona. Junio 2017. [Tesis doctoral][ citado en 2020 Mar 31]. [Internet] | Link | Xaubet A, Molina-Molina M, Ancochea J. Fibrosis pulmonar relacionada con el tabaco. Medicina respiratoria. 2015 [citado 2020 Mar 31]; 8 (1): 39-46. [citado 2020 Abr 21]. [Internet]. | Link |
Xaubet A, Molina-Molina M, Ancochea J. Fibrosis pulmonar relacionada con el tabaco. Medicina respiratoria. 2015 [citado 2020 Mar 31]; 8 (1): 39-46. [citado 2020 Abr 21]. [Internet]. | Link | Trinidad GD, Landin LC, Hamdan PN, et al. Interstitial lung disease associated to metrotexate. Neumol Cir Torax. 2009; 68(1):35-40. [Internet] | Link |
Trinidad GD, Landin LC, Hamdan PN, et al. Interstitial lung disease associated to metrotexate. Neumol Cir Torax. 2009; 68(1):35-40. [Internet] | Link | Raghu G, Freudenberger TD, Yang S, Curtis JR, Spada C, Hayes J, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006 Jan;27(1):136-42. | CrossRef | PubMed |
Raghu G, Freudenberger TD, Yang S, Curtis JR, Spada C, Hayes J, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006 Jan;27(1):136-42. | CrossRef | PubMed | Gaudiano J, Betolaza S de, Amaral M. Descripción de una población de pacientes portadores de enfermedad pulmonar intersticial asistidos en el Hospital Pasteur de Montevideo, Uruguay. Rev Urug Med Interna. 2020;5(2):9-16. | CrossRef |
Gaudiano J, Betolaza S de, Amaral M. Descripción de una población de pacientes portadores de enfermedad pulmonar intersticial asistidos en el Hospital Pasteur de Montevideo, Uruguay. Rev Urug Med Interna. 2020;5(2):9-16. | CrossRef | Buitrón UF, Rojas DA. Validez de dos escalas pronosticas de mortalidad por enfermedad pulmonar intersticial difusa en pacientes de la altura - Huancayo del 2014 al 2015. [Tesis de titulación]. Huancayo: Universidad Nacional del centro, Facultad de medicina; 2015. [citado 2020 Abr 21]. [Internet]. | Link |
Buitrón UF, Rojas DA. Validez de dos escalas pronosticas de mortalidad por enfermedad pulmonar intersticial difusa en pacientes de la altura - Huancayo del 2014 al 2015. [Tesis de titulación]. Huancayo: Universidad Nacional del centro, Facultad de medicina; 2015. [citado 2020 Abr 21]. [Internet]. | Link | Raghu G, Meyer KC. Silent gastro-oesophageal reflux and microaspiration in IPF: mounting evidence for anti-reflux therapy? Eur Respir J. 2012 Feb;39(2):242-5. | CrossRef | PubMed |
Raghu G, Meyer KC. Silent gastro-oesophageal reflux and microaspiration in IPF: mounting evidence for anti-reflux therapy? Eur Respir J. 2012 Feb;39(2):242-5. | CrossRef | PubMed | Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002 Aug 15;166(4):510-3. | CrossRef | PubMed |
Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002 Aug 15;166(4):510-3. | CrossRef | PubMed | Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, et al. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol. 2003 Jun;41(6):2633-40. | CrossRef | PubMed |
Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, et al. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol. 2003 Jun;41(6):2633-40. | CrossRef | PubMed | Zamò A, Poletti V, Reghellin D, Montagna L, Pedron S, Piccoli P, et al. HHV-8 and EBV are not commonly found in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005 Jun;22(2):123-8. | CrossRef |
Zamò A, Poletti V, Reghellin D, Montagna L, Pedron S, Piccoli P, et al. HHV-8 and EBV are not commonly found in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005 Jun;22(2):123-8. | CrossRef | Arase Y, Ikeda K, Tsubota A, Saitoh S, Suzuki Y, Kobayashi M, et al. Usefulness of serum KL-6 for early diagnosis of idiopathic pulmonary fibrosis in patients with hepatitis C virus. Hepatol Res. 2003 Oct;27(2):89-94. | CrossRef | PubMed |
Arase Y, Ikeda K, Tsubota A, Saitoh S, Suzuki Y, Kobayashi M, et al. Usefulness of serum KL-6 for early diagnosis of idiopathic pulmonary fibrosis in patients with hepatitis C virus. Hepatol Res. 2003 Oct;27(2):89-94. | CrossRef | PubMed | Raghu G, Amatto VC, Behr J, Stowasser S. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J. 2015 Oct;46(4):1113-30. | CrossRef | PubMed |
Raghu G, Amatto VC, Behr J, Stowasser S. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J. 2015 Oct;46(4):1113-30. | CrossRef | PubMed | Margaritopoulos GA, Antoniou KM, Wells AU. Comorbidities in interstitial lung diseases. Eur Respir Rev. 2017 Jan 3;26(143):160027. | CrossRef | PubMed |
Margaritopoulos GA, Antoniou KM, Wells AU. Comorbidities in interstitial lung diseases. Eur Respir Rev. 2017 Jan 3;26(143):160027. | CrossRef | PubMed | Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018 Sep 1;198(5):e44-e68. | CrossRef | PubMed |
Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018 Sep 1;198(5):e44-e68. | CrossRef | PubMed | Devaraj A. Imaging: how to recognise idiopathic pulmonary fibrosis. Eur Respir Rev. 2014 Jun;23(132):215-9. | CrossRef | PubMed |
Devaraj A. Imaging: how to recognise idiopathic pulmonary fibrosis. Eur Respir Rev. 2014 Jun;23(132):215-9. | CrossRef | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis









