Key Words: phacoemulsification, cataract, endothelial cells
Abstract
Introduction
Phacoeresis is the procedure through which the lens is surgically removed to treat cataracts. A corneal endothelial loss is a recognized sequel. Although several factors associated with this harm have been described, the surgeon’s prior experience has been scarcely evaluated.
Objectives
To assess the association between the surgeon’s experience and other variables associated with a corneal endothelial cell loss in the context of phacoeresis.
Methods
Clinical records of 198 patients undergoing cataract operations were prospectively reviewed. The experience of the surgeon and other variables were recorded, including cumulative dissipated energy, viscoelastic type, the use of trypan blue, amount of fluidics, ultrasound time, combined phacoemulsification energy, and pre- and postoperative corneal endothelial cell counts.
Results
No differences were observed in the postoperative corneal endothelial cell count between surgeons with more or less than five years of experience. Nevertheless, ophthalmologists with more than five years’ experience used less trypan blue, but more cumulative dissipated energy in each procedure, while less experienced ophthalmologists used less fluidics.
Conclusions
Although there were differences in the surgical management regarding the surgeons’ experience in factors known to influence corneal endothelial cell loss, no differences in endothelial cell loss were observed as an outcome.
| Main ideas
|
Introduction
It is estimated that 285 million people worldwide have some degree of visual disability, of which 33% is caused by cataracts[1]. This pathology is estimated to be the second highest costing visual disorder[2]. Data from the Chilean National Health Survey report a prevalence of 4.5%, reaching up to 23.9% in older adults[3].
The treatment for cataracts consists in the surgical extraction of the lens (phacoeresis), an effective and safe procedure, where it is imperative that the integrity of the capsule and the corneal endothelium is preserved[4],[5],[6],[7]. Phacoeresis can be associated with an additional loss of corneal endothelial cells per se, beyond the physiological loss, which ranges from 0.6% to 2.5% per year[8],[9],[10]. When the corneal endothelium count drops below 500-700 cells per square millimeter, edema occurs, triggering loss of visual acuity associated to bullous keratopathy[8],[11],[12].
The most widely used form of phacoeresis, phacoemulsification, consists in the emulsification of the lens using ultrasonic frequency[5],[13], which is also used in refractive surgery. Ultrasonic energy may be expressed in different ways, such as cumulative dissipated energy, a combined estimator that associates the power of ultrasound longitudinal/torsional energy with time[14]. Several authors have described an association between cumulative dissipated energy and corneal endothelial cell loss linked to phacoemulsification[15],[16],[17].
In addition to cumulative dissipated energy, other variables related to the surgical procedure might influence endothelial cell loss, including the surgical technique, the type of cataract, the use of staining elements such as trypan blue, the age of the patient, the type of viscoelastic used to protect the endothelium, the position of the phaco tip[18], as well as prior cell density of the cornea[19],[20]. However, a variable not explored in the literature to date is previous experience of the surgeon. The objective of the present study is, therefore, to determine the association between the surgeon experience and endothelial cell loss in the context of variables associated with cataract surgery known to influence this outcome.
Methods
Clinical records of 198 cataract surgeries at the Ophthalmology Service of the Carlos van Buren Hospital, Valparaíso, between March and September 2015, were prospectively reviewed. The study protocol was approved by the Institutional Review Board of the Valparaíso-San Antonio Health Service (Act Nº27, 2017). Eligibility criteria were: 1) patients over 18 years; 2) Phacoheresis performed with monofocal intraocular lens in the capsular bag; 3) Endothelial cell count performed by specular microscopy prior to the procedure and at 90 days following the procedure; 4) Preoperative endothelial count over 1,500 cells per square millimeter. Exclusion criteria were: 1) Patients with any other type of ocular pathology; 2) Those with intraoperative lesions of the corneal endothelium; 3) Rupture of posterior lens capsule; 4) Small pupil that requires for some intervention to increase the diameter; 5) Pediatric or secondary cataract; 6) Surgeries performed by residents in ophthalmology; 7) Patients with incomplete data in the clinical record.
All patients underwent surgery with the same phacoemulsification system, phaco tip, balanced salt solution as irrigating solution and anesthetic eye drops of proparacaine (only in particular cases intracameral anesthesia was applied). The surgical technique was defined by the surgeon according to the individual characteristics of each patient. In case of a poor pupillary red reflex, intracameral trypan blue through air bubble was used in order to stain the anterior lens capsule in all patients. In included cases, intracameral antibiotic was not used as prophylaxis of postoperative endophthalmitis. None of the patients were pre-medicated during the days before the intervention and, after that, all received a tobramycin/dexamethasone regime for 14 days at least.
The following variables were evaluated with respect to the surgeon’s experience (greater or less than 5 years): type of cataract (according to Lens Opacities Classification System III, LOCS III), cumulative dissipated energy, type of viscoelastic, use of trypan blue, amount of fluidics, total time of ultrasound and combined phacoemulsification energy. The outcome variable was cell loss at 90 days (difference between pre- and post-surgical endothelial cell count). Additionally, the proportion of eyes that lost more than 500 cells per square millimeter was determined.
For the descriptive statistics, absolute and relative frequencies, proportions and averages with standard deviation are reported. For inferential analyses, Student's T was used for numerical variables and Fisher's exact test for categorical variables. A p-value lower than 0.05 was considered statistically significant. Data analyses were performed using Stata 15 software (StataCorp, Texas, USA).
Results
One hundred and ninety-eight eye records corresponding to 158 patients undergoing cataract operation were included. The average age was 74.1 ± 8.5 years. The characteristics of included patients are shown in Table 1.
Table 1. Characteristics of patients included in the study.
One hundred and twelve surgeries were performed by ophthalmologists with greater than five years of experience, while 86 were performed by surgeons with less than five years of experience (Table 2). No differences were observed in terms of age, gender or type of cataract among patients grouped by surgeon’s experience. There was no difference in global endothelial loss (p = 0.1945) between surgeons with more than five years of experience compared to those with less than five years of experience (446.17 ± 342 cells per square millimeter and 511.1 ± 354.8 cells per square millimeter respectively, p = 0.1945). One patient presented a count of 700 cells per square millimeter at 90 days.
Tabla 2. Variables evaluadas según experiencia del oftalmólogo.
The viscoelastic that was most commonly used by both groups of ophthalmologists was 3% sodium hyaluronate plus 4% chondroitin sulphate, followed by 1% sodium hyaluronate plus 3% hyaluronate and 4% chondroitin sulfate: there was no difference in global endothelial loss between the patients who received one type of viscoelastic or the other.
The cumulated dissipated energy estimated for ophthalmologists with more than five years of experience was 25.5 ± 17.3%-s, while the group with fewer experience reached an average of 21.3 ± 11.6%-s, a significant difference (p = 0.0268).
The use of fluidics by ophthalmologists with more than five years of professional practice was 75.36 mL ± 26.08, while those less than five years used on average 92 mL ± 47.64 (p = 0.002).
Use of trypan blue was significantly higher amongst ophthalmologists with less experience (p < 0.001). Regarding the endothelial cell loss usually associated to its use, the patients who received trypan blue had a cell loss on average 513 ± 352 cells per square millimeter, compared to 388 ± 325 cells per square millimeter in those who did not (p = 0.019).
Regarding the remaining variables, no differences among groups were found. There were not complications related to the use of trypan blue, neither to other procedures.
Discussion
In the present study, we found no impact of ophthalmologist experience on post-phacoeresis endothelial cell loss, nor on loss greater than 500 cells per square millimeter at 90 days. Although there have been no studies comparing post-operative results according to surgeon experience, it might be argued that surgeons with less experience may cause greater cell loss, but it has not been corroborated by this research. The present result might have been achieved as a consequence that less experienced ophthalmologists operated less complex cataracts, however, the LOCS III classification regarding the type of cataract was comparable amongst the procedures assigned to both groups.
Some procedural differences were detected among the experience-based groups: ophthalmologists with more than five years of experience used more 3% sodium hyaluronate plus 4% chondroitin sulphate viscoelastic than their less experienced colleagues (p = 0.006). A possible interpretation is that this type of viscoelastic has a lower cost and greater availability in public hospitals, so it might be elected by surgeons with greater experience. This viscoelastic presents properties that could reduce the efficiency of the operative procedure, since the cohesive and dispersive characteristics are favorable for the mixed type. Despite this, it becomes necessary to consider that this election may be conditioned by the availability at the moment of the surgery.
A relevant finding in our study was that ophthalmologists with more than five years’ experience apply greater cumulative dissipated energy compared to their less experienced colleagues, which could expose patients to an increased risk of corneal edema[15],[16]. In turn, the more experienced group used trypan blue in a very small proportion of patients (p < 0.001), probably due to a reduced need to identify the anterior capsule by staining. Greater use of cumulative dissipated energy and reduced use of trypan blue by experienced surgeons may have generate a compensatory phenomenon that would explain comparable final cell loss between the two groups, especially considering that both variables have been associated with post-surgical endothelial cell loss. In our study, the use of trypan blue was not related to complications, although several publications have associated its use to toxicity in animal[21] and cell culture models[22], as well as vitreous spots[23]. Indeed, amongst patients in whom trypan blue was applied there was a significantly greater loss of endothelial cells than in those in whom it was not used (p = 0.019).
Another important variable in the postoperative results is fluidics. High fluidity values during interventions produce turbulence in the anterior chamber, which can trigger damage to endothelial cells, producing post-operative inflammation and increasing the thickness of the central macula[24],[25],[26]. In the present analysis, ophthalmologists with less than five years’ experience used more fluidics, which could have been compensated by the lower use of cumulative dissipated energy, explaining the observed endothelial cell count. A similar situation was observed in a randomized clinical trial carried out by Sudeep Das and colleagues, where it was determined that in the group where lower cumulative dissipated energy had been applied, higher fluidics were used during surgery[27].
One of the limitations of the present study is that preferential use of some viscoelastics may have been determined by availability at the time of the procedure, so might not necessarily depend on ophthalmologists’ preference according to their experience. It becomes desirable that in the near future other methodological designs are conducted in order to analyze other variables, such as surgical technique, the use of drugs inside the chamber, and other clinically relevant outcomes, such as corneal edema, visual acuity and quality of life after the surgery.
Conclusion
In this research, which has an adequate sample size, we contribute to the limited knowledge about the clinical results according to the surgeon’s experience in a very frequent but scarcely assessed type of surgery from this point of view.
In the present investigation, years of experience of ophthalmologists were significantly associated with the application of greater cumulative dissipated energy, lower use of trypan blue and lower use of fluidics during phacoemulsification. Nevertheless, the years of experience showed no association with the post-operative corneal endothelial cell loss.
Notes
From the editor
The authors originally submitted this article in Spanish and subsequently translated it into English. The Journal has not copyedited this version.
Roles and contributions of authorship
FC: conceptualization, methodology, data validation, research, materials, writing (preparation of the original draft), writing (review and editing), review and presentation, supervision, project management.
CB: conceptualization, methodology, data validation, formal analysis, research, materials, writing (preparation of the original draft), writing (review and editing), review and presentation, supervision, project management.
CL: conceptualization, methodology, data validation, formal analysis, research, materials, writing (preparation of the original draft), writing (review and editing), review and presentation, supervision, project management.
AL: conceptualization, methodology, materials, writing (preparation of the original draft), writing (revision and editing), revision and presentation.
MA: conceptualization, methodology, data validation, formal analysis, research, writing (preparation of the original draft), writing (review and editing), review and presentation, supervision, project management.
JS: conceptualization, methodology, writing (preparation of the original draft), writing (revision and editing), revision and presentation.
EM: conceptualization, methodology, data validation, formal analysis, research, writing (preparation of the original draft), writing (review and editing), review and presentation, supervision, project management.
Funding
The authors state that there were no external sources of funding.
Competing interests
The authors have completed the ICMJE conflict of interest declaration form, and declare that they have not received funding for the completion of the report; have no financial relationships with organizations that might have an interest in the published article in the last three years; and have no other relationships or activities that could influence the published article. Forms can be requested by contacting the responsible author or the editorial board of the Journal.
Ethical aspects
The Journal is aware that this study was approved by the ethics review board of the Valparaíso-San Antonio Health Service and issued Resolution N° 8409 of July 10, 2017.
Editor's note
The principal or corresponding author claims that this manuscript is an honest, accurate, and transparent transcript of the reported study; that no important aspect of the study has been omitted; and that discrepancies between the study results and those planned (if relevant) have been recorded and explained.

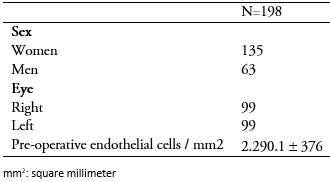 Table 1. Characteristics of patients included in the study.
Table 1. Characteristics of patients included in the study.

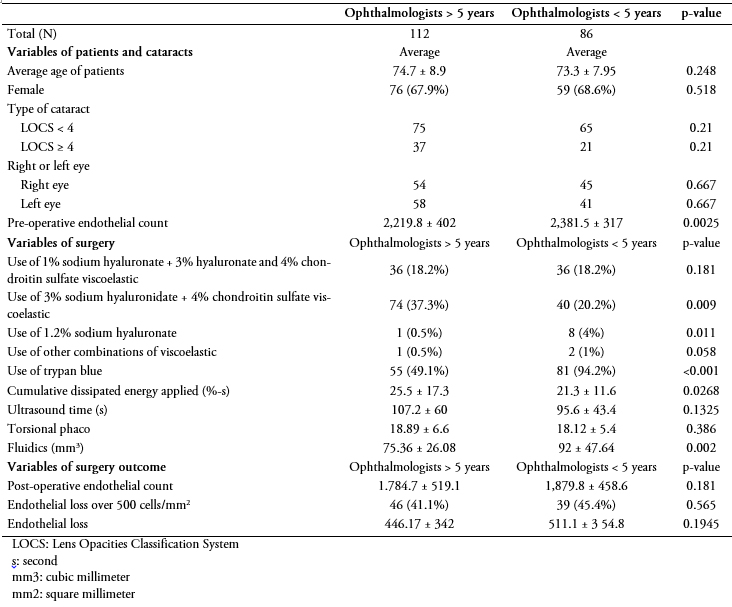 Tabla 2. Variables evaluadas según experiencia del oftalmólogo.
Tabla 2. Variables evaluadas según experiencia del oftalmólogo.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Introducción
La facoéresis es el procedimiento en que se extrae quirúrgicamente el cristalino para tratar las cataratas. La pérdida endotelial corneal es una complicación reconocida. Si bien se han descrito diversos factores asociados a este daño, la experiencia del cirujano ha sido poco explorada.
Objetivos
Evaluar la asociación entre la experiencia del cirujano y otras variables asociadas a la pérdida celular endotelial en el contexto de la facoéresis.
Métodos
Se analizaron prospectivamente los registros clínicos de 198 pacientes operados por cataratas, evaluando el efecto de la experiencia del cirujano y otras variables asociadas: energía disipada acumulada, tipo de viscoelástico empleado, uso de azul tripán, cantidad de fluídica, tiempo de ultrasonido, energía de facoemulsificación combinada y recuento celular endotelial pre y postoperatorio.
Resultados
No se observaron diferencias en el conteo postoperatorio de células endoteliales. Los oftalmólogos con más de cinco años de experiencia presentaron menor uso de azul tripán pero mayor cantidad de energía disipada acumulada en cada procedimiento, mientras que los oftalmólogos con menor experiencia utilizaron mayor cantidad de fluídica.
Conclusiones
Aunque hubo diferencias en el manejo de algunos factores influyentes sobre la pérdida endotelial cornal según la experiencia de los oftalmólogos, no se hallaron diferencias en relación a dicha pérdida como resultado final.
 Authors:
Fernando Chamorro[1], Cristóbal Briones[1], Cristóbal Loézar[2], Álex León[3], Marcelo Arancibia[2], Jana Stojanova[2], Eva Madrid[2]
Authors:
Fernando Chamorro[1], Cristóbal Briones[1], Cristóbal Loézar[2], Álex León[3], Marcelo Arancibia[2], Jana Stojanova[2], Eva Madrid[2]
Affiliation:
[1] Escuela de Medicina, Facultad de Medicina, Universidad de Valparaíso, Chile
[2] Centro Interdisciplinario de Estudios en Salud (CIESAL), Universidad de Valparaíso, Chile
[3] Servicio de Oftalmología, Hospital Carlos van Buren, Valparaíso, Chile
E-mail: marcelo.arancibiame@uv.cl
Author address:
[1] Facultad de Medicina
Universidad de Valparaíso
Angamos 655
Viña del Mar
Chile

Citation: Chamorro F, Briones C, Loézar C, León A, Arancibia M, Stojanova J, et al. Corneal endothelial cell loss associated to phacoemulsification and ophthalmologist experience: prospective analysis of individual secondary data. Medwave 2018;18(6) doi: 10.5867/medwave.2018.06.7314
Submission date: 6/7/2018
Acceptance date: 21/9/2018
Publication date: 29/10/2018
Origin: not requested
Type of review: reviewed by three external peer reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012 May;96(5):614-8. | CrossRef | PubMed |
- Wittenborn JS, Zhang X, Feagan CW, Crouse WL, Shrestha S, Kemper AR, Hoerger TJ, et al. The economic burden of vision loss and eye disorders among the United States population younger than 40 years. Ophthalmology. 2013 Sep;120(9):1728-35. | CrossRef | PubMed |
- Gobierno de Chile, Ministerio de Salud. Encuesta Nacional de Salud 2009-2010. Santiago, Chile:MINSAL; 2010.
- Sorrentino FS, Bonifazzi C, Parmeggiani F, Perri P. A Pilot Study to Propose a "Harm Scale", a New Method to Predict Risk of Harm to the Corneal Endothelium Caused by Longitudinal Phacoemulsification, and the Subsequent Effect of Endothelial Damage on Post Operative Visual Acuity. PLoS One. 2016 Jan 13;11(1):e0146580. | CrossRef | PubMed |
- Kelman CD. Phaco-Emulsification and Aspiration: A New Technique of Cataract emoval: A Preliminary Report. Am J Ophthalmol. 2018 Jul;191:xxx-xl. | CrossRef | PubMed |
- Bourne RR, Minassian DC, Dart JK, Rosen P, Kaushal S, Wingate N. Effect of cataract surgery on the corneal endothelium: modern phacoemulsification compared with extracapsular cataract surgery. Ophthalmology. 2004 Apr;111(4):679-85. | PubMed |
- Rosado-Adames N, Afshari NA. The changing fate of the corneal endothelium in cataract surgery. Curr Opin Ophthalmol. 2012 Jan;23(1):3-6. | CrossRef | PubMed |
- DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011 Mar;37(3):588-98. | CrossRef | PubMed |
- McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008 Jan;27(1):1-16. | CrossRef | PubMed |
- Bourne WM. Biology of the corneal endothelium in health and disease. Eye (Lond). 2003 Nov;17(8):912-8. | PubMed |
- Powe NR, Schein OD, Gieser SC, Tielsch JM, Luthra R, Javitt J, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Cataract Patient Outcome Research Team. Arch Ophthalmol. 1994 Feb;112(2):239-52. Erratum in: Arch Ophthalmol 1994 Jul;112(7):889. | PubMed |
- Cheng H, Law AB, McPherson K, Price NC. Longitudinal study of intraocular lens implants after intracapsular cataract extraction. Complete follow-up of the first 7 years. Trans Ophthalmol Soc U K. 1981;101(1):79-83. | PubMed |
- Bowling B. Kanski's Clinical Ophtalmology 8th Edition. Philadelphia, US. Saunders; 2015. | Link |
- Lee RY, Chen RI, Kasuga T, Cui QN, Porco TC, Lin SC. The Effect of Cumulative Dissipated Energy on Changes in Intraocular Pressure After Uncomplicated Cataract Surgery by Phacoemulsification. J Glaucoma. 2016 Jul;25(7):565-70. | CrossRef | PubMed |
- Pirazzoli G, D'Eliseo D, Ziosi M, Acciarri R. Effects of phacoemulsification time on the corneal endothelium using phacofracture and phaco chop techniques. J Cataract Refract Surg. 1996 Sep;22(7):967-9. | PubMed |
- Gonen T, Sever O, Horozoglu F, Yasar M, Keskinbora KH. Endothelial cell loss: Biaxial small-incision torsional phacoemulsification versus biaxial small-incision longitudinal phacoemulsification. J Cataract Refract Surg. 2012 Nov;38(11):1918-24. | CrossRef | PubMed |
- Sorrentino FS, Matteini S, Imburgia A, Bonifazzi C, Sebastiani A, Parmeggiani F. Torsional phacoemulsification: A pilot study to revise the "harm scale" evaluating the endothelial damage and the visual acuity after cataract surgery. PLoS One. 2017 Oct 26;12(10):e0186975. | CrossRef | PubMed |
- Faramarzi A, Javadi MA, Karimian F, Jafarinasab MR, Baradaran-Rafii A, Jafari F, Yaseri M. Corneal endothelial cell loss during phacoemulsification: bevel-up versus bevel-down phaco tip. J Cataract Refract Surg. 2011 Nov;37(11):1971-6. | CrossRef | PubMed |
- Cho YK, Chang HS, Kim MS. Risk factors for endothelial cell loss after phacoemulsification: comparison in different anterior chamber depth groups. Korean J Ophthalmol. 2010 Feb;24(1):10-5. | CrossRef | PubMed |
- Hayashi K, Hayashi H, Nakao F, Hayashi F. Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg. 1996 Oct;22(8):1079-84. | PubMed |
- Veckeneer M, van Overdam K, Monzer J, Kobuch K, van Marle W, Spekreijse H, et al. Ocular toxicity study of trypan blue injected into the vitreous cavity of rabbit eyes. Graefes Arch Clin Exp Ophthalmol. 2001 Sep;239(9):698-704. | PubMed |
- van Dooren BT, Beekhuis WH, Pels E. Biocompatibility of trypan blue with human corneal cells. Arch Ophthalmol. 2004 May;122(5):736-42. | PubMed |
- Pelit A. Unintentional staining of the posterior lens capsule with trypan blue dye during phacoemulsification: case report. Int Ophthalmol. 2012 Apr;32(2):187-9. | CrossRef | PubMed |
- Verges C, Cazal J, Lavin C. Surgical strategies in patients with cataract and glaucoma. Curr Opin Ophthalmol. 2005 Feb;16(1):44-52. | PubMed |
- Rossetti L, Chaudhuri J, Dickersin K. Medical prophylaxis and treatment of cystoid macular edema after cataract surgery. The results of a meta-analysis. Ophthalmology. 1998 Mar;105(3):397-405. | PubMed |
- Baradaran-Rafii A, Rahmati-Kamel M, Eslani M, Kiavash V, Karimian F. Effect of hydrodynamic parameters on corneal endothelial cell loss after phacoemulsification. J Cataract Refract Surg. 2009 Apr;35(4):732-7. | CrossRef | PubMed |
- Das S, Nanaiah SG, Kummelil MK, Nagappa S, Shetty R, Shetty BK. Effect of fluidics on corneal endothelial cell density, central corneal thickness, and central macular thickness after phacoemulsification with torsional ultrasound. Indian journal of ophthalmology. 2015;63(8):641-4. | CrossRef |
 Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012 May;96(5):614-8. | CrossRef | PubMed |
Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012 May;96(5):614-8. | CrossRef | PubMed | Wittenborn JS, Zhang X, Feagan CW, Crouse WL, Shrestha S, Kemper AR, Hoerger TJ, et al. The economic burden of vision loss and eye disorders among the United States population younger than 40 years. Ophthalmology. 2013 Sep;120(9):1728-35. | CrossRef | PubMed |
Wittenborn JS, Zhang X, Feagan CW, Crouse WL, Shrestha S, Kemper AR, Hoerger TJ, et al. The economic burden of vision loss and eye disorders among the United States population younger than 40 years. Ophthalmology. 2013 Sep;120(9):1728-35. | CrossRef | PubMed | Gobierno de Chile, Ministerio de Salud. Encuesta Nacional de Salud 2009-2010. Santiago, Chile:MINSAL; 2010.
Gobierno de Chile, Ministerio de Salud. Encuesta Nacional de Salud 2009-2010. Santiago, Chile:MINSAL; 2010.  Sorrentino FS, Bonifazzi C, Parmeggiani F, Perri P. A Pilot Study to Propose a "Harm Scale", a New Method to Predict Risk of Harm to the Corneal Endothelium Caused by Longitudinal Phacoemulsification, and the Subsequent Effect of Endothelial Damage on Post Operative Visual Acuity. PLoS One. 2016 Jan 13;11(1):e0146580. | CrossRef | PubMed |
Sorrentino FS, Bonifazzi C, Parmeggiani F, Perri P. A Pilot Study to Propose a "Harm Scale", a New Method to Predict Risk of Harm to the Corneal Endothelium Caused by Longitudinal Phacoemulsification, and the Subsequent Effect of Endothelial Damage on Post Operative Visual Acuity. PLoS One. 2016 Jan 13;11(1):e0146580. | CrossRef | PubMed | Kelman CD. Phaco-Emulsification and Aspiration: A New Technique of Cataract emoval: A Preliminary Report. Am J Ophthalmol. 2018 Jul;191:xxx-xl. | CrossRef | PubMed |
Kelman CD. Phaco-Emulsification and Aspiration: A New Technique of Cataract emoval: A Preliminary Report. Am J Ophthalmol. 2018 Jul;191:xxx-xl. | CrossRef | PubMed | Bourne RR, Minassian DC, Dart JK, Rosen P, Kaushal S, Wingate N. Effect of cataract surgery on the corneal endothelium: modern phacoemulsification compared with extracapsular cataract surgery. Ophthalmology. 2004 Apr;111(4):679-85. | PubMed |
Bourne RR, Minassian DC, Dart JK, Rosen P, Kaushal S, Wingate N. Effect of cataract surgery on the corneal endothelium: modern phacoemulsification compared with extracapsular cataract surgery. Ophthalmology. 2004 Apr;111(4):679-85. | PubMed | Rosado-Adames N, Afshari NA. The changing fate of the corneal endothelium in cataract surgery. Curr Opin Ophthalmol. 2012 Jan;23(1):3-6. | CrossRef | PubMed |
Rosado-Adames N, Afshari NA. The changing fate of the corneal endothelium in cataract surgery. Curr Opin Ophthalmol. 2012 Jan;23(1):3-6. | CrossRef | PubMed | DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011 Mar;37(3):588-98. | CrossRef | PubMed |
DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011 Mar;37(3):588-98. | CrossRef | PubMed | McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008 Jan;27(1):1-16. | CrossRef | PubMed |
McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008 Jan;27(1):1-16. | CrossRef | PubMed | Bourne WM. Biology of the corneal endothelium in health and disease. Eye (Lond). 2003 Nov;17(8):912-8. | PubMed |
Bourne WM. Biology of the corneal endothelium in health and disease. Eye (Lond). 2003 Nov;17(8):912-8. | PubMed | Powe NR, Schein OD, Gieser SC, Tielsch JM, Luthra R, Javitt J, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Cataract Patient Outcome Research Team. Arch Ophthalmol. 1994 Feb;112(2):239-52. Erratum in: Arch Ophthalmol 1994 Jul;112(7):889. | PubMed |
Powe NR, Schein OD, Gieser SC, Tielsch JM, Luthra R, Javitt J, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Cataract Patient Outcome Research Team. Arch Ophthalmol. 1994 Feb;112(2):239-52. Erratum in: Arch Ophthalmol 1994 Jul;112(7):889. | PubMed | Cheng H, Law AB, McPherson K, Price NC. Longitudinal study of intraocular lens implants after intracapsular cataract extraction. Complete follow-up of the first 7 years. Trans Ophthalmol Soc U K. 1981;101(1):79-83. | PubMed |
Cheng H, Law AB, McPherson K, Price NC. Longitudinal study of intraocular lens implants after intracapsular cataract extraction. Complete follow-up of the first 7 years. Trans Ophthalmol Soc U K. 1981;101(1):79-83. | PubMed | Lee RY, Chen RI, Kasuga T, Cui QN, Porco TC, Lin SC. The Effect of Cumulative Dissipated Energy on Changes in Intraocular Pressure After Uncomplicated Cataract Surgery by Phacoemulsification. J Glaucoma. 2016 Jul;25(7):565-70. | CrossRef | PubMed |
Lee RY, Chen RI, Kasuga T, Cui QN, Porco TC, Lin SC. The Effect of Cumulative Dissipated Energy on Changes in Intraocular Pressure After Uncomplicated Cataract Surgery by Phacoemulsification. J Glaucoma. 2016 Jul;25(7):565-70. | CrossRef | PubMed | Pirazzoli G, D'Eliseo D, Ziosi M, Acciarri R. Effects of phacoemulsification time on the corneal endothelium using phacofracture and phaco chop techniques. J Cataract Refract Surg. 1996 Sep;22(7):967-9. | PubMed |
Pirazzoli G, D'Eliseo D, Ziosi M, Acciarri R. Effects of phacoemulsification time on the corneal endothelium using phacofracture and phaco chop techniques. J Cataract Refract Surg. 1996 Sep;22(7):967-9. | PubMed | Gonen T, Sever O, Horozoglu F, Yasar M, Keskinbora KH. Endothelial cell loss: Biaxial small-incision torsional phacoemulsification versus biaxial small-incision longitudinal phacoemulsification. J Cataract Refract Surg. 2012 Nov;38(11):1918-24. | CrossRef | PubMed |
Gonen T, Sever O, Horozoglu F, Yasar M, Keskinbora KH. Endothelial cell loss: Biaxial small-incision torsional phacoemulsification versus biaxial small-incision longitudinal phacoemulsification. J Cataract Refract Surg. 2012 Nov;38(11):1918-24. | CrossRef | PubMed | Sorrentino FS, Matteini S, Imburgia A, Bonifazzi C, Sebastiani A, Parmeggiani F. Torsional phacoemulsification: A pilot study to revise the "harm scale" evaluating the endothelial damage and the visual acuity after cataract surgery. PLoS One. 2017 Oct 26;12(10):e0186975. | CrossRef | PubMed |
Sorrentino FS, Matteini S, Imburgia A, Bonifazzi C, Sebastiani A, Parmeggiani F. Torsional phacoemulsification: A pilot study to revise the "harm scale" evaluating the endothelial damage and the visual acuity after cataract surgery. PLoS One. 2017 Oct 26;12(10):e0186975. | CrossRef | PubMed | Faramarzi A, Javadi MA, Karimian F, Jafarinasab MR, Baradaran-Rafii A, Jafari F, Yaseri M. Corneal endothelial cell loss during phacoemulsification: bevel-up versus bevel-down phaco tip. J Cataract Refract Surg. 2011 Nov;37(11):1971-6. | CrossRef | PubMed |
Faramarzi A, Javadi MA, Karimian F, Jafarinasab MR, Baradaran-Rafii A, Jafari F, Yaseri M. Corneal endothelial cell loss during phacoemulsification: bevel-up versus bevel-down phaco tip. J Cataract Refract Surg. 2011 Nov;37(11):1971-6. | CrossRef | PubMed | Cho YK, Chang HS, Kim MS. Risk factors for endothelial cell loss after phacoemulsification: comparison in different anterior chamber depth groups. Korean J Ophthalmol. 2010 Feb;24(1):10-5. | CrossRef | PubMed |
Cho YK, Chang HS, Kim MS. Risk factors for endothelial cell loss after phacoemulsification: comparison in different anterior chamber depth groups. Korean J Ophthalmol. 2010 Feb;24(1):10-5. | CrossRef | PubMed | Hayashi K, Hayashi H, Nakao F, Hayashi F. Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg. 1996 Oct;22(8):1079-84. | PubMed |
Hayashi K, Hayashi H, Nakao F, Hayashi F. Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg. 1996 Oct;22(8):1079-84. | PubMed | Veckeneer M, van Overdam K, Monzer J, Kobuch K, van Marle W, Spekreijse H, et al. Ocular toxicity study of trypan blue injected into the vitreous cavity of rabbit eyes. Graefes Arch Clin Exp Ophthalmol. 2001 Sep;239(9):698-704. | PubMed |
Veckeneer M, van Overdam K, Monzer J, Kobuch K, van Marle W, Spekreijse H, et al. Ocular toxicity study of trypan blue injected into the vitreous cavity of rabbit eyes. Graefes Arch Clin Exp Ophthalmol. 2001 Sep;239(9):698-704. | PubMed | van Dooren BT, Beekhuis WH, Pels E. Biocompatibility of trypan blue with human corneal cells. Arch Ophthalmol. 2004 May;122(5):736-42. | PubMed |
van Dooren BT, Beekhuis WH, Pels E. Biocompatibility of trypan blue with human corneal cells. Arch Ophthalmol. 2004 May;122(5):736-42. | PubMed | Pelit A. Unintentional staining of the posterior lens capsule with trypan blue dye during phacoemulsification: case report. Int Ophthalmol. 2012 Apr;32(2):187-9. | CrossRef | PubMed |
Pelit A. Unintentional staining of the posterior lens capsule with trypan blue dye during phacoemulsification: case report. Int Ophthalmol. 2012 Apr;32(2):187-9. | CrossRef | PubMed | Verges C, Cazal J, Lavin C. Surgical strategies in patients with cataract and glaucoma. Curr Opin Ophthalmol. 2005 Feb;16(1):44-52. | PubMed |
Verges C, Cazal J, Lavin C. Surgical strategies in patients with cataract and glaucoma. Curr Opin Ophthalmol. 2005 Feb;16(1):44-52. | PubMed | Rossetti L, Chaudhuri J, Dickersin K. Medical prophylaxis and treatment of cystoid macular edema after cataract surgery. The results of a meta-analysis. Ophthalmology. 1998 Mar;105(3):397-405. | PubMed |
Rossetti L, Chaudhuri J, Dickersin K. Medical prophylaxis and treatment of cystoid macular edema after cataract surgery. The results of a meta-analysis. Ophthalmology. 1998 Mar;105(3):397-405. | PubMed | Baradaran-Rafii A, Rahmati-Kamel M, Eslani M, Kiavash V, Karimian F. Effect of hydrodynamic parameters on corneal endothelial cell loss after phacoemulsification. J Cataract Refract Surg. 2009 Apr;35(4):732-7. | CrossRef | PubMed |
Baradaran-Rafii A, Rahmati-Kamel M, Eslani M, Kiavash V, Karimian F. Effect of hydrodynamic parameters on corneal endothelial cell loss after phacoemulsification. J Cataract Refract Surg. 2009 Apr;35(4):732-7. | CrossRef | PubMed | Das S, Nanaiah SG, Kummelil MK, Nagappa S, Shetty R, Shetty BK. Effect of fluidics on corneal endothelial cell density, central corneal thickness, and central macular thickness after phacoemulsification with torsional ultrasound. Indian journal of ophthalmology. 2015;63(8):641-4. | CrossRef |
Das S, Nanaiah SG, Kummelil MK, Nagappa S, Shetty R, Shetty BK. Effect of fluidics on corneal endothelial cell density, central corneal thickness, and central macular thickness after phacoemulsification with torsional ultrasound. Indian journal of ophthalmology. 2015;63(8):641-4. | CrossRef |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








