Key Words: Arterial hypertension, hypertensive cardiopathy, prognostic factors, prognostic model
Abstract
Introduction
By definition, hypertensive cardiopathy is a series of complex and variable effects responsible for the chronic elevation of blood pressure in the heart. It stands out within a broad spectrum of cardiovascular diseases associated with hypertension.
Objective
To evaluate the capacity to predict the development of adaptive changes to hypertensive cardiopathy within ten years following diagnosis of the condition, using a model based on prognostic factors.
Methods
A prospective cohort study was conducted in hypertensive patients. The patients were followed at the specialized hypertension physician’s office of the specialty policlinic attached to Carlos Manuel de Céspedes University Hospital, in the Bayamo Municipality, Granma Province, Cuba, from 1 January 2008 to 31 December 2018.
Results
Cox’s proportional regression model showed a significant statistical relationship between most of the factors and the development of the adaptive changes in hypertensive cardiopathy within ten years of follow-up after the diagnosis of this condition. The lack of blood pressure control (Hazard ratio: 2.090; confidence interval 95%: 1.688 to 2.588; p: 0.000) followed by stage 2 of hypertension (hazard ratio: 1.987; confidence interval 95%: 1.584 to 2.491; p: 0.000) were the main factors. Internal validation of the model, discriminant capacity (C- statistic: 0.897) and calibration Hosmer-Lemeshow (χ2: 5.384; p: 0.716), was acceptable.
Conclusions
We develop a model to predict the progression of hypertensive cardiopathy from grade I to grade IV with adequate discriminatory capacity. The model is based on prognostic factors, from which the characteristic effects of arterial hypertension, diabetes mellitus, and chronic kidney disease stood out.
|
Main messages
|
Introduction
The relationship between blood pressure and cardiovascular complications is continuous. However, cut-point values of blood pressure to be taken as high risk do not exist, which makes the distinction between normotension and hypertension somehow arbitrary. In fact, cardiovascular risk can increase from very low blood pressure values (systolic blood pressure greater than 115 millimeters of mercury). Likewise, hypertension is defined as the blood pressure level in which treatment benefits (either life-style interventions or pharmacological treatment) clearly outweigh the risks[1].
It is important to remark that hypertension seldom appears alone; instead, it comes frequently accompanied by other cardiovascular risk factors, a fact that multiplies risk. So, it is necessary to quantify the probability for an individual to suffer from a cardiovascular complication in any given period of time for the stratification of risk in people with hypertension[1],[2].
Hypertensive cardiopathy stands out in the wide variety of cardiovascular diseases associated with high blood pressure. It is defined as a complex and variable group of effects that causes chronic elevation of blood pressure in the heart, and it is characterized by the presence of anatomic or biochemical signs of left ventricular hypertrophy or diastolic or systolic ventricular dysfunction, myocardial ischemia and heart rhythm disorders[3],[4].
In short, high blood pressure affects relaxation of left ventricle and it is an important predictor of cardiac insufficiency, even if the systolic function of the left ventricle is normal and there is no prior myocardial infarction. Other contributing factors are fibrosis caused by hypertension and structural disorders of large and small vessels[1],[5].
Despite the increasing number of studies about the risk factors for hypertensive cardiopathy and the important contribution of the knowledge on this topic, there exists a high prevalence of hypertensive cardiopathy and an increase in patients’ mortality and incapacity.
However, it is not known yet whether it could be possible to estimate the evolution of hypertensive cardiopathy from mild diastolic dysfunction to depressed systolic function by means of a model based on prognostic factors.
It is taken as a point of departure that the hypothesis that a model based on hypertension factors, comorbidity, and biological markers can be used to establish the prognosis of the evolution of hypertensive cardiopathy from mild diastolic dysfunction to depressed systolic function. Consequently, the study aims at evaluating the capacity of a model based on prognostic factors to predict the development of hypertensive cardiopathy with depressed systolic function in a ten-year period following the diagnosis of mild diastolic dysfunction.
Consequently, with the present study we would like to evaluate the capacity to predict the development of hypertensive cardiopathy in the ten years following its diagnosis by means of a model based on prognostic factors.
Methods
Study design
A prospective cohort study, in patients with hypertensive cardiopathy, was performed at the specialized hypertension physician’s office of the specialty policlinic attached to “Carlos Manuel de Céspedes” Hospital, in the Bayamo Municipality, Granma Province, Cuba, from 1 January 2008 to 31 December 2018. The patients followed had at least four appointments per year.
Inclusion and exclusion criteria
The study included patients over 20 years old who suffered from essential hypertension and grade I hypertensive cardiopathy (diastolic dysfunction without left ventricular hypertrophy, as diagnosed by echocardiogram). The diagnosis and classification used was based on the criteria proposed by the Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure[6] and by the European Guidelines for the Diagnosis and Treatment of Hypertension[1].
Patients with ischemic cardiopathy were excluded. Despite its high frequency in hypertensive patients, this study aims to evaluate the isolated effects of high blood pressure. For this reason, the inclusion of patients with ischemic cardiopathy could lead to confusion. By the same token, patients with interventricular and atrial-ventricular conduction disorders were excluded.
Likewise, patients with systematic diseases that by their natural evolution or by taking medication could result in heart disease were excluded, such as: thyroid diseases, chronic intestinal inflammatory diseases, collagen diseases, cancer or any other disease that by its characteristics could lead to heart disease.In addition, patients found to be in cytostatic treatment when being evaluated for the study were excluded, since these drugs result in heart disease by structurally damaging the heart muscle.
Patient source
All the patients included in the cohort were selected from the specialized hypertension physician’s office of our hospital who had been followed-up for many years prior to the beginning of the study; the cohort began evaluation on 1 January 2008.
In the first appointment each patient was interviewed, and a thorough physical exam was performed to obtain the necessary data for the study. Later, we performed clinical evaluations every six months while including the analysis of paraclinical data; meanwhile, echocardiogram and electrocardiogram were done yearly, unless the patient’s situation demanded to have them done before that time.
During the study, all patients received an initial uniform medical treatment based on therapeutic protocol approved by the hospital’s Research Ethics Committee, which was also based on the latest scientific evidence on high blood pressure[6].
The treatment was personalized according to the patient’s age, skin color, other risk factors and possible contraindications. On the whole, the treatment protocol included an angiotensin II-converting enzyme inhibitor plus a diuretic; antacids alone or combined with a diuretic or a beta-blocker alone or combined with a diuretic; or several combinations with the drugs mentioned, according to the patient’s hypertension stage. Angiotensin II receptor antagonists and other therapeutic options were prescribed according to the patient’s tolerance and associated conditions.
Participants
For several years prior to the initial evaluation of the cohort, 5019 patients were seen, 3517 (70.07%) from urban areas and 1502 (29.93%) from rural areas—most of the patients were from the Granma province. With observation of the criteria previously stated, 1637 patients were included in the study. During the ten-year follow-up of patients by the hypertension physician’s office, there were 196 deaths by several causes (cardiovascular complications and diseases were among the most significant ones) and 246 dropouts by different reasons (they did not attend appointments anymore, moved to another province, or were diagnosed by an excluding condition). Finally, 1194 individuals were included in the study.
1 January 2008 was defined as zero hour or the beginning of the cohort. Once the cohort was started, it was decided not to include any more patients (closed cohort). Each individual evaluation concluded when the patient developed grade IV hypertensive cardiopathy, or at the end of the ten-year follow-up period in patients who did not develop the condition.
Dependent variable
Two categories were considered: development of grade IV hypertensive cardiopathy or lack of development (left ventricular hypertrophy with depressed systolic function). Every hypertensive patient was diagnosed with this condition provided that they met these criteria[7],[8].
The diagnosis of grade IV hypertensive cardiopathy was considered in all patients with left ventricular hypertrophy and left ventricle ejection fraction smaller than 35% (LVEF: percentage of blood expelled from the ventricle with every beat).
The echocardiogram was done by two cardiologists specialized in echocardiography and with more than 15 years of experience. An ASAOTE Caris PLUS machine was used under the norms of the American Association of Echocardiography.
Independent variables
Are those factors whose influence on the onset of hypertensive cardiopathy was under evaluation. They are described below. All variables were dichotomized. One category represented what was considered “exposed” which, according to previous knowledge, implied a greater likelihood for the development of grade IV hypertensive cardiopathy. In the other category were included those considered “non-exposed”.
Age was quantified in years. Patients over 60 years old were considered exposed. Sex was divided by male (exposed) and female.
Smoking was grouped into two categories: smokers (exposed) if they smoked daily or nearly daily cigarettes, cigars, or pipe, regardless of the number smoked; and ex-smokers who had quit for less than a year. Nonsmokers were those who were not in the habit or had quit for more than a year.
Alcoholism (exposed) was considered as the ingestion of more than one ounce of pure alcohol daily, equivalent to an ounce (20 ml) of ethanol, eight ounces (240 ml) of wine, 24 ounces (720 ml) of beer, or 1½ ounce (45 ml) of rum. In the case of women and patients, underweight for their size, were considered exposed if they consumed 15 ml per day or more of any kind of alcoholic beverage[9].
Obesity was established by calculating the body mass index (BMI: weight in kilograms divided by size in square meters). Every subject with a muscular mass index greater or equal to 30 or with a waist circumference greater or equal to 102 centimeters for men and 88 centimeters for women was considered exposed.
Excessive sodium in the diet. A subject with a salt ingestion greater than five grams a day was considered exposed. This is equivalent to more than a teaspoonful of salt, distributed among the dishes prepared for lunch and dinner. Patients who consumed bakery products or used table salt (three or more times per week) were also included as exposed[9],[10].
A survey was used to obtain accurate information taking into consideration the amount and frequency of the foods consumed and their sodium content, as well as the amount of salt added while cooking and at the table (Annex I). Patients who answered affirmatively any of the items labeled 1a, 2a or 3a were considered to have excessive sodium consumption in their diet (exposed).
To make sure that the information on the patients’ sodium intake was accurate, urine sodium was estimated by means of a 24-hour urine cytochemical test every three months; patients were unaware of the aim of the urine study.
The biological markers selected as possible risk factors were cholesterol, triglycerides, HDL-cholesterol, glycemia, C-reactive protein, creatinine, microalbuminuria and cholesterol/HDL coefficient. The blood samples for each of the laboratory tests were taken in fasting state (8 to 12 hours) and were centrifuged at room temperature at 2000 rpm for 10 minutes. Creatinine, uric acid, cholesterol, HDL-cholesterol, triglycerides and glycemia were measured using a HITACHI 902® self-analyzer in the first 24 hours after their extraction; the first was expressed in μmol/l and the rest were expressed in mmol/l. All the study determinations were done by enzymatic methods.
The cut-points for the dichotomization of each laboratory variable used in the bivariate and multivariate statistical analysis were established according to a method used to find optimum cutpoints proposed in the literature explained below. In this way cut-points that defined the “exposed” cases at the following values were established: serum cholesterol greater than 4.8 mmol/l, triglycerides greater than 1.7 mmol/l, HDL-cholesterol lower than 1.5 mmol/l, cholesterol/HDL coefficient greater than 4, fasting glycemia greater than 5 mmol/l, creatinine greater than 80 μmol/l and uric acid greater than 375 μmol/l. C-reactive protein was determined using the quantitative turbidimetric determination method, considering the patients exposed with values over 4 mg/l.
Patients with microalbuminuria were considered exposed when their figures ranged from 0.02 to 0.2 g/l in 24 hours[1] and it was quantified by means of the Microalb-Latex technique (consisting in the measurement of this substance in the first morning urine void).
The value of the quantitative variables resulted from an average of three results in the first three appointments during the first year after the patient’s inclusion in the study.
Hypertension control: controlled patients were those with blood pressure figures smaller than 140 and 90 mmHg (systolic and diastolic respectively) in 100% of the samples obtained in their appointments during each year of follow-up until the onset of hypertensive cardiopathy or until the study was concluded (at least four determinations per year) under medical treatment, and not controlled if the patients did not meet the previous criteria[9],[11].
Every patient whose blood pressure determinations were 140 mmHg systolic and 90 diastolic or higher at the inception of the cohort was considered “not controlled.” This variable underwent changes during follow-up because some of the subjects included in the study were initially controlled; for this reason, time was included in the model as a dependent variable.
Other blood pressure determinations from contacts with the healthcare system outside of the hospital outpatient department were, for whatever reason, also taken into account (for this purpose, the patients were instructed to bring, in writing, their blood pressure values); to guarantee the authenticity of this variable, each patient was given a form demanding the following information: date, time, blood pressure values, and the doctor’s signature and seal. This document would be presented to the hypertension specialist on the day of the follow-up appointment.
The time of evolution and the hypertension stage were also considered as possible prognostic factors. Concerning the time of evolution, the patients were grouped into two categories: patients with an evolution time between 5 and 14 years, and patients with more than 15 years of evolution (exposed). Hypertension stage was classified according to the high blood pressure 7th report proposal (Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure Hypertension), taking into account the measures proposed for the correct classification and for blood pressure determination[6].
For blood pressure determinations aneroid and mercury sphygmomanometers previously calibrated by the Territorial Quality and Normalization Office were used, accredited in Cuba for such purposes.
Chronic kidney disease was also considered (patients in terminal stage were excluded) as a kidney structural or functional lesion, shown by damage markers (urine, blood or images), independent of the cause that caused it, for a period of three or more months[12].
For the diagnosis of diabetes mellitus, independent of its type, up-to-date diagnostic criteria were considered. (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus)[13].
The data were obtained in different appointments by means of interviews conducted by the authors with the prior knowledge and consent of the patients.
Bias control
To increase accuracy of the necessary data for the study the following biases were considered:
Selection biases
The out-patient’s clinical history was used to minimize the use of memory. Two different authors interviewed each patient separately and compared their results later. Structured questionnaires were used to make the interviews more homogeneous.
Information biases
An adequate data processing and quality control was achieved by using validated measurement instruments and standardized criteria for sample collection.
Accuracy biases
The data were processed by the authors and an extrinsic biostatistician; the results obtained coincided.
Statistical analysis
The first step in the statistical analysis consisted of sample characterization and the description of variable. For the quantitative variables, the means and standard deviation were described, as well as the minimum and maximum values for each distribution. For the qualitative variables, absolute and relative frequencies (percentages) were obtained.
Bivariate analysis
To evaluate the association among qualitative variables and the risk of developing grade IV hypertensive cardiopathy, Mantel’s chi-square test was used.
The magnitude of associations was estimated by calculating the relative risks (exposed/non-exposed) of developing grade IV hypertensive cardiopathy after the first year until the end of ten years of follow-up. Punctual estimations were obtained and by confidence interval (95%) of the relative risks. The hypothesis that the population relative risk equaled 1 with a significance level of 0.05 was tested for each variable.
For the bivariate analysis the quantitative variables were dichotomized looking for the optimal cut-points. In the searching of possible cut-points, the most extreme values of the variables on both ends were not considered, that is, under percentile 5 and above percentile 95. Likewise, due to the increased probability of type I error when using several hypothesis test, the following formula was used to correct it: p = −3.13 pmin (1 + 1.65 Ln (pmin)), where pmin is the minimum probability value obtained and p is the corrected value.
The C value was chosen as optimal cut-point since it had the highest chi square result (that is, it was the one with the lowest p value) for all the values of the dichotomized variables[14].
So, it was selected as cut-point for each variable the value that best differentiated patients between those who developed hypertensive cardiopathy and those who did not.
Multivariate analysis
Cox’s proportional risk model was used with all independent variables. The regression coefficients (β), and the standard error of each coefficient (S) were estimated. The significance of each coefficient was proved (null hypothesis β=0) with Wald’s statistics and the corresponding square chi test. The hazard ratio as exp (B) with confidence interval (CI) of 95% was also estimated. Cox’s proportional risk function permitted to model the prognosis of patients with hypertensive cardiopathy (evolution to grade IV cardiopathy) in each instant of time for different co-variables. At the same time that the rest of the variables are controlled, the HRs estimate how many times the risk to develop grade IV hypertensive cardiopathy is greater in exposed as compared to non-exposed in each variable.
The adjustment of Cox’s regression function, equivalent to the estimation of its parameters, was done by the maximum verisimilitude method (omnibus test in SPSS). All patients who did not develop grade IV hypertensive cardiopathy during the ten years that the cohort lasted were considered censured cases (both bivariate and multivariate analyses were used), and those who met the exit criteria from the cohort were also included in the multivariate analysis as censured data. All the statistical calculations were done with Windows SPSS software (version 25.0).
Finally, the discriminative capacity and calibration were determined to evaluate the internal validity of the model.
C statistics was calculated to evaluate the discriminative capacity of the prognostic model, to separate the patients who developed grade IV hypertensive cardiopathy from the ones who did not. C statistics is analogous to the area under the ROC curve, operative characteristic of the receptor based on a logistic regression. A C statistic value greater or equal to 0.7 indicates a good discriminative capacity and, the closer to 1 the C value, the better the discriminative capacity[15],[16].
A version of the Hosmer-Lemeshow χ2 statistic test (HL-statistic) was calculated to evaluate the model calibration capacity (or how close to the expected probabilities were to the actual risk). The participants were divided into deciles according to the probabilities previewed for developing grade IV hypertensive cardiopathy, within 10 years, using the proposed predictive model. The HL-statistic was calculated to compare the differences between the expected and actual proportions to develop grade IV hypertensive cardiopathy. It was considered that an HL statistic value greater than 0.05 was indicative of a good calibration[17].
The use of the re-sampling technique (bootstrap) made possible to quantify the degree of excessive optimism of the regression coefficients and, therefore, the amount of necessary simplification to correct it.
In this way, it was possible to distinguish the patients who developed grade IV hypertensive cardiopathy from those who did not develop the condition. The statistical analysis described above was done using the Stata 14.1 software.
Ethics
In the present study the basic ethical principles of the clinical and epidemiological research processes for observational studies were met. The board of directors and the ethics committee of the hospital gave their approval for the study. Potentially eligible patients were informed about the study and their consent was obtained. They were also guaranteed that their personal data would not be revealed. In addition, the patients received pertinent treatment for their disease during the time that the study lasted. No patient declined to participate in the study.
Results
Table 1 shows the baseline values of the qualitative variables, where there is a slight predominance of the male sex with 50.6% of the sample. It is significant that in more than 40% of the patients their disease was not controlled and that, in spite of the insistence on the harmful effects of smoking, 42.7% were smokers.
Table 1. Sample characterization. Qualitative variables.
The average values of the quantitative variables are represented in Table 2. Pulse pressure and age were the variables with greater standard deviation, 17.86287 and 12.08650 respectively.
Table 2. Average values of quantitative variables.
The association between chronic kidney disease and the bad prognosis of the patients with hypertensive cardiopathy is shown in Table 3; where it is seen that a patient that suffers from this disease has a threefold risk of developing grade IV hypertensive cardiopathy (relative risk: 3.503; confidence interval: 2.931 to 4.187; p: 0.00). Diabetes mellitus (relative risk: 2.762; confidence interval: 2.185 to 3.491; p: 0.00) and uncontrolled high blood pressure (relative risk: 2.758; confidence interval: 2.241 to 3.393; p: 0.00) were also associated to the mentioned risk in a very significant way.
Table 3. Bivariate analysis of qualitative variables.
The quantitative variable with greater influence in the development of grade IV hypertensive cardiopathy was the time of evolution of high blood pressure, with a period longer than 10 years suffering from this condition, increasing the risk 4.667 times (confidence interval: 2.539 to 8.578; p: 0.00) followed by a C-reactive protein value greater than 4mg/L (relative risk: 4.534; confidence interval: 3.699 to 5.557; p: 0.00) and glycemia greater than 5 mmol/L (relative risk: 2.763; confidence interval: 2.279 to 3.351; p: 0.00). Table 4.
Table 4. Bivariate analysis of quantitative variables.
The application of Cox’s multivariate model (table 5) to evaluate the independent effect of the variables studied on the evolution of grade IV hypertensive cardiopathy showed that the most important variable was uncontrolled hypertension (Hazard ratio: 2.090; confidence interval: 1.688 to 2.588; p: 0.000) followed by the classification of high blood pressure as stage 2 (Hazard ratio: 1.987; confidence interval: 1.584 to 2.491; p: 0.000).
Table 5. Cox’s multivariate model results.
Finally, the discriminative capacity of the elaborated model was adequate (C statistic: 0.897). Likewise, the calibration was examined (X2: 5.384; p = 0.716), as is shown by Table 6.
Table 6. Model internal validity (calibration and discrimination).
Discussion
Hypertensive cardiopathy is one of the lesions of target organs with higher morbidity and mortality. Nevertheless, despite the large number of studies carried out, the independent contribution of the factors that provoke the evolvable changes of this disease is not known exactly.
Both treatment and prevention are complex processes in which the study of the prognostic factors is crucial[18]. It can be expected then that the results of the present study contribute to the prevention of the evolution of grade I hypertensive cardiopathy to grade IV in patients with high blood pressure.
Nielsen et al[19] found that 12% of the patients in hemodialysis had cardiac insufficiency with an ejection fraction lower than 40%. Meanwhile, Schneider et al[20] stated that 9.3% of their patients had dilatation of the left ventricle and hypertrophy and that, in patients with mild to moderate kidney disease, 7.2% had a left ventricle fraction under 50%. In this study, it was found that a past history of chronic kidney disease is one of the most important prognostic factors, which coincides with the authors quoted above.
Subjects with chronic kidney disease should be considered of high or very high cardiovascular risk, without having to apply risk scales. So, in presence of chronic kidney disease with FG < 60 ml/min/1,73 m2 the subject classifies as of very high cardiovascular risk[21].
In patients with chronic kidney disease resistant and masked hypertensions, as well as high nocturnal blood pressure are frequent, which are associated with a low glomerular filtration rate, greater levels of albuminuria and organic damage[22],[23].
Undoubtedly, the strong connection between renal and cardiac diseases provides evidence for the complex kidney-heart interaction (the renin-angiotensin system, several inflammatory mediators, reactive species of oxygen, provoke histological and functional changes which are characteristic of the hypertensive renal and cardiac lesion)[1],[3],[24] which can explain the findings in this study, becoming a vicious cycle: the higher the blood pressure, the greater the renal and cardiac damage. Nevertheless, the physiopathological mechanisms of this reciprocal relation remain ambiguous[24].
The presence of diabetes mellitus in the hypertensive individual increases the risk of myocardial function depression, as it was demonstrated in the study. Tovillas-Moránet al[25] found that diabetes mellitus was one of the main risk factors for cardiovascular disease in patients with high blood pressure.
Other authors also coincide with our results since they found a higher incidence of cardiac insufficiency with reduced ejection fraction in diabetic and hypertensive patients[26],[27],[28],[29].
Disorders of cardiac metabolism are present before or simultaneously to declination of cardiac function, the data suggest that there is an excess of metabolites which are not used in the production of adenosine triphosphate and then oxidative stress increases, which can trigger inflammation and cause an early cardiac dysfunction. The decrease of sensitivity to insulin, hyperinsulinism, and resistance to insulin are facts characteristic of several processes of high cardiovascular risk for hypertension and diabetes mellitus and could be the basis for cardiac metabolomic disorders; but this association has been seen only in essential hypertension, which suggests a common genetic component in these two conditions[30],[31].
In coincidence with our study, several researchers find a relation between uncontrolled hypertension and the risk of cardiac insufficiency[32],[33],[34]. High blood pressure is the most important risk factor for cardiac insufficiency. In fact, the majority of the patients with cardiac insufficiency have a past history of hypertension, which causes left ventricular hypertrophy and, consequently, diastolic dysfunction which is, in turn, an important predictor of cardiac insufficiency, even when the left ventricle systolic function is normal and there is no prior myocardial infarction[1].
Other contributing factors are fibrosis resulting from high blood pressure and structural disorders of large and small vessels (microvascular disease). Consequently, the treatment of hypertension allows for control of the disease and has an important impact on reducing the risk and hospitalization for cardiac insufficiency, especially in adults and the elderly[1],[33]. These aspects can explain our findings.
As in this study, other authors[36],[37] confirmed that the patients with blood pressure values equal to or higher than 160/100 mmHg were associated with higher rates of cardiac dysfunction and death. These results indicate a continuous, consistent, and independent relationship between hypertension and the risk of cardiovascular complications. High blood pressure means a greater likelihood of suffering a heart attack or developing cardiac insufficiency (for every increase of 20 mmHg in systolic arterial pressure, the likelihood is twofold)[38].
As we had mentioned before, hypertension is an important predictor of cardiac insufficiency, even when the left ventricle systolic function is normal and there is no previous myocardial infarction[1]. However, in spite of these findings and the comments made before, it is still necessary to keep researching the topic, since the evolution of these patients from hypertension to cardiac insufficiency with depressed systolic function (grade IV hypertensive cardiopathy) is complex and the existing knowledge at present not only challenge the existing paradigms established for years, but also compel to seek new answers.
The consequences of sustained chronic hypertension on the heart and the blood vessels have been well demonstrated. For that reason it is necessary to reduce systolic blood pressure below 150 mmHg in all patients, including patients over 60 years old[1].
An increase in albuminuria indicates a progressive impairment of the renal function and it is an independent and cumulative predictor of an increase in cardiovascular risk[1]. There is evidence that microalbuminuria is an important factor to predict cardiovascular risk. In such a matter, there is a high prevalence of subclinical deterioration of the left ventricle in subjects with microalbuminuria[1],[39]. These facts coincide with our study.
The reduction of albuminuria has also been considered a therapeutic goal. In the analysis of data from clinical essays it has been found that changes in excretion of albumin in the urine are predictors of renal and cardiovascular complications[1],[40]. However, in some studies in which treatment was less effective for the reduction of albuminuria[41], it was more effective in the reduction of cardiovascular complications, and vice versa[42],[43],[44]. So, it has not been proven if the reduction of albuminuria per se can be useful for the prevention of cardiovascular disease.
Notwithstanding, prior to these discrepancies, the authors consider that the results can be explained by the close relation of microalbuminuria with different pathological disorders, such as insulin resistance, endothelial dysfunction, dyslipidemias, sensitivity to salt and increase in angiotensin II. In this way, an inflammatory process occurs that causes dysfunction of the renal and cardiac cell membranes[45].
Although it has not been demonstrated that quitting smoking decreases the risk for cardiac insufficiency in any condition, it is true that it has an epidemiological association with the onset and prognosis of cardiovascular diseases; therefore, it is recommended not only to avoid smoking, but also to avoid exposure to tobacco smoke (passive smoker), since it elevates significantly the risk of cardiovascular diseases. In smokers, quitting tobacco use is the most efficient measure to prevent cardiovascular diseases[46],[47] which means that this measure can be beneficial. What has been detailed above could be the explanation to our results.
Coinciding with the present study, obesity is considered a risk factor for cardiac insufficiency[46]. The loss of weight in hypertensive patients can improve the efficacy of antihypertensive medications and the cardiovascular risk profile[1].
It is recommended that patients who are hypertensive, overweight, or obese lose weight to control factors for metabolic risk. This fact is important for the complex relationship between obesity and cardiovascular diseases, as various pathological mechanisms involve many factors and have a complex interaction; among them sodium retention, resistance to insulin, subclinical inflammation, neurohormonal activation, high concentrations of leptin, increase of free fatty acid oxidation in the myocardium, and deposit of fat in the heart stand out, as well as the capacity of adipose tissue to synthesize a great deal of hormones and interleukins[48],[49],[50].
In similar fashion to our results, other studies show that there is an association among excessive salt in the diet, uncontrolled hypertension, and the development of hypertensive cardiopathy[1],[46],[50]. The link between the high intake of salt in the diet and high blood pressure complications, because an increase in the plasmatic activity of renin and other components of the axle, reduces cerebral natriuretic peptide and creatinine clearance, as well as creates an increase in liquid retention and arterial rigidity[51].
In this study no differences were found concerning the risk of developing grade IV cardiopathy in alcoholic individuals or by sex. Although high blood pressure appears earlier in men, associated to other cardiovascular risk factors, it has been observed that after menopause, the risk is the same in both sexes and can be even higher in the female sex[50].
Regarding alcoholism, the explanation could be related to the magnitude of the intake—since most of our patients were light drinkers and were perhaps influenced by the suggestions and sanitary education provided by their appointments over many years.
In hypertensive patients, long-term hypertension causes a chronic increase of workload for the left ventricle which produces left ventricular hypertension, associated to other morphological changes which increase the risk of cardiac insufficiency with a conserved or reduced ejection fraction[1],[49]. These aspects are consistent with our results.
A constant finding in the myocardium of patients with insufficiency is a disorder of the deposit of type I and III of collagen fibers. These disorders reflect the loss of physiological balance between the synthesis and degradation of the fibrillar collagen molecules, as a result of the fact that in the hypertensive myocardium there is a regulation disorder of both the factors that stimulate synthesis and inhibit degradation, and the factors with opposite actions[52].
So, due to the highly versatile nature of hypertension and its progressive impact, it is necessary to change the approach from a casual arterial pressure to a better long-term overload profile[50].
In short, hypertension is a progressive cardiovascular syndrome in which the chronic effect on the heart and other organs will always be present, even in controlled patients. Therefore, it is necessary not only to focus on the control of high blood pressure but also on its prevention.
Several elements can explain our results, where high levels of C-reactive protein are associated to the development of hypertensive cardiopathy—among them it is quoted that this biological marker speeds up the ventricular remodeling—increases the deterioration of the endothelial vasodilator function, activates platelets, and increases hypoxia-induced apoptosis through a dependent via of the mitochondria, as well as the high levels found in cardiac insufficiency[50],[53],[54]. Definitively, C-reactive protein is a biomarker of active vascular processes, which exerts a direct action upon the morphological and cardiac function and can contribute causally to the development of grade IV hypertensive cardiopathy; the effects are independent of any other cardiovascular risk factor[53].
Concerning glucose metabolism, Cuspidi[35] and Álvarez Aliaga[50] showed that values over 5 mmol/L in fasting state in non-diabetic but hypertensive individuals was a risk factor independent of cardiovascular complications, which coincides with the findings in this study.
Either alone or associated to hypertension, hyperglycemia can be related to cardiac hypertrophy interstitial fibrosis, arteriosclerosis, and coronary endothelial dysfunction, which predisposes to ischemia in absence of stenosis of these arteries. There is also a greater activation of the CNS, as well as disorders of the systolic and diastolic functions occur in absence of coronary or valvular disease[53]. Therefore, a glycemia level even lower than the diagnostic threshold of diabetes mellitus is, undoubtedly, an important risk factor for hypertensive cardiopathy and its evolution to a final stage.
Review of the evidence that shows the European guide about hypertension[1] indicates that a great number of patients with high blood pressure could benefit from the treatment with statins. Above all, patients with metabolic syndrome, diabetes mellitus, and several risk factors for atherosclerosis could benefit. This statement coincides up to some extent with our work, where higher risk for cardiopathy was found in patients with high cholesterol and cholesterol/HDL coefficient. However, high cholesterol concentrations in combination with low density lipoproteins are not frequent in cardiac insufficiency with reduced ejection fraction; patients in an advanced state of this condition can exhibit low concentrations of low-density lipoproteins, which is associated to a worse prognosis and, although the treatment did not reduce morbidity and mortality, it did not increase the risk either, yet reduced hospitalizations[46].
It is true that the patients previously mentioned can be associated to worse prognoses; however, it is a fact that patients with several risk factors for cardiovascular diseases can benefit[55],[56].
Before these differences in outcomes, the authors consider that lipid metabolism disorders predispose for the onset of hypertensive cardiopathy (ventricular hypertrophy, mild diastolic dysfunction) and it is associated to its progression to terminal cardiopathy (grade IV) due to several reasons: they favor the progression of the atherosclerotic lesion since it starts a local inflammatory process, worsen the hemodynamic and non-hemodynamic disorders, which take part in the genesis of hypertensive cardiopathy, and provokes the lesion of other organs such as the kidney. This increases the risk of greater cardiac lesion[24],[30],[50].
On the other hand, some studies associate the poor prognoses of patients who suffer from cardiac insufficiency with reduced ejection fraction with low cholesterol levels, but many of these patients already have other complications like cachexia (a generalized debilitating process of all the body compartments) which can appear in 5 to 15% of the patients with a more advanced stage of the disease whose cholesterol levels is usually low[46]. Similarly, serious cachexia is associated to more serious symptoms: reduced functional capacity, more frequent hospitalizations, and lower survival rates. And although in its complex etiology stand out insufficient nutrition and absorption, caloric and protein imbalance, anabolic hormonal resistance, diminished anabolic impulse, prolonged immobilization and lack of physical shape[46].
The arguments stated above should lead us to a reflection about the true value of lipid metabolism disorder and the evolvable changes of hypertensive cardiopathy, as well as toward new studies where the variable cachexia is controlled, to avoid confusion.
Likewise, Barter et al[57] have been quoted as stating that cholesterol/HDL coefficient doubles the risk for cardiovascular complications. Meanwhile, Ashen and Blumenthal[58] demonstrated that it is not possible to achieve a significant reduction of the atheroma plaques in individuals with treatment to reduce the levels of total cholesterol total and LDL, if the HDL values are not increased. These results also coincide with our findings.
Pulse pressure (closely related to arterial rigidity) increases with age, both in men and women, parallel to the increase of arterial systolic pressure, above all in the population of individuals over 60 years old. Pulse pressure increase is associated to a greater cardiovascular morbidity and mortality, and it is an independent marker of cardiovascular risk[59],[60].
There are two main ways to explain the relation of arterial rigidity with cardiac dysfunction: rigidity makes the incident and the reflex wave travel faster, which provokes an early return of the reflex wave with an increase of the left aortic and ventricular pressure in the systole. An elevated left ventricular load predisposes for the development of left ventricular hypertrophy, progressing to left ventricular dysfunction and cardiac insufficiency, and an unfavorable oxygen offer-demand proportion. The other factor is the reduction of aortic pressure during diastole, which reduces coronary perfusion pressure, and contributes to myocardial ischemia, even in absence of an atherosclerotic stenosis of the coronary artery[61].
Hypertensive patients may present atherogenic dyslipidemia, which is characterized by high concentrations of triglycerides and low concentrations of cholesterol combined with high density lipoproteins. These disorders are associated with higher cardiovascular risk[1], which can explain the outcomes found in our patients.
The present study shows the role of the hemodynamic effects produced by high blood pressure, a history of diabetes mellitus and chronic kidney disease as the most significant factors in the progress of hypertensive cardiopathy from grade I to grade IV.
We have quoted several explanations for our results; however, it is important to add that the hemodynamic effects that bring about myocardial hypertension, that is, the pressure overload of the left ventricle reduces the maximum shortening speed with a reduction of the heat produced during contraction, which, although it seems to be a beneficial process initially, is one of the first steps towards terminal cardiac insufficiency[62].
Eventually, functional changes (changes in cellular permeability, increase in the synthesis of intracellular proteins, and increase of the extracellular matrix) and structural changes (left ventricular hypertrophy, remodeling, fibrosis, diastolic dysfunction and microcirculation dysfunction to terminal cardiopathy) of the myocardium will appear. These are consequences of, in part, the pressure overload and of, in another part, the neurohormonal activation which accompanies hypertension and its worse prognosis[55],[62],[63],[64]. The previous arguments indicate the importance not only of an early diagnosis of hypertension, but also of its strict control. Likewise, it is necessary to maintain the surveillance and control of diabetes mellitus and chronic kidney disease to standardize the medical treatment and promote changes that bring about healthier lifestyles.
Strengths and limitations
We present a unique and original model, based on prognostic factors to predict the evolvable changes of hypertensive cardiopathy; from mild diastolic dysfunction to depressed systolic function. It was also demonstrated the pathogenic importance of the factors studied, according to their role in the model.
As limitations of the present study we should note that it was not possible to study new predictors of cardiovascular risk such as hypersensitive C-reactive protein, endostatin, homocysteine, among others. However, the long-term results of the interventions on the new risk markers are mostly hypothetic. Furthermore, its contribution to improve the area under the receiver operating curve is modest compared to the function that includes classical factors exclusively[4],[47]. Another limitation was that it was not possible to quantify the excretion of daily urine sodium (only on trimestral basis) to evaluate salt intake more objectively.
Conclusion
The present study has demonstrated the role played by the effects of hypertension, comorbidity (diabetes mellitus and chronic kidney disease), as well as the presence of microalbuminuria as major factors in the progress from grade I to grade IV hypertensive cardiopathy. In addition, a model was obtained with adequate discriminative capacity and calibration, which can be used to predict the outcome of the individuals studied.
Notes
Authorship contributions
All the authors participated in the sample collection, as well as in the elaboration and revision of the manuscript.
Ethics
The Ethical and Scientific Committee from Carlos Manuel de Céspedes University Hospital approved this study with a letter dated September 12, 2019.
Funding
The authors claim that there were no external funding sources.
Acknowledgment
The authors acknowledge the important work done by the Clinical Laboratory and Cardiology Departments from Carlos Manuel de Céspedes University Hospital to obtain crucial information for this study.
From the editors
The original version of this article was submitted and published in Spanish. This English translation, submitted by the authors, has been lightly copyedited by the Journal.

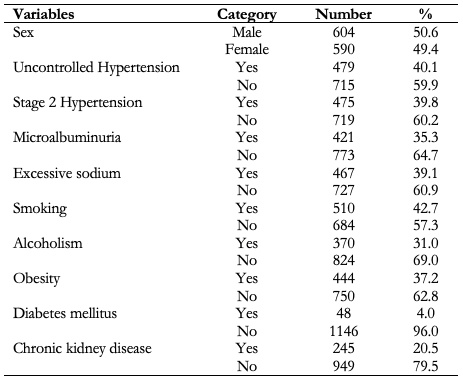 Table 1. Sample characterization. Qualitative variables.
Table 1. Sample characterization. Qualitative variables.

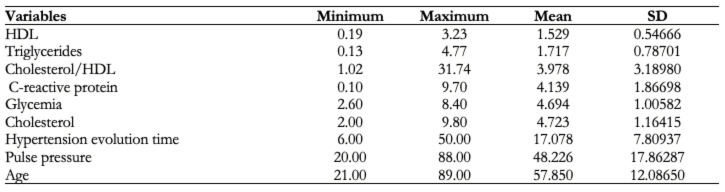 Table 2. Average values of quantitative variables.
Table 2. Average values of quantitative variables.

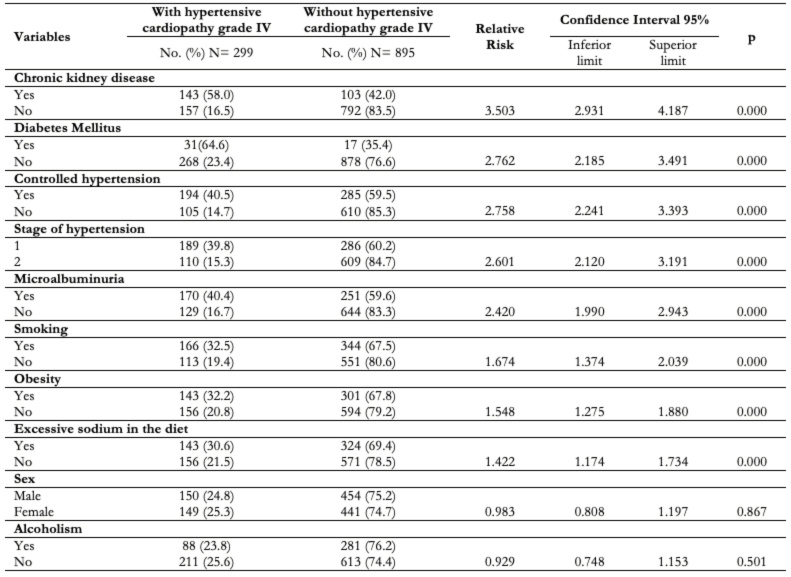 Table 3. Bivariate analysis of qualitative variables.
Table 3. Bivariate analysis of qualitative variables.

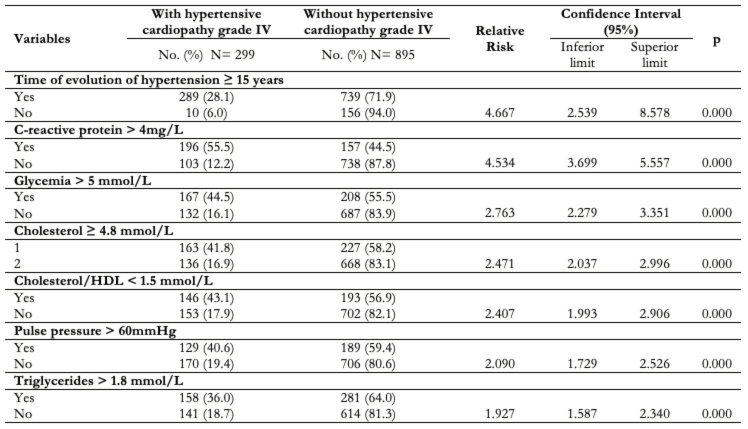 Table 4. Bivariate analysis of quantitative variables.
Table 4. Bivariate analysis of quantitative variables.

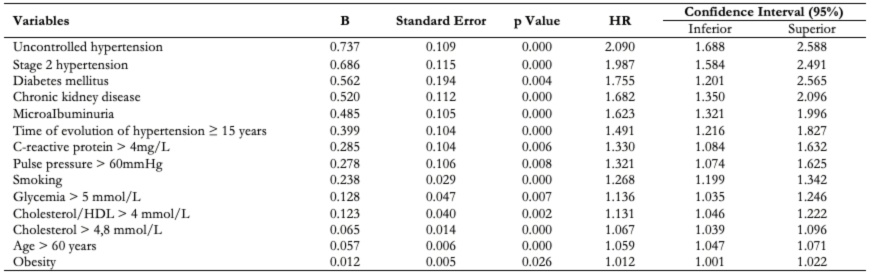 Table 5. Cox’s multivariate model results.
Table 5. Cox’s multivariate model results.

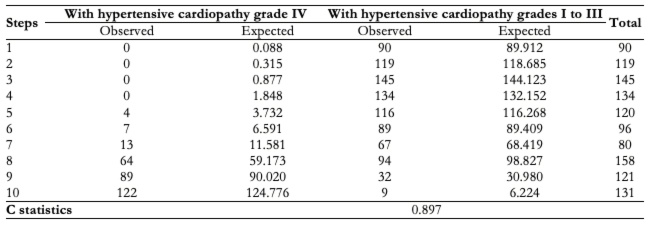 Table 6. Model internal validity (calibration and discrimination).
Table 6. Model internal validity (calibration and discrimination).
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Introduction
By definition, hypertensive cardiopathy is a series of complex and variable effects responsible for the chronic elevation of blood pressure in the heart. It stands out within a broad spectrum of cardiovascular diseases associated with hypertension.
Objective
To evaluate the capacity to predict the development of adaptive changes to hypertensive cardiopathy within ten years following diagnosis of the condition, using a model based on prognostic factors.
Methods
A prospective cohort study was conducted in hypertensive patients. The patients were followed at the specialized hypertension physician’s office of the specialty policlinic attached to Carlos Manuel de Céspedes University Hospital, in the Bayamo Municipality, Granma Province, Cuba, from 1 January 2008 to 31 December 2018.
Results
Cox’s proportional regression model showed a significant statistical relationship between most of the factors and the development of the adaptive changes in hypertensive cardiopathy within ten years of follow-up after the diagnosis of this condition. The lack of blood pressure control (Hazard ratio: 2.090; confidence interval 95%: 1.688 to 2.588; p: 0.000) followed by stage 2 of hypertension (hazard ratio: 1.987; confidence interval 95%: 1.584 to 2.491; p: 0.000) were the main factors. Internal validation of the model, discriminant capacity (C- statistic: 0.897) and calibration Hosmer-Lemeshow (χ2: 5.384; p: 0.716), was acceptable.
Conclusions
We develop a model to predict the progression of hypertensive cardiopathy from grade I to grade IV with adequate discriminatory capacity. The model is based on prognostic factors, from which the characteristic effects of arterial hypertension, diabetes mellitus, and chronic kidney disease stood out.
 Authors:
Alexis Álvarez-Aliaga[1,2], Adonis Frómeta-Guerra[1,2], Alexis Suárez-Quesada[1,2], David del Llano-Sosa[1,2], Joel Berdú-Saumell[1,2], Yasel Alberto Lago-Santiesteban[1,2]
Authors:
Alexis Álvarez-Aliaga[1,2], Adonis Frómeta-Guerra[1,2], Alexis Suárez-Quesada[1,2], David del Llano-Sosa[1,2], Joel Berdú-Saumell[1,2], Yasel Alberto Lago-Santiesteban[1,2]
Affiliation:
[1] Hospital General Universitario Carlos Manuel de Céspedes, Bayamo, Granma, Cuba
[2] Universidad de Ciencias Médicas de Granma, Bayamo, Cuba
E-mail: alexis.grm@infomed.sld.cu
Author address:
[1] Calle E № 7 entre 3 y 5
Reparto Carlos Manuel de Céspedes
Bayamo, Granma
Cuba, 85100.

Citation: Álvarez-Aliaga A, Frómeta-Guerra A, Suárez-Quesada A, del Llano-Sosa D, Berdú-Saumell J, Lago-Santiesteban YA. Prognostic model of the adaptive changes from hypertensive cardiopathy: from mild diastolic dysfunction to depressed systolic function. Medwave 2020;20(3):e7873 doi: 10.5867/medwave.2020.03.7873
Submission date: 4/11/2019
Acceptance date: 18/3/2020
Publication date: 9/4/2020
Origin: Not commissioned.
Type of review: Externally peer-reviewed by four reviewers, double-blind.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. Guía ESC/ESH 2018 sobre el diagnóstico y tratamiento de la hipertensión arterial. Rev Esp Cardiol. 2019;72(2): 160.e1-e78.
- Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012 Jan 26;366(4):321-9. | CrossRef | PubMed |
- Díez J, Frohlich ED. A translational approach to hypertensive heart disease. Hypertension. 2010;55:1- 8. [Internet] | Link |
- Álvarez-Aliaga A, Quesada-Vázquez AJ, Suárez-Quesada A, de Llano Sosa D. Design and validation of an Index to predict the development of Hypertensive Cardiopathy. J Cardiol Cardiovasc Med. 2018;(3):008-022. [Internet] | CrossRef |
- Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014 May 31;383(9932):1899-911. | CrossRef | PubMed |
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206-52. | PubMed |
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440-63. | PubMed |
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006 Mar;7(2):79-108. | PubMed |
- Pérez Caballero MD, Dueñas Herrera A, Alfonso Guerra JP, Vázquez Vigoa A, Navarro Despaigne D, Hernández Cueto M, et al. Hipertensión arterial. Guía para la prevención, diagnóstico y tratamiento. Comisión Nacional Técnica Asesora del Programa de Hipertensión Arterial. La Habana: Editorial Ciencias Médicas; 2008. [Internet] | Link |
- Zhao W, Hasegawa K, Chen J. The use of food-frequency questionnaires for various purposes in China. Public Health Nutr. 2002 Dec;5(6A):829-33. | PubMed |
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010 May 26;303(20):2043-50. | CrossRef | PubMed |
- Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005 Jun;67(6):2089-100. | PubMed |
- Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003 Nov;26(11):3160-7. | PubMed |
- Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000 Jan 15;19(1):113-32. | PubMed |
- Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996 Feb 28;15(4):361-87. | PubMed |
- Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. Springer; 2009:255–79.
- May S, Hosmer DW. Hosmer and Lemeshow type goodness-of-fit statistics for the Cox proportional hazards model. Elsevier; 2004:383–94.
- Georgiopoulou VV, Kalogeropoulos AP, Raggi P, Butler J. Prevention, diagnosis, and treatment of hypertensive heart disease. Cardiol Clin. 2010 Nov;28(4):675-91. | CrossRef | PubMed |
- Nielsen TL, Plesner LL, Warming PE, Mortensen OH, Iversen KK, Heaf JG. FGF23 in hemodialysis patients is associated with left ventricular hypertrophy and reduced ejection fraction. Nefrologia. 2019 May - Jun;39(3):258-268. | CrossRef | PubMed |
- Schneider MP, Scheppach JB, Raff U, Toncar S, Ritter C, Klink T, et al. Left Ventricular Structure in Patients With Mild-to-Moderate CKD-a Magnetic Resonance Imaging Study. Kidney Int Rep. 2018 Oct 11;4(2):267-274. | CrossRef | PubMed |
- Martínez-Castelao A, Górriz JL, Segura-de la Morena J, Cebollada J, Escalada J, Esmatjes E, et al. Consensus document for the detection and management of chronic kidney disease. Nefrologia. 2014;34(2):243-62. | CrossRef | PubMed |
- Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, et al. Masked Hypertension and Elevated Nighttime Blood Pressure in CKD: Prevalence and Association with Target Organ Damage. Clin J Am Soc Nephrol. 2016 Apr 7;11(4):642-52. | CrossRef | PubMed |
- Rossignol P, Massy ZA, Azizi M, Bakris G, Ritz E, Covic A, et al. The double challenge of resistant hypertension and chronic kidney disease. Lancet. 2015 Oct 17;386(10003):1588-98. | CrossRef | PubMed |
- Núñez J, Miñana G, Santas E, Bertomeu-González V. Cardiorenal Syndrome in Acute Heart Failure: Revisiting Paradigms. Rev Esp Cardiol (Engl Ed). 2015 May;68(5):426-35. | CrossRef | PubMed |
- Tovillas-Morán FJ, Zabaleta-del-Olmo E, Dalfó-Baqué A, Vilaplana-Cosculluela M, Galcerán JM, Coca A. Morbimortalidad cardiovascular y patrones geométricos del ventrículo izquierdo en pacientes hipertensos atendidos en atención primaria. Rev Esp Cardiol. 2009; 62:246-54. | CrossRef |
- Lam CSP, Voors AA, de Boer RA, Solomon SD, van Veldhuisen DJ. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J. 2018 Aug 7;39(30):2780-2792. | CrossRef | PubMed |
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 Nov 26;373(22):2117-28. | CrossRef | PubMed |
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017 Aug 17;377(7):644-657. | CrossRef | PubMed |
- Fitchett D, Butler J, van de Borne P, Zinman B, Lachin JM, Wanner C, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J. 2018 Feb 1;39(5):363-370. | CrossRef | PubMed |
- Li J, Kemp BA, Howell NL, Massey J, Mińczuk K, Huang Q, et al. Metabolic Changes in Spontaneously Hypertensive Rat Hearts Precede Cardiac Dysfunction and Left Ventricular Hypertrophy. J Am Heart Assoc. 2019 Feb 19;8(4):e010926. | CrossRef | PubMed |
- American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019 Jan;42(Suppl 1):S103-S123. | CrossRef | PubMed |
- SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 Nov 26;373(22):2103-16. | CrossRef | PubMed |
- Beckett N, Peters R, Leonetti G, Duggan J, Fagard R, Thijs L, et al. Subgroup and per-protocol analyses from the Hypertension in the Very Elderly Trial. J Hypertens. 2014 Jul;32(7):1478-87; discussion 1487. | CrossRef | PubMed |
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering treatment. 6. Prevention of heart failure and new-onset heart failure--meta-analyses of randomized trials. J Hypertens. 2016 Mar;34(3):373-84; discussion 384. | CrossRef | PubMed |
- Cuspidi C, Giudici V, Negri F, Meani S, Sala C, Zanchetti A, et al. Improving cardiovascular risk stratification in essential hypertensive patients by indexing left ventricular mass to height(2.7). J Hypertens. 2009 Dec;27(12):2465-71. | CrossRef | PubMed |
- Gu Q, Dillon CF, Burt VL, Gillum RF. Association of hypertension treatment and control with all-cause and cardiovascular disease mortality among US adults with hypertension. Am J Hypertens. 2010 Jan;23(1):38-45. | CrossRef | PubMed |
- Peralta CA, Katz R, Newman AB, Psaty BM, Odden MC. Systolic and diastolic blood pressure, incident cardiovascular events, and death in elderly persons: the role of functional limitation in the Cardiovascular Health Study. Hypertension. 2014 Sep;64(3):472-80. | CrossRef | PubMed |
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010 May 26;303(20):2043-50. | CrossRef | PubMed |
- Maione A, Annemans L, Strippoli G. Proteinuria and clinical outcomes in hypertensive patients. Am J Hypertens. 2009 Nov;22(11):1137-47 | CrossRef |
- Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001 Jul 25;286(4):421-6. | PubMed |
- Schmieder RE, Mann JF, Schumacher H, Gao P, Mancia G, Weber MA, et al. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol. 2011 Jul;22(7):1353-64. | CrossRef | PubMed |
- Bakris GL, Sarafidis PA, Weir MR, Dahlöf B, Pitt B, Jamerson K, et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010 Apr 3;375(9721):1173-81. | CrossRef | PubMed |
- Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, Menne J, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011 Mar 10;364(10):907-17. | CrossRef | PubMed |
- Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012 Dec 6;367(23):2204-13. | CrossRef | PubMed |
- Zhang Z, Dzau VJ. Angiotensin II type 1 receptor-associated protein is an endogenous inhibitor of angiotensin II type 1 receptor action in cardiac hypertrophy: role in check and balance. Hypertension. 2010 May;55(5):1086-7. | CrossRef | PubMed |
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS. Guia ESC 2016 sobre el diagnóstico y tratamiento de la insuficiencia cardiaca aguda y crónica. Rev Esp Cardiol. 2016;69(12): 1167.e1-e85. [Internet] | CrossRef |
- Royo-Bordonada MÁ, Armario P, Lobos Bejarano JM, Pedro-Botet J, Villar Alvarez F, Elosua R, et al. Adaptación española de las guías europeas de 2016 sobre prevención de la enfermedad cardiovascular en la práctica clínica. Hipertens Riesgo Vasc. 2017;34(1):24-40. [Internet] | CrossRef |
- López-Jiménez F, Cortés-Bergoderi M. Update: systemic diseases and the cardiovascular system (i): obesity and the heart. Rev Esp Cardiol. 2011 Feb;64(2):140-9. | CrossRef | PubMed |
- Zhang Y, Ren J. Role of cardiac steatosis and lipotoxicity in obesity cardiomyopathy. Hypertension. 2011 Feb;57(2):148-50. | CrossRef | PubMed |
- Álvarez Aliaga A, González Aguilera JC, Maceo Gómez Ldel R. Factors associated to hypertensive heart disease development: a prospective cohort study in Bayamo, Cuba. Medwave. 2016 Jul 7;16(6):e6492. | CrossRef | PubMed |
- Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell'Italia LJ, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009 Sep;54(3):475-81. | CrossRef | PubMed |
- López Salazar B, Ravassa Albéniz S, Arias Guedón T, González Miqueo A, Querejeta R, Díez Martínez J. [Altered fibrillar collagen metabolism in hypertensive heart failure. Current understanding and future prospects]. Rev Esp Cardiol. 2006 Oct;59(10):1047-57. | CrossRef | PubMed |
- Schulz R, Heusch G. C-reactive protein: just a biomarker of inflammation or a pathophysiological player in myocardial function and morphology? Hypertension. 2011; 57:151-3. [Internet] | Link |
- Nagai T, Anzai T, Kaneko H, Mano Y, Anzai A, Maekawa Y, et al. C-reactive protein overexpression exacerbates pressure overload-induced cardiac remodeling through enhanced inflammatory response. Hypertension. 2011 Feb;57(2):208-15. | CrossRef | PubMed |
- Frohlich ED, González A, Díez J. Hypertensive left ventricular hypertrophy risk: beyond adaptive cardiomyocytic hypertrophy. J Hypertens. 2011 Jan;29(1):17-26. | CrossRef | PubMed |
- Downs JR, O'Malley PG. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2014 U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guideline. Ann Intern Med. 2015 Aug 18;163(4):291-7. | CrossRef | PubMed |
- Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007 Sep 27;357(13):1301-10. | PubMed |
- Ashen MD, Blumenthal RS. Clinical practice. Low HDL cholesterol levels. N Engl J Med. 2005 Sep 22;353(12):1252-60. Review. Erratum in: N Engl J Med. 2006 Jan 12;354(2):215. | PubMed |
- Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013 Jul;31(7):1281-357. | CrossRef | PubMed |
- Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011 May 17;57(20):2037-114. | CrossRef | PubMed |
- Adji A, O'Rourke MF, Namasivayam M. Arterial stiffness, its assessment, prognostic value, and implications for treatment. Am J Hypertens. 2011 Jan;24(1):5-17. | CrossRef | PubMed |
- Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070-7. [Internet] | Link |
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008 Mar 27;358(13):1370-80. | CrossRef | PubMed |
- Gradman AH, Alfayoumi F. From left ventricular hypertrophy to congestive heart failure: management of hypertensive heart disease. Prog Cardiovasc Dis. 2006 Mar-Apr;48(5):326-41. | PubMed |
 Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. Guía ESC/ESH 2018 sobre el diagnóstico y tratamiento de la hipertensión arterial. Rev Esp Cardiol. 2019;72(2): 160.e1-e78.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. Guía ESC/ESH 2018 sobre el diagnóstico y tratamiento de la hipertensión arterial. Rev Esp Cardiol. 2019;72(2): 160.e1-e78.  Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012 Jan 26;366(4):321-9. | CrossRef | PubMed |
Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012 Jan 26;366(4):321-9. | CrossRef | PubMed | Díez J, Frohlich ED. A translational approach to hypertensive heart disease. Hypertension. 2010;55:1- 8. [Internet] | Link |
Díez J, Frohlich ED. A translational approach to hypertensive heart disease. Hypertension. 2010;55:1- 8. [Internet] | Link | Álvarez-Aliaga A, Quesada-Vázquez AJ, Suárez-Quesada A, de Llano Sosa D. Design and validation of an Index to predict the development of Hypertensive Cardiopathy. J Cardiol Cardiovasc Med. 2018;(3):008-022. [Internet] | CrossRef |
Álvarez-Aliaga A, Quesada-Vázquez AJ, Suárez-Quesada A, de Llano Sosa D. Design and validation of an Index to predict the development of Hypertensive Cardiopathy. J Cardiol Cardiovasc Med. 2018;(3):008-022. [Internet] | CrossRef | Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014 May 31;383(9932):1899-911. | CrossRef | PubMed |
Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014 May 31;383(9932):1899-911. | CrossRef | PubMed | Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206-52. | PubMed |
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206-52. | PubMed | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440-63. | PubMed |
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440-63. | PubMed | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006 Mar;7(2):79-108. | PubMed |
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006 Mar;7(2):79-108. | PubMed | Pérez Caballero MD, Dueñas Herrera A, Alfonso Guerra JP, Vázquez Vigoa A, Navarro Despaigne D, Hernández Cueto M, et al. Hipertensión arterial. Guía para la prevención, diagnóstico y tratamiento. Comisión Nacional Técnica Asesora del Programa de Hipertensión Arterial. La Habana: Editorial Ciencias Médicas; 2008. [Internet] | Link |
Pérez Caballero MD, Dueñas Herrera A, Alfonso Guerra JP, Vázquez Vigoa A, Navarro Despaigne D, Hernández Cueto M, et al. Hipertensión arterial. Guía para la prevención, diagnóstico y tratamiento. Comisión Nacional Técnica Asesora del Programa de Hipertensión Arterial. La Habana: Editorial Ciencias Médicas; 2008. [Internet] | Link | Zhao W, Hasegawa K, Chen J. The use of food-frequency questionnaires for various purposes in China. Public Health Nutr. 2002 Dec;5(6A):829-33. | PubMed |
Zhao W, Hasegawa K, Chen J. The use of food-frequency questionnaires for various purposes in China. Public Health Nutr. 2002 Dec;5(6A):829-33. | PubMed | Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010 May 26;303(20):2043-50. | CrossRef | PubMed |
Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010 May 26;303(20):2043-50. | CrossRef | PubMed | Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005 Jun;67(6):2089-100. | PubMed |
Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005 Jun;67(6):2089-100. | PubMed | Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003 Nov;26(11):3160-7. | PubMed |
Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003 Nov;26(11):3160-7. | PubMed | Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000 Jan 15;19(1):113-32. | PubMed |
Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000 Jan 15;19(1):113-32. | PubMed | Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996 Feb 28;15(4):361-87. | PubMed |
Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996 Feb 28;15(4):361-87. | PubMed | Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. Springer; 2009:255–79.
Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. Springer; 2009:255–79.  May S, Hosmer DW. Hosmer and Lemeshow type goodness-of-fit statistics for the Cox proportional hazards model. Elsevier; 2004:383–94.
May S, Hosmer DW. Hosmer and Lemeshow type goodness-of-fit statistics for the Cox proportional hazards model. Elsevier; 2004:383–94.  Georgiopoulou VV, Kalogeropoulos AP, Raggi P, Butler J. Prevention, diagnosis, and treatment of hypertensive heart disease. Cardiol Clin. 2010 Nov;28(4):675-91. | CrossRef | PubMed |
Georgiopoulou VV, Kalogeropoulos AP, Raggi P, Butler J. Prevention, diagnosis, and treatment of hypertensive heart disease. Cardiol Clin. 2010 Nov;28(4):675-91. | CrossRef | PubMed | Nielsen TL, Plesner LL, Warming PE, Mortensen OH, Iversen KK, Heaf JG. FGF23 in hemodialysis patients is associated with left ventricular hypertrophy and reduced ejection fraction. Nefrologia. 2019 May - Jun;39(3):258-268. | CrossRef | PubMed |
Nielsen TL, Plesner LL, Warming PE, Mortensen OH, Iversen KK, Heaf JG. FGF23 in hemodialysis patients is associated with left ventricular hypertrophy and reduced ejection fraction. Nefrologia. 2019 May - Jun;39(3):258-268. | CrossRef | PubMed | Schneider MP, Scheppach JB, Raff U, Toncar S, Ritter C, Klink T, et al. Left Ventricular Structure in Patients With Mild-to-Moderate CKD-a Magnetic Resonance Imaging Study. Kidney Int Rep. 2018 Oct 11;4(2):267-274. | CrossRef | PubMed |
Schneider MP, Scheppach JB, Raff U, Toncar S, Ritter C, Klink T, et al. Left Ventricular Structure in Patients With Mild-to-Moderate CKD-a Magnetic Resonance Imaging Study. Kidney Int Rep. 2018 Oct 11;4(2):267-274. | CrossRef | PubMed | Martínez-Castelao A, Górriz JL, Segura-de la Morena J, Cebollada J, Escalada J, Esmatjes E, et al. Consensus document for the detection and management of chronic kidney disease. Nefrologia. 2014;34(2):243-62. | CrossRef | PubMed |
Martínez-Castelao A, Górriz JL, Segura-de la Morena J, Cebollada J, Escalada J, Esmatjes E, et al. Consensus document for the detection and management of chronic kidney disease. Nefrologia. 2014;34(2):243-62. | CrossRef | PubMed | Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, et al. Masked Hypertension and Elevated Nighttime Blood Pressure in CKD: Prevalence and Association with Target Organ Damage. Clin J Am Soc Nephrol. 2016 Apr 7;11(4):642-52. | CrossRef | PubMed |
Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, et al. Masked Hypertension and Elevated Nighttime Blood Pressure in CKD: Prevalence and Association with Target Organ Damage. Clin J Am Soc Nephrol. 2016 Apr 7;11(4):642-52. | CrossRef | PubMed | Rossignol P, Massy ZA, Azizi M, Bakris G, Ritz E, Covic A, et al. The double challenge of resistant hypertension and chronic kidney disease. Lancet. 2015 Oct 17;386(10003):1588-98. | CrossRef | PubMed |
Rossignol P, Massy ZA, Azizi M, Bakris G, Ritz E, Covic A, et al. The double challenge of resistant hypertension and chronic kidney disease. Lancet. 2015 Oct 17;386(10003):1588-98. | CrossRef | PubMed | Núñez J, Miñana G, Santas E, Bertomeu-González V. Cardiorenal Syndrome in Acute Heart Failure: Revisiting Paradigms. Rev Esp Cardiol (Engl Ed). 2015 May;68(5):426-35. | CrossRef | PubMed |
Núñez J, Miñana G, Santas E, Bertomeu-González V. Cardiorenal Syndrome in Acute Heart Failure: Revisiting Paradigms. Rev Esp Cardiol (Engl Ed). 2015 May;68(5):426-35. | CrossRef | PubMed | Tovillas-Morán FJ, Zabaleta-del-Olmo E, Dalfó-Baqué A, Vilaplana-Cosculluela M, Galcerán JM, Coca A. Morbimortalidad cardiovascular y patrones geométricos del ventrículo izquierdo en pacientes hipertensos atendidos en atención primaria. Rev Esp Cardiol. 2009; 62:246-54. | CrossRef |
Tovillas-Morán FJ, Zabaleta-del-Olmo E, Dalfó-Baqué A, Vilaplana-Cosculluela M, Galcerán JM, Coca A. Morbimortalidad cardiovascular y patrones geométricos del ventrículo izquierdo en pacientes hipertensos atendidos en atención primaria. Rev Esp Cardiol. 2009; 62:246-54. | CrossRef | Lam CSP, Voors AA, de Boer RA, Solomon SD, van Veldhuisen DJ. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J. 2018 Aug 7;39(30):2780-2792. | CrossRef | PubMed |
Lam CSP, Voors AA, de Boer RA, Solomon SD, van Veldhuisen DJ. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J. 2018 Aug 7;39(30):2780-2792. | CrossRef | PubMed | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 Nov 26;373(22):2117-28. | CrossRef | PubMed |
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 Nov 26;373(22):2117-28. | CrossRef | PubMed | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017 Aug 17;377(7):644-657. | CrossRef | PubMed |
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017 Aug 17;377(7):644-657. | CrossRef | PubMed | Fitchett D, Butler J, van de Borne P, Zinman B, Lachin JM, Wanner C, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J. 2018 Feb 1;39(5):363-370. | CrossRef | PubMed |
Fitchett D, Butler J, van de Borne P, Zinman B, Lachin JM, Wanner C, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J. 2018 Feb 1;39(5):363-370. | CrossRef | PubMed | Li J, Kemp BA, Howell NL, Massey J, Mińczuk K, Huang Q, et al. Metabolic Changes in Spontaneously Hypertensive Rat Hearts Precede Cardiac Dysfunction and Left Ventricular Hypertrophy. J Am Heart Assoc. 2019 Feb 19;8(4):e010926. | CrossRef | PubMed |
Li J, Kemp BA, Howell NL, Massey J, Mińczuk K, Huang Q, et al. Metabolic Changes in Spontaneously Hypertensive Rat Hearts Precede Cardiac Dysfunction and Left Ventricular Hypertrophy. J Am Heart Assoc. 2019 Feb 19;8(4):e010926. | CrossRef | PubMed | American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019 Jan;42(Suppl 1):S103-S123. | CrossRef | PubMed |
American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019 Jan;42(Suppl 1):S103-S123. | CrossRef | PubMed | SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 Nov 26;373(22):2103-16. | CrossRef | PubMed |
SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 Nov 26;373(22):2103-16. | CrossRef | PubMed | Beckett N, Peters R, Leonetti G, Duggan J, Fagard R, Thijs L, et al. Subgroup and per-protocol analyses from the Hypertension in the Very Elderly Trial. J Hypertens. 2014 Jul;32(7):1478-87; discussion 1487. | CrossRef | PubMed |
Beckett N, Peters R, Leonetti G, Duggan J, Fagard R, Thijs L, et al. Subgroup and per-protocol analyses from the Hypertension in the Very Elderly Trial. J Hypertens. 2014 Jul;32(7):1478-87; discussion 1487. | CrossRef | PubMed | Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering treatment. 6. Prevention of heart failure and new-onset heart failure--meta-analyses of randomized trials. J Hypertens. 2016 Mar;34(3):373-84; discussion 384. | CrossRef | PubMed |
Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering treatment. 6. Prevention of heart failure and new-onset heart failure--meta-analyses of randomized trials. J Hypertens. 2016 Mar;34(3):373-84; discussion 384. | CrossRef | PubMed | Cuspidi C, Giudici V, Negri F, Meani S, Sala C, Zanchetti A, et al. Improving cardiovascular risk stratification in essential hypertensive patients by indexing left ventricular mass to height(2.7). J Hypertens. 2009 Dec;27(12):2465-71. | CrossRef | PubMed |
Cuspidi C, Giudici V, Negri F, Meani S, Sala C, Zanchetti A, et al. Improving cardiovascular risk stratification in essential hypertensive patients by indexing left ventricular mass to height(2.7). J Hypertens. 2009 Dec;27(12):2465-71. | CrossRef | PubMed | Gu Q, Dillon CF, Burt VL, Gillum RF. Association of hypertension treatment and control with all-cause and cardiovascular disease mortality among US adults with hypertension. Am J Hypertens. 2010 Jan;23(1):38-45. | CrossRef | PubMed |
Gu Q, Dillon CF, Burt VL, Gillum RF. Association of hypertension treatment and control with all-cause and cardiovascular disease mortality among US adults with hypertension. Am J Hypertens. 2010 Jan;23(1):38-45. | CrossRef | PubMed | Peralta CA, Katz R, Newman AB, Psaty BM, Odden MC. Systolic and diastolic blood pressure, incident cardiovascular events, and death in elderly persons: the role of functional limitation in the Cardiovascular Health Study. Hypertension. 2014 Sep;64(3):472-80. | CrossRef | PubMed |
Peralta CA, Katz R, Newman AB, Psaty BM, Odden MC. Systolic and diastolic blood pressure, incident cardiovascular events, and death in elderly persons: the role of functional limitation in the Cardiovascular Health Study. Hypertension. 2014 Sep;64(3):472-80. | CrossRef | PubMed | Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010 May 26;303(20):2043-50. | CrossRef | PubMed |
Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010 May 26;303(20):2043-50. | CrossRef | PubMed | Maione A, Annemans L, Strippoli G. Proteinuria and clinical outcomes in hypertensive patients. Am J Hypertens. 2009 Nov;22(11):1137-47 | CrossRef |
Maione A, Annemans L, Strippoli G. Proteinuria and clinical outcomes in hypertensive patients. Am J Hypertens. 2009 Nov;22(11):1137-47 | CrossRef | Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001 Jul 25;286(4):421-6. | PubMed |
Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001 Jul 25;286(4):421-6. | PubMed | Schmieder RE, Mann JF, Schumacher H, Gao P, Mancia G, Weber MA, et al. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol. 2011 Jul;22(7):1353-64. | CrossRef | PubMed |
Schmieder RE, Mann JF, Schumacher H, Gao P, Mancia G, Weber MA, et al. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol. 2011 Jul;22(7):1353-64. | CrossRef | PubMed | Bakris GL, Sarafidis PA, Weir MR, Dahlöf B, Pitt B, Jamerson K, et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010 Apr 3;375(9721):1173-81. | CrossRef | PubMed |
Bakris GL, Sarafidis PA, Weir MR, Dahlöf B, Pitt B, Jamerson K, et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010 Apr 3;375(9721):1173-81. | CrossRef | PubMed | Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, Menne J, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011 Mar 10;364(10):907-17. | CrossRef | PubMed |
Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, Menne J, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011 Mar 10;364(10):907-17. | CrossRef | PubMed | Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012 Dec 6;367(23):2204-13. | CrossRef | PubMed |
Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012 Dec 6;367(23):2204-13. | CrossRef | PubMed | Zhang Z, Dzau VJ. Angiotensin II type 1 receptor-associated protein is an endogenous inhibitor of angiotensin II type 1 receptor action in cardiac hypertrophy: role in check and balance. Hypertension. 2010 May;55(5):1086-7. | CrossRef | PubMed |
Zhang Z, Dzau VJ. Angiotensin II type 1 receptor-associated protein is an endogenous inhibitor of angiotensin II type 1 receptor action in cardiac hypertrophy: role in check and balance. Hypertension. 2010 May;55(5):1086-7. | CrossRef | PubMed | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS. Guia ESC 2016 sobre el diagnóstico y tratamiento de la insuficiencia cardiaca aguda y crónica. Rev Esp Cardiol. 2016;69(12): 1167.e1-e85. [Internet] | CrossRef |
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS. Guia ESC 2016 sobre el diagnóstico y tratamiento de la insuficiencia cardiaca aguda y crónica. Rev Esp Cardiol. 2016;69(12): 1167.e1-e85. [Internet] | CrossRef | Royo-Bordonada MÁ, Armario P, Lobos Bejarano JM, Pedro-Botet J, Villar Alvarez F, Elosua R, et al. Adaptación española de las guías europeas de 2016 sobre prevención de la enfermedad cardiovascular en la práctica clínica. Hipertens Riesgo Vasc. 2017;34(1):24-40. [Internet] | CrossRef |
Royo-Bordonada MÁ, Armario P, Lobos Bejarano JM, Pedro-Botet J, Villar Alvarez F, Elosua R, et al. Adaptación española de las guías europeas de 2016 sobre prevención de la enfermedad cardiovascular en la práctica clínica. Hipertens Riesgo Vasc. 2017;34(1):24-40. [Internet] | CrossRef | López-Jiménez F, Cortés-Bergoderi M. Update: systemic diseases and the cardiovascular system (i): obesity and the heart. Rev Esp Cardiol. 2011 Feb;64(2):140-9. | CrossRef | PubMed |
López-Jiménez F, Cortés-Bergoderi M. Update: systemic diseases and the cardiovascular system (i): obesity and the heart. Rev Esp Cardiol. 2011 Feb;64(2):140-9. | CrossRef | PubMed | Zhang Y, Ren J. Role of cardiac steatosis and lipotoxicity in obesity cardiomyopathy. Hypertension. 2011 Feb;57(2):148-50. | CrossRef | PubMed |
Zhang Y, Ren J. Role of cardiac steatosis and lipotoxicity in obesity cardiomyopathy. Hypertension. 2011 Feb;57(2):148-50. | CrossRef | PubMed | Álvarez Aliaga A, González Aguilera JC, Maceo Gómez Ldel R. Factors associated to hypertensive heart disease development: a prospective cohort study in Bayamo, Cuba. Medwave. 2016 Jul 7;16(6):e6492. | CrossRef | PubMed |
Álvarez Aliaga A, González Aguilera JC, Maceo Gómez Ldel R. Factors associated to hypertensive heart disease development: a prospective cohort study in Bayamo, Cuba. Medwave. 2016 Jul 7;16(6):e6492. | CrossRef | PubMed | Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell'Italia LJ, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009 Sep;54(3):475-81. | CrossRef | PubMed |
Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell'Italia LJ, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009 Sep;54(3):475-81. | CrossRef | PubMed | López Salazar B, Ravassa Albéniz S, Arias Guedón T, González Miqueo A, Querejeta R, Díez Martínez J. [Altered fibrillar collagen metabolism in hypertensive heart failure. Current understanding and future prospects]. Rev Esp Cardiol. 2006 Oct;59(10):1047-57. | CrossRef | PubMed |
López Salazar B, Ravassa Albéniz S, Arias Guedón T, González Miqueo A, Querejeta R, Díez Martínez J. [Altered fibrillar collagen metabolism in hypertensive heart failure. Current understanding and future prospects]. Rev Esp Cardiol. 2006 Oct;59(10):1047-57. | CrossRef | PubMed | Schulz R, Heusch G. C-reactive protein: just a biomarker of inflammation or a pathophysiological player in myocardial function and morphology? Hypertension. 2011; 57:151-3. [Internet] | Link |
Schulz R, Heusch G. C-reactive protein: just a biomarker of inflammation or a pathophysiological player in myocardial function and morphology? Hypertension. 2011; 57:151-3. [Internet] | Link | Nagai T, Anzai T, Kaneko H, Mano Y, Anzai A, Maekawa Y, et al. C-reactive protein overexpression exacerbates pressure overload-induced cardiac remodeling through enhanced inflammatory response. Hypertension. 2011 Feb;57(2):208-15. | CrossRef | PubMed |
Nagai T, Anzai T, Kaneko H, Mano Y, Anzai A, Maekawa Y, et al. C-reactive protein overexpression exacerbates pressure overload-induced cardiac remodeling through enhanced inflammatory response. Hypertension. 2011 Feb;57(2):208-15. | CrossRef | PubMed | Frohlich ED, González A, Díez J. Hypertensive left ventricular hypertrophy risk: beyond adaptive cardiomyocytic hypertrophy. J Hypertens. 2011 Jan;29(1):17-26. | CrossRef | PubMed |
Frohlich ED, González A, Díez J. Hypertensive left ventricular hypertrophy risk: beyond adaptive cardiomyocytic hypertrophy. J Hypertens. 2011 Jan;29(1):17-26. | CrossRef | PubMed | Downs JR, O'Malley PG. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2014 U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guideline. Ann Intern Med. 2015 Aug 18;163(4):291-7. | CrossRef | PubMed |
Downs JR, O'Malley PG. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2014 U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guideline. Ann Intern Med. 2015 Aug 18;163(4):291-7. | CrossRef | PubMed | Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007 Sep 27;357(13):1301-10. | PubMed |
Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007 Sep 27;357(13):1301-10. | PubMed | Ashen MD, Blumenthal RS. Clinical practice. Low HDL cholesterol levels. N Engl J Med. 2005 Sep 22;353(12):1252-60. Review. Erratum in: N Engl J Med. 2006 Jan 12;354(2):215. | PubMed |
Ashen MD, Blumenthal RS. Clinical practice. Low HDL cholesterol levels. N Engl J Med. 2005 Sep 22;353(12):1252-60. Review. Erratum in: N Engl J Med. 2006 Jan 12;354(2):215. | PubMed | Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013 Jul;31(7):1281-357. | CrossRef | PubMed |
Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013 Jul;31(7):1281-357. | CrossRef | PubMed | Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011 May 17;57(20):2037-114. | CrossRef | PubMed |
Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011 May 17;57(20):2037-114. | CrossRef | PubMed | Adji A, O'Rourke MF, Namasivayam M. Arterial stiffness, its assessment, prognostic value, and implications for treatment. Am J Hypertens. 2011 Jan;24(1):5-17. | CrossRef | PubMed |
Adji A, O'Rourke MF, Namasivayam M. Arterial stiffness, its assessment, prognostic value, and implications for treatment. Am J Hypertens. 2011 Jan;24(1):5-17. | CrossRef | PubMed | Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070-7. [Internet] | Link |
Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070-7. [Internet] | Link | Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008 Mar 27;358(13):1370-80. | CrossRef | PubMed |
Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008 Mar 27;358(13):1370-80. | CrossRef | PubMed | Gradman AH, Alfayoumi F. From left ventricular hypertrophy to congestive heart failure: management of hypertensive heart disease. Prog Cardiovasc Dis. 2006 Mar-Apr;48(5):326-41. | PubMed |
Gradman AH, Alfayoumi F. From left ventricular hypertrophy to congestive heart failure: management of hypertensive heart disease. Prog Cardiovasc Dis. 2006 Mar-Apr;48(5):326-41. | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








