Key Words: stress fracture, magnetic resonance imaging, athletic injury, overuse injury
Abstract
Introduction
Stress fractures are injuries produced by the overuse of certain extremities, generating repetitive fatigue in the bone with insufficient rest periods and hormonal disorders, among others. High osteoclastic activity and lower activity of the osteoblasts at the cortical level occurs.
Objective
To determine the factors associated with a stress fracture in a single medical center of the Peruvian navy.
Methods
We conducted an observational, analytical case-control study. The dependent variable was stress fracture confirmed by magnetic resonance imaging of the patients; the independent variables were age, sex, calcemia, socioeconomic status, and time of daily physical activity. All data were extracted from the medical records. Crude and adjusted odds ratios were calculated with 95% confidence intervals.
Results
The sample was comprised of 238 patients (119 cases and 119 controls), of which 79.8% were male, and 20.2% were female; the average age was 20.25. In the bivariate analysis, stress fractures were associated with male sex (odds ratio 3.00; 95% confidence interval 1.51 to 5.95), hypocalcemia (2.83; 2.32 to 3.44), more than two hours of daily physical activity (24.7; 12.51 to 48.95) and socioeconomic level C (6.66; 2.82 to 15.74). Time dedicated to physical activity (adjusted odds ratio 44.46; 95% confidence interval 17.93 to 110.22) and socioeconomic level C (adjusted odds ratio 22.57; 95% confidence interval 7.03 to 72.74) were associated in the multivariate analysis.
Conclusion
We found that stress fractures were associated with physical activity time and a lower socioeconomic level. Further studies are needed to evaluate the relationship with other factors in the military population of Peru.
|
Main messages
|
Introduction
Stress fractures are injuries that generally occur when overusing certain extremities or when applying excessive high-intensity training with heavy loads to the bone without adequate resting periods. This process can cause an increase in the activity of osteoclasts, generating microtraumas and microfractures, and finally producing a stress fracture[1]. The continuous stress produces microfractures that could subsequently be aggravated in a cortical rupture, or stress fracture[1]. This type of fracture is responsible for 0.7 to 20% of all injuries in the clinical sports medicine practice[2], and they represent approximately 10% of all overuse injuries in athletes, dancers, and military recruits[3],[4].
Typically, the bone adapts to mechanical loads through a remodeling process in which osteoclasts reabsorb the laminar bone, creating resorption cavities that are subsequently replaced by denser bones by osteoblasts[1]. However, because there is a delay between the increase in osteoclast activity and osteoblastic activity, the bone weakens during this time, increasing the risk of microfractures[5]. Furthermore, there is evidence to suggest that these stress fractures may appear as a consequence of pure cyclic overload without the bone remodeling process[6].
Risk factors for stress fracture can be classified as extrinsic (external) or intrinsic (internal). Injuries occur as a result of a convergence of several factors at a given point in time. The type of exercise, we find, is among the possible extrinsic factors for stress fracture, the most frequent being running or jogging[7]. Some intrinsic factors can be mechanical, such as malformations in the lower limbs or muscle fatigue[8]. There can also be disturbances in sex hormones or other hormones that affect the balance between bone resorption and formation[9],[10], or nutritional factors, such as diets with calorie restriction, alcohol, calcium, or vitamin D deficiency[9]. A meta-analysis compared the serum level of this fat-soluble vitamin and the incidence of stress fracture in nine observational studies and the results suggest an association between these variables[15] because calcium and vitamin D can have a positive influence on bone health since vitamin D contributes to bone resorption and the absorption of calcium and phosphorus from the intestine[23]. In addition to the independent effects of a given risk factor and its mechanism for contributing to bone injury, interactions between risk factors are possible.
Stress fractures usually occur in young people who are active or undergoing training, such as athletes, dancers, or military recruits[5]. However, most studies conducted on this subject focus on the athlete population, while fewer studies focus on the military population. Of the studies carried out on the military population, almost all of them are American or European. For example, in the US armed forces, the cumulative incidence of stress fracture over four years was 5.7% for male cadets and 19.1% for female cadets[4], contributing to a substantial loss of training time. Similarly, stress fractures in military recruits typically occur in the tibia, femur, metatarsals, and pelvis, and present with the insidious onset of localized pain that worsens with activity[11].
However, there are very few studies on stress fractures in the Latin American military population. As such, the present study aims to determine the factors associated with stress fractures in a medical center of the Peruvian Navy (in Spanish, Marina de Guerra del Perú).
Methods
Study design and context
An observational, analytical investigation of age matching in cases-controls studies was conducted. We define as a case group the military personnel in training, diagnosed with stress fracture by medical evaluation and confirmed by magnetic resonance (MRI), and hospitalized in the traumatology service. The control group was defined as military personnel in training hospitalized in the traumatology service without a diagnosis of a stress fracture. All patients, both cases and control groups, were obtained from the trauma and orthopedic service of the Naval Medical Center, with controls being taken simultaneously in the same study period.
The dependent variable was stress fracture, which had diagnostic confirmation by magnetic resonance imaging, one of the most sensitive and specific methods for the diagnosis of stress fracture. The sign considered to diagnose a stress fracture on an MRI was the diminished signal visualized in the T1 and T2 sequences, usually in the form of a trace that arises from the bone cortex and extends perpendicular to the surface, accompanied by a periosteal reaction[12].
Regarding the independent variables, age, gender and serum calcium levels were obtained through the clinical history of the patients; the socioeconomic status and the time of daily physical activity were obtained using a survey of the patients, with prior verbal consent during their hospital stay or by telephone if they were already discharged.
The level of calcaemia was classified as hypocalcemia if it was less than eight milligrams per deciliter; normocalcemia if it was between eight and 10 milligrams per deciliter and hypercalcemia if it was greater than 10 milligrams per deciliter. The socioeconomic status was categorized according to the monthly average income of the patient's family according to the Peruvian Association of Market Research Companies (in Spanish, Asociación Peruana de Empresas de Investigación de Mercado)[13], being as follows:
Population and sample
The population consisted of patients treated in the traumatology service of the Cirujano Mayor Santiago Tavara Naval Medical Center (in Spanish, Centro Médico Naval Cirujano Mayor Santiago Távara) during 2016. Patients that did not have a history of complete variables or that did not answer calls for data collection were excluded. The sample size was calculated with a formula for case-control studies, taking an expected frequency of 20%, a predicted odds ratio of 3.2, a statistical power of 80%, and a confidence level of 95%, resulting in an estimated sample size of 119 cases and 119 controls.
Before data collection, the corresponding authorizations were requested from the Research Ethics Committee of the Cirujano Mayor Santiago Tavara Naval Medical Center. Once granted, the medical records of the selected patients were collected by randomizing the sample from the total population in 2016. The data was entered into a Microsoft Excel 2016 matrix.
Statistical analysis
For statistical analysis, the qualitative variables were described by using frequencies and percentages. Likewise, for the quantitative variables, measures of central tendency and dispersion were used after evaluating their normality. For the bivariate analysis, a chi-square test was used to determine the association between two qualitative variables and the student's t-test to determine the association between a qualitative variable and a quantitative variable with normal distribution; or the Mann-Whitney U test if it was not of normal distribution. A p < 0.05 was considered statistically significant.
For the multivariate analysis, logistic regression was used between the variables that were associated in the bivariate analysis; those variables of more than two categories and the quantitative ones were dichotomized, qualitative variables with very small frequencies that make their analysis difficult were excluded from the multivariate model. The adjusted odds ratios were found with their respective 95% confidence intervals.
Ethical aspects
This research received institutional approval from the Cirujano Mayor Santiago Távara Naval Medical Center and the Institute of Research in Biomedical Sciences of the Ricardo Palma University. The data were confidential and managed only by the researchers through a database without access to third parties. No personal data, or any other data that could identify patients in the study, were published.
Results
A total of 238 people (119 cases and 119 controls) were in the study, of whom 79.8% were men, and 20.2% were women; the mean age was 20.25 ± 1.5. Regarding the level of calcemia, the most frequent was normocalcemia with a total of 58.4%, and, finally, the majority belonged to socioeconomic status B with 77.3% (Table 2.). One hundred five men with a diagnosis of stress fracture and 85 men who did not present this pathology were included in the present study, for a total of 190 male patients. Likewise, 14 women with a diagnosis of stress fracture and 34 women without this pathology were included, for a total of 48 female patients. From the studied group, 54 patients presented hypocalcemia, 139 normocalcemia, and 45 hypercalcemia. Twelve patients belonged to socioeconomic status A, 184 to socioeconomic status B, and 42 to socioeconomic status C (Table 3.).
In the bivariate analysis, a statistically significant association was found between stress fracture and males, hypocalcemia, and socioeconomic status C (Table 3). Likewise, a relationship was found between the hours of physical activity per day and the stress fracture (Figure 1). In the binary logistic regression, an association was found with performing a daily physical activity for more than two hours and belonging to the socioeconomic status C (Table 4).
Table 2. General characteristics of the patients included in the study.
Table 3. Factors associated with stress fracture in the patients included in the study.
Figure 1. Association between age and hours of physical activity in patients with stress fracture.
Discussion
Stress fractures are injuries caused by physical stress, which is a stimulus for bone remodeling, affecting the structure and complex function through mechanotransduction mechanisms. This continuous stress produces microfractures that, eventually, can result in cortical rupture, or stress fracture[1]. These effects are likely to be exerted by modulation of the activity of the hypothalamic-pituitary-adrenal axis, resulting in an altered release of growth hormones, glucocorticoids, and cytokines, as demonstrated in human and animal studies.
In Latin America, and especially in Peru, there are few studies on stress fracture; there are even fewer studies among the military population[14]. Despite being a relatively common pathology in certain population groups, previous local data on risk factors for this condition are not available.
In our study, we found no association of stress fracture with gender probably because males have a higher frequency than females since the study was performed in a military population, and the proportion of military personnel is mostly male. Gender, therefore, is not an independent risk factor for a stress fracture in our study.
These results differ from the results reported by a study from the Defense Medical Epidemiological Database in the United States Navy from 2009 to 2012, where they found that the female gender had a higher risk of stress fracture compared to males (relative risk of 3.11)[4]. Moreover, another study conducted of the military population, of recruits of the Israeli anti-aircraft defense, found that the risk of suffering from stress fracture was higher in women, with a relative risk of 2.13[16]. This was confirmed by a systematic review that, in addition to finding a difference by gender, reported the difference in athletes as well as in the military population[17]. The difference in the incidence of stress fracture found in the literature concerning gender can be explained by studies that show that women have smaller bone structures and less muscle strength[18], a lower bone mineral density[17],[18], and hormonal irregularities characteristic of the menstrual cycle compared to men[19]. Studies involving a larger female population in the armed forces would help to elucidate this issue.
The variable that was associated in the bivariate analysis was the level of calcemia, with a higher proportion of stress fracture in the group with hypocalcemia, compared to those with normocalcemia and hypercalcemia; however, this variable could not be included in the multivariate model because it did not have the required expected frequencies. Few studies have sought to correlate the level of serum calcium with the incidence of stress fracture in the military population, so fractures associated with hypocalcemia are usually reported as atypical and pathological fractures, as related to other morbid conditions[20],[21]. Although serum calcium acts specifically on other biological functions, the variable calcemia was considered in the present study because of its direct relationship with parathormone. Low serum calcium levels are related to decreased bone density, especially in the cortical bone[26].
In adults, approximately 5% of the cortical bone and 30% of the trabecular bone is replaced within one year[27]. Therefore, to replace the trabecular bone almost in its entirety will require three years of proper nutrition, while replacing the cortical bone would need approximately 20 years. Adequate nutrition, consequently, is important to maintain the appropriate levels, which could intervene in the appearance of stress fracture.
It is in these populations that constant monitoring of serum calcium and supplementation of calcium and vitamin D in the diet is recommended to prevent this type of fractures[21]. These recommendations are also valid for populations that may be at risk of stress fracture due to their physical activity, such as military recruits; some studies corroborate that an adequate diet in calcium and vitamin D consumption significantly decreases the risk of stress fracture[22].
Also, an association between socioeconomic status and stress fracture was found, which was maintained in the multivariate analysis as an independent association. It shows that people with a lower socioeconomic status, in the present study, are more likely to have a stress fracture. The relationship could be explained through the quality of the diet, understanding that a higher socioeconomic status may have a greater likelihood of having better nutrition.
It has been found that diet quality can influence the incidence of stress fractures. A study by Frusztajer et al. in ballet dancers found that in the majority (80%) of the ten recent stress fracture, the dancers had weights less than 75% of the ideal (p < 0.05), as well as a higher incidence of eating disorders (p < 0.05). Thus, these dancers had a lower intake of fats and a higher intake of low-calorie foods (p < 0.05)[24].
On the other hand, it is known that less muscle strength in the lower limbs is associated with a higher incidence of stress fracture, mainly due to mechanical stress that the muscles are unable to contain if they do not have the proper strength; this could be worsened by a low-protein diet[25]. This would prevent proper muscle formation, which exposes the bone tissue to greater mechanical stress and, therefore, a higher rate of stress fracture. It shows that not only is the consumption of calcium and vitamin D supplements important, but a balanced diet with the right amount of macro and micronutrients to maintain adequate bone health is required[18].
Finally, the multivariate analysis found an association between hours of physical activity per day and stress fracture. This is explained by the fact that people who expose their bone tissue to mechanical stress for a longer time stimulate bone resorption, creating cavities within the bone tissue that, when given enough rest, the osteoblasts do not get to fill (a process is known as bone remodeling). This process generates microfractures from these points of lower bone density that, when the excessive stress on the bone continues, end up worsening and become stress fractures[5]. In addition, as mentioned above, muscular fatigue from a physical activity beyond the limits of the person who performs it, generates less protection of the muscle against the mechanical load of the sport or activity performed[8].
Some of the limitations of this study are related to the retrospective, observational design of a single institution, with a bias in the selection of study units given the male preference in the Peruvian armed forces. We recommend continuing this line of research, including a larger female population, quantifying calcium and vitamin D in the blood, and developing studies with more statistical power, in multiple sites and prospective to confirm the results of this study.
Conclusions
We conclude that stress fracture is associated with the amount of daily physical activity, the level of calcemia (hypocalcemia), and lower socioeconomic status.
Further studies are needed to assess the relationship with other factors in the Peruvian military population, such as the inclusion of a female population, the relationship with calcium and vitamin D, and the execution of multicenter and prospective investigations, with greater statistical power.
Notes
Roles of authorship
JBS: concept or design of the work; or to the acquisition, analysis or interpretation of the data of the work, drafting or critical revision of the manuscript, with important intellectual contributions, final approval of the version to be published, consent to assume responsibility for all aspects of the work. JDV: concept or design of the work; or to the acquisition, analysis or interpretation of the data of the work, drafting or critical revision of the manuscript, with important intellectual contributions, final approval of the version to be published. LVH: Drafting or critical revision of the manuscript, with important intellectual contributions.
Conflicts of interest
The authors declare that they have no conflicts of interest with the subject matter of this article.
Funding
There was no funding for the preparation of this review.
From the editors
The original version of this manuscript was submitted in Spanish. This English version was submitted by the authors and has been copyedited by the Journal.

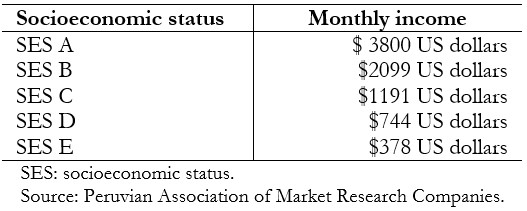 Table 1. Socioeconomic study.
Table 1. Socioeconomic study.

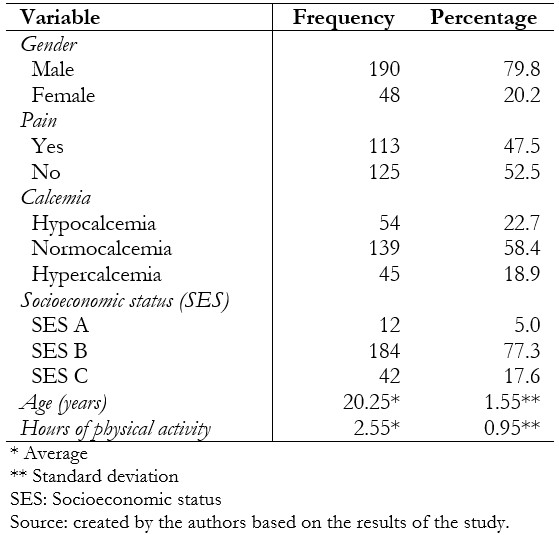 Table 2. General characteristics of the patients included in the study.
Table 2. General characteristics of the patients included in the study.

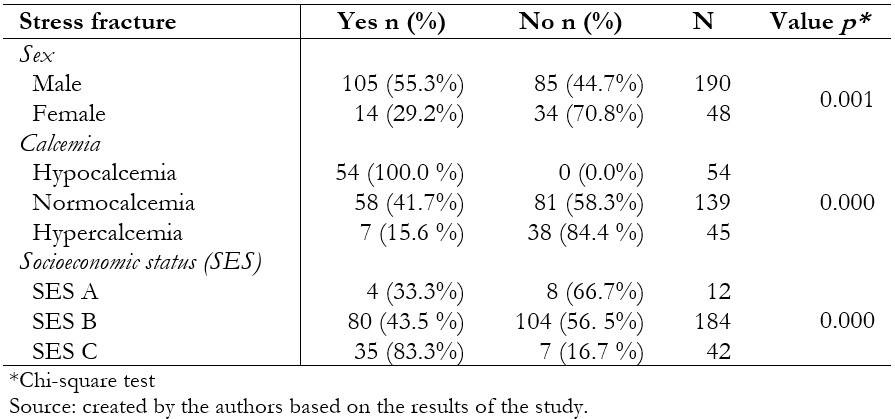 Table 3. Factors associated with stress fracture in the patients included in the study.
Table 3. Factors associated with stress fracture in the patients included in the study.

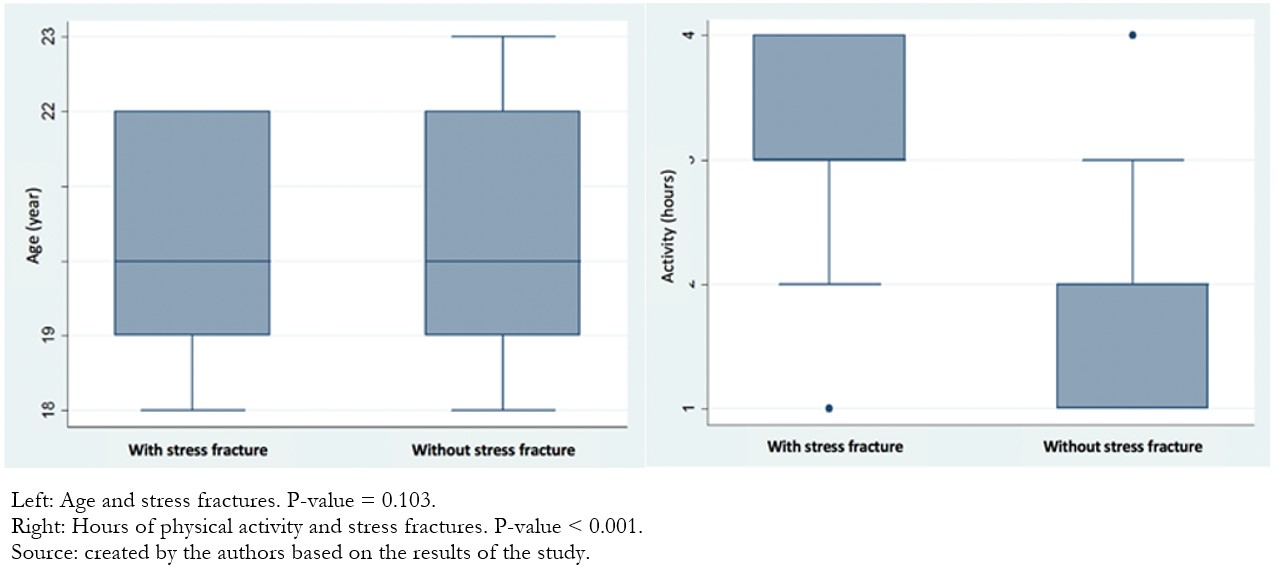 Figure 1. Association between age and hours of physical activity in patients with stress fracture.
Figure 1. Association between age and hours of physical activity in patients with stress fracture.

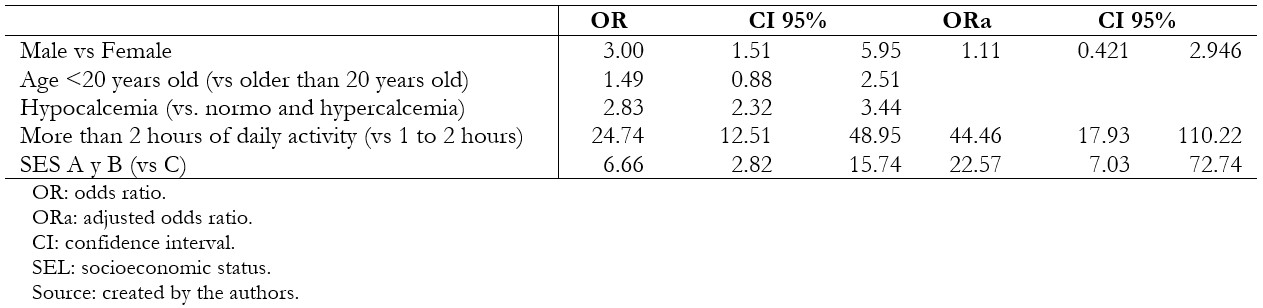 Table 4. Logistic regression of bivariate and multivariate analysis of factors associated with stress fracture in patients included in the study.
Table 4. Logistic regression of bivariate and multivariate analysis of factors associated with stress fracture in patients included in the study.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Introducción
Las fracturas por estrés son lesiones producidas por el sobreuso de ciertas extremidades, generando fatigas repetitivas en el hueso con insuficientes periodos de descanso, trastornos hormonales, entre otras. Se produce una elevada actividad osteoclástica y una menor actividad de los osteoblastos a nivel cortical.
Objetivo
Determinar los factores asociados a fractura por estrés en un centro médico de la Marina de Guerra de Perú.
Metodología
Se realizó una investigación de tipo observacional analítica de casos y controles. La variable dependiente fue la fractura por estrés, confirmada por resonancia magnética de los pacientes. Las variables independientes fueron edad, sexo y nivel de calcemia tomadas a partir de la historia clínica; el nivel socioeconómico y tiempo de actividad física diaria fueron datos recogidos mediante encuesta. Se obtuvieron los Odds ratio crudos y ajustados con un intervalo de confianza de 95%.
Resultados
Se trabajó con un total de 238 pacientes (119 casos y 119 controles), de los cuales 79,8% fueron varones y 20,2% fueron mujeres; la media de edad fue de 20,25 años. En el análisis bivariado se encontró asociación de fracturas por estrés con el sexo masculino (Odds ratio: 3,00; intervalo de confianza 95%: 1,51 a 5,95), hipocalcemia (Odds ratio: 2,83; intervalo de confianza 95%: 2,32 a 3,44), más de dos horas de actividad física diaria (Odds ratio: 24,74; intervalo de confianza 95%: 12,51 a 48,95) y un nivel socioeconómico C (Odds ratio: 6,66; intervalo de confianza 95%: 2,82 a 15,74). Mantuvieron su asociación en el análisis multivariado el tiempo de actividad física (Odds ratio: 44,46; intervalo de confianza 95%: 17,93 a 110,22) y el nivel socioeconómico C (Odds ratio: 22,57; intervalo de confianza 95%: 7,03 a 72,74).
Conclusión
Las fracturas por estrés estuvieron asociadas al tiempo de actividad física y a un nivel socioeconómico menor. Son necesarios más estudios para evaluar la relación con otros factores en la población militar de Perú.
 Authors:
John C Becerra Sandoval [1], Lissette Ventura Huamán [1], Jhony A De La Cruz-Vargas [2]
Authors:
John C Becerra Sandoval [1], Lissette Ventura Huamán [1], Jhony A De La Cruz-Vargas [2]
Affiliation:
[1] Centro Médico Naval Cirujano Mayor Santiago Távara, Lima, Perú
[2] Instituto de Investigación en Ciencias Biomédicas, Universidad Ricardo Palma, Lima, Perú
E-mail: jcbs_5@hotmail.com
Author address:
[1] Mz. C Lt 5 Urb. 7 de Agosto 2do programa Callao, Lima Perú

Citation: Becerra Sandoval JC, Ventura Huamán L, De La Cruz-Vargas JA. Factors associated with stress fracture: A case-control study in a Peruvian navy medical center. Medwave 2020;20(5):e7936 doi: 10.5867/medwave.2020.05.7936
Submission date: 24/11/2019
Acceptance date: 6/6/2020
Publication date: 25/6/2020
Origin: Not commissioned
Type of review: Externally peer-reviewed by three reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Melton W, Jackson JB. Stress fractures of the foot and ankle. En: Foot and Ankle Sports Orthopaedics. Springer International Publishing; 2017. p. 161–9. | CrossRef |
- Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006 Oct;17(5):309-25. | CrossRef | PubMed |
- Korpelainen R, Orava S, Karpakka J, Siira P, Hulkko A. Risk factors for recurrent stress fractures in athletes. Am J Sports Med. 2001 May-Jun;29(3):304-10. | CrossRef | PubMed |
- Waterman BR, Gun B, Bader JO, Orr JD, Belmont PJ Jr. Epidemiology of Lower Extremity Stress Fractures in the United States Military. Mil Med. 2016 Oct;181(10):1308-1313. | CrossRef | PubMed |
- Matcuk GR Jr, Mahanty SR, Skalski MR, Patel DB, White EA, Gottsegen CJ. Stress fractures: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol. 2016 Aug;23(4):365-75. | CrossRef | PubMed |
- Astur DC, Zanatta F, Arliani GG, Moraes ER, Pochini Ade C, Ejnisman B. Stress fractures: definition, diagnosis and treatment. Rev Bras Ortop. 2015 Dec 30;51(1):3-10. | CrossRef | PubMed |
- Yong JR, Silder A, Montgomery KL, Fredericson M, Delp SL. Acute changes in foot strike pattern and cadence affect running parameters associated with tibial stress fractures. J Biomech. 2018 Jul 25;76:1-7. | CrossRef | PubMed |
- Hadid A, Epstein Y, Shabshin N, Gefen A. Biomechanical Model for Stress Fracture-related Factors in Athletes and Soldiers. Med Sci Sports Exerc. 2018 Sep;50(9):1827-1836. | CrossRef | PubMed |
- Goolsby MA, Boniquit N. Bone Health in Athletes. Sports Health. 2017 Mar/Apr;9(2):108-117. | CrossRef | PubMed |
- Campbell EJ, Campbell GM, Hanley DA. The effect of parathyroid hormone and teriparatide on fracture healing. Expert Opin Biol Ther. 2015 Jan;15(1):119-29. | CrossRef | PubMed |
- Bulathsinhala L, Hughes JM, McKinnon CJ, Kardouni JR, Guerriere KI, Popp KL, et al. Risk of Stress Fracture Varies by Race/Ethnic Origin in a Cohort Study of 1.3 Million US Army Soldiers. J Bone Miner Res. 2017 Jul;32(7):1546-1553. | CrossRef | PubMed |
- Kahanov L, Eberman LE, Games KE, Wasik M. Diagnosis, treatment, and rehabilitation of stress fractures in the lower extremity in runners. Open Access J Sports Med. 2015 Mar 27;6:87-95. | CrossRef | PubMed |
- Asociación Peruana de Empresas de Investigación de Mercados. Informes NSE. APEIM;2019. [On line]. | Link |
- Becerra J, Mendieta J, De La Cruz Vargas JA. Fractura por estrés: fractura incompleta de fémur derecho. Rev Fac Med Humana. 2017;17(2):104-6. | CrossRef |
- Dao D, Sodhi S, Tabasinejad R, Peterson D, Ayeni OR, Bhandari M, et al. Serum 25-Hydroxyvitamin D Levels and Stress Fractures in Military Personnel: A Systematic Review and Meta-analysis. Am J Sports Med. 2015 Aug;43(8):2064-72. | CrossRef | PubMed |
- Gam A, Goldstein L, Karmon Y, Mintser I, Grotto I, Guri A, et al. Comparison of stress fractures of male and female recruits during basic training in the Israeli anti-aircraft forces. Mil Med. 2005 Aug;170(8):710-2. | CrossRef | PubMed |
- Wentz L, Liu PY, Haymes E, Ilich JZ. Females have a greater incidence of stress fractures than males in both military and athletic populations: a systemic review. Mil Med. 2011 Apr;176(4):420-30. | CrossRef | PubMed |
- Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000 Sep;27(3):437-44. | CrossRef | PubMed |
- Kelsey JL, Bachrach LK, Procter-Gray E, Nieves J, Greendale GA, Sowers M, et al. Risk factors for stress fracture among young female cross-country runners. Med Sci Sports Exerc. 2007 Sep;39(9):1457-63. | CrossRef | PubMed |
- Thakur A, Sood R, Gupta A, Thakker V, Thakur R. Femoral Neck Fatigue Fracture as the First Manifestation of Celiac Disease: A Case Report. IJSS Case Rep Rev. 2016;2(10):13-5. [On line]. | Link |
- Pittman K, Antill YC, Goldrick A, Goh J, de Boer RH. Denosumab: Prevention and management of hypocalcemia, osteonecrosis of the jaw and atypical fractures. Asia Pac J Clin Oncol. 2017 Aug;13(4):266-276. | CrossRef | PubMed |
- Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008 May;23(5):741-9. | CrossRef | PubMed |
- McInnis KC, Ramey LN. High-Risk Stress Fractures: Diagnosis and Management. PM R. 2016 Mar;8(3 Suppl):S113-24. | CrossRef | PubMed |
- Frusztajer NT, Dhuper S, Warren MP, Brooks-Gunn J, Fox RP. Nutrition and the incidence of stress fractures in ballet dancers. Am J Clin Nutr. 1990 May;51(5):779-83. | CrossRef | PubMed |
- Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016 May;75(2):188-98. | CrossRef | PubMed |
- López Gavilanez E, Huamán Garaycoa F, Garcés Santos JC, Marengo Baquerizo C, Reyes Aguirre G. Osteoporosis, PTH elevada y calcio iónico inadecuadamente “normal.” Rev Esp Enfermedades Metab Oseas. 2005 May;14(3):52–4. | CrossRef |
- Restrepo-Giraldo LM, Arévalo-Novoa J, Toro-Ramos M. Metabolismo mineral y óseo: visión general y sus métodos de medición. Bone and mineral metabolism: Overview and methods of measurement. Medicina y Laboratorio. 2015;21(11-12):511-38. [On line]. | Link |
 Melton W, Jackson JB. Stress fractures of the foot and ankle. En: Foot and Ankle Sports Orthopaedics. Springer International Publishing; 2017. p. 161–9. | CrossRef |
Melton W, Jackson JB. Stress fractures of the foot and ankle. En: Foot and Ankle Sports Orthopaedics. Springer International Publishing; 2017. p. 161–9. | CrossRef | Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006 Oct;17(5):309-25. | CrossRef | PubMed |
Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006 Oct;17(5):309-25. | CrossRef | PubMed | Korpelainen R, Orava S, Karpakka J, Siira P, Hulkko A. Risk factors for recurrent stress fractures in athletes. Am J Sports Med. 2001 May-Jun;29(3):304-10. | CrossRef | PubMed |
Korpelainen R, Orava S, Karpakka J, Siira P, Hulkko A. Risk factors for recurrent stress fractures in athletes. Am J Sports Med. 2001 May-Jun;29(3):304-10. | CrossRef | PubMed | Waterman BR, Gun B, Bader JO, Orr JD, Belmont PJ Jr. Epidemiology of Lower Extremity Stress Fractures in the United States Military. Mil Med. 2016 Oct;181(10):1308-1313. | CrossRef | PubMed |
Waterman BR, Gun B, Bader JO, Orr JD, Belmont PJ Jr. Epidemiology of Lower Extremity Stress Fractures in the United States Military. Mil Med. 2016 Oct;181(10):1308-1313. | CrossRef | PubMed | Matcuk GR Jr, Mahanty SR, Skalski MR, Patel DB, White EA, Gottsegen CJ. Stress fractures: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol. 2016 Aug;23(4):365-75. | CrossRef | PubMed |
Matcuk GR Jr, Mahanty SR, Skalski MR, Patel DB, White EA, Gottsegen CJ. Stress fractures: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol. 2016 Aug;23(4):365-75. | CrossRef | PubMed | Astur DC, Zanatta F, Arliani GG, Moraes ER, Pochini Ade C, Ejnisman B. Stress fractures: definition, diagnosis and treatment. Rev Bras Ortop. 2015 Dec 30;51(1):3-10. | CrossRef | PubMed |
Astur DC, Zanatta F, Arliani GG, Moraes ER, Pochini Ade C, Ejnisman B. Stress fractures: definition, diagnosis and treatment. Rev Bras Ortop. 2015 Dec 30;51(1):3-10. | CrossRef | PubMed | Yong JR, Silder A, Montgomery KL, Fredericson M, Delp SL. Acute changes in foot strike pattern and cadence affect running parameters associated with tibial stress fractures. J Biomech. 2018 Jul 25;76:1-7. | CrossRef | PubMed |
Yong JR, Silder A, Montgomery KL, Fredericson M, Delp SL. Acute changes in foot strike pattern and cadence affect running parameters associated with tibial stress fractures. J Biomech. 2018 Jul 25;76:1-7. | CrossRef | PubMed | Hadid A, Epstein Y, Shabshin N, Gefen A. Biomechanical Model for Stress Fracture-related Factors in Athletes and Soldiers. Med Sci Sports Exerc. 2018 Sep;50(9):1827-1836. | CrossRef | PubMed |
Hadid A, Epstein Y, Shabshin N, Gefen A. Biomechanical Model for Stress Fracture-related Factors in Athletes and Soldiers. Med Sci Sports Exerc. 2018 Sep;50(9):1827-1836. | CrossRef | PubMed | Goolsby MA, Boniquit N. Bone Health in Athletes. Sports Health. 2017 Mar/Apr;9(2):108-117. | CrossRef | PubMed |
Goolsby MA, Boniquit N. Bone Health in Athletes. Sports Health. 2017 Mar/Apr;9(2):108-117. | CrossRef | PubMed | Campbell EJ, Campbell GM, Hanley DA. The effect of parathyroid hormone and teriparatide on fracture healing. Expert Opin Biol Ther. 2015 Jan;15(1):119-29. | CrossRef | PubMed |
Campbell EJ, Campbell GM, Hanley DA. The effect of parathyroid hormone and teriparatide on fracture healing. Expert Opin Biol Ther. 2015 Jan;15(1):119-29. | CrossRef | PubMed | Bulathsinhala L, Hughes JM, McKinnon CJ, Kardouni JR, Guerriere KI, Popp KL, et al. Risk of Stress Fracture Varies by Race/Ethnic Origin in a Cohort Study of 1.3 Million US Army Soldiers. J Bone Miner Res. 2017 Jul;32(7):1546-1553. | CrossRef | PubMed |
Bulathsinhala L, Hughes JM, McKinnon CJ, Kardouni JR, Guerriere KI, Popp KL, et al. Risk of Stress Fracture Varies by Race/Ethnic Origin in a Cohort Study of 1.3 Million US Army Soldiers. J Bone Miner Res. 2017 Jul;32(7):1546-1553. | CrossRef | PubMed | Kahanov L, Eberman LE, Games KE, Wasik M. Diagnosis, treatment, and rehabilitation of stress fractures in the lower extremity in runners. Open Access J Sports Med. 2015 Mar 27;6:87-95. | CrossRef | PubMed |
Kahanov L, Eberman LE, Games KE, Wasik M. Diagnosis, treatment, and rehabilitation of stress fractures in the lower extremity in runners. Open Access J Sports Med. 2015 Mar 27;6:87-95. | CrossRef | PubMed | Asociación Peruana de Empresas de Investigación de Mercados. Informes NSE. APEIM;2019. [On line]. | Link |
Asociación Peruana de Empresas de Investigación de Mercados. Informes NSE. APEIM;2019. [On line]. | Link | Becerra J, Mendieta J, De La Cruz Vargas JA. Fractura por estrés: fractura incompleta de fémur derecho. Rev Fac Med Humana. 2017;17(2):104-6. | CrossRef |
Becerra J, Mendieta J, De La Cruz Vargas JA. Fractura por estrés: fractura incompleta de fémur derecho. Rev Fac Med Humana. 2017;17(2):104-6. | CrossRef | Dao D, Sodhi S, Tabasinejad R, Peterson D, Ayeni OR, Bhandari M, et al. Serum 25-Hydroxyvitamin D Levels and Stress Fractures in Military Personnel: A Systematic Review and Meta-analysis. Am J Sports Med. 2015 Aug;43(8):2064-72. | CrossRef | PubMed |
Dao D, Sodhi S, Tabasinejad R, Peterson D, Ayeni OR, Bhandari M, et al. Serum 25-Hydroxyvitamin D Levels and Stress Fractures in Military Personnel: A Systematic Review and Meta-analysis. Am J Sports Med. 2015 Aug;43(8):2064-72. | CrossRef | PubMed | Gam A, Goldstein L, Karmon Y, Mintser I, Grotto I, Guri A, et al. Comparison of stress fractures of male and female recruits during basic training in the Israeli anti-aircraft forces. Mil Med. 2005 Aug;170(8):710-2. | CrossRef | PubMed |
Gam A, Goldstein L, Karmon Y, Mintser I, Grotto I, Guri A, et al. Comparison of stress fractures of male and female recruits during basic training in the Israeli anti-aircraft forces. Mil Med. 2005 Aug;170(8):710-2. | CrossRef | PubMed | Wentz L, Liu PY, Haymes E, Ilich JZ. Females have a greater incidence of stress fractures than males in both military and athletic populations: a systemic review. Mil Med. 2011 Apr;176(4):420-30. | CrossRef | PubMed |
Wentz L, Liu PY, Haymes E, Ilich JZ. Females have a greater incidence of stress fractures than males in both military and athletic populations: a systemic review. Mil Med. 2011 Apr;176(4):420-30. | CrossRef | PubMed | Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000 Sep;27(3):437-44. | CrossRef | PubMed |
Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000 Sep;27(3):437-44. | CrossRef | PubMed | Kelsey JL, Bachrach LK, Procter-Gray E, Nieves J, Greendale GA, Sowers M, et al. Risk factors for stress fracture among young female cross-country runners. Med Sci Sports Exerc. 2007 Sep;39(9):1457-63. | CrossRef | PubMed |
Kelsey JL, Bachrach LK, Procter-Gray E, Nieves J, Greendale GA, Sowers M, et al. Risk factors for stress fracture among young female cross-country runners. Med Sci Sports Exerc. 2007 Sep;39(9):1457-63. | CrossRef | PubMed | Thakur A, Sood R, Gupta A, Thakker V, Thakur R. Femoral Neck Fatigue Fracture as the First Manifestation of Celiac Disease: A Case Report. IJSS Case Rep Rev. 2016;2(10):13-5. [On line]. | Link |
Thakur A, Sood R, Gupta A, Thakker V, Thakur R. Femoral Neck Fatigue Fracture as the First Manifestation of Celiac Disease: A Case Report. IJSS Case Rep Rev. 2016;2(10):13-5. [On line]. | Link | Pittman K, Antill YC, Goldrick A, Goh J, de Boer RH. Denosumab: Prevention and management of hypocalcemia, osteonecrosis of the jaw and atypical fractures. Asia Pac J Clin Oncol. 2017 Aug;13(4):266-276. | CrossRef | PubMed |
Pittman K, Antill YC, Goldrick A, Goh J, de Boer RH. Denosumab: Prevention and management of hypocalcemia, osteonecrosis of the jaw and atypical fractures. Asia Pac J Clin Oncol. 2017 Aug;13(4):266-276. | CrossRef | PubMed | Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008 May;23(5):741-9. | CrossRef | PubMed |
Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008 May;23(5):741-9. | CrossRef | PubMed | McInnis KC, Ramey LN. High-Risk Stress Fractures: Diagnosis and Management. PM R. 2016 Mar;8(3 Suppl):S113-24. | CrossRef | PubMed |
McInnis KC, Ramey LN. High-Risk Stress Fractures: Diagnosis and Management. PM R. 2016 Mar;8(3 Suppl):S113-24. | CrossRef | PubMed | Frusztajer NT, Dhuper S, Warren MP, Brooks-Gunn J, Fox RP. Nutrition and the incidence of stress fractures in ballet dancers. Am J Clin Nutr. 1990 May;51(5):779-83. | CrossRef | PubMed |
Frusztajer NT, Dhuper S, Warren MP, Brooks-Gunn J, Fox RP. Nutrition and the incidence of stress fractures in ballet dancers. Am J Clin Nutr. 1990 May;51(5):779-83. | CrossRef | PubMed | Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016 May;75(2):188-98. | CrossRef | PubMed |
Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016 May;75(2):188-98. | CrossRef | PubMed | López Gavilanez E, Huamán Garaycoa F, Garcés Santos JC, Marengo Baquerizo C, Reyes Aguirre G. Osteoporosis, PTH elevada y calcio iónico inadecuadamente “normal.” Rev Esp Enfermedades Metab Oseas. 2005 May;14(3):52–4. | CrossRef |
López Gavilanez E, Huamán Garaycoa F, Garcés Santos JC, Marengo Baquerizo C, Reyes Aguirre G. Osteoporosis, PTH elevada y calcio iónico inadecuadamente “normal.” Rev Esp Enfermedades Metab Oseas. 2005 May;14(3):52–4. | CrossRef | Restrepo-Giraldo LM, Arévalo-Novoa J, Toro-Ramos M. Metabolismo mineral y óseo: visión general y sus métodos de medición. Bone and mineral metabolism: Overview and methods of measurement. Medicina y Laboratorio. 2015;21(11-12):511-38. [On line]. | Link |
Restrepo-Giraldo LM, Arévalo-Novoa J, Toro-Ramos M. Metabolismo mineral y óseo: visión general y sus métodos de medición. Bone and mineral metabolism: Overview and methods of measurement. Medicina y Laboratorio. 2015;21(11-12):511-38. [On line]. | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








