Key Words: cost-effectiveness analysis, duloxetine, pregabalin, painful diabetic neuropathy, Venezuela.
Abstract
INTRODUCTION
Painful diabetic peripheral neuropathy affects 40-50% of patients with diabetic neuropathy, leading to impaired quality of life and substantial costs. Duloxetine and pregabalin have evidence-based support, and are formally approved for controlling painful diabetic peripheral neuropathy.
METHODS
We used a 12-week decision model for examining painful diabetic peripheral neuropathy first-line therapy with daily doses of duloxetine 60mg or pregabalin 300mg, under the perspective of the Instituto Venezolano de los Seguros Sociales. We gathered model parameters from published literature and expert´s opinion, focusing on the magnitude of pain relief, the presence of adverse events, the possibility of withdrawal owing to intolerable adverse events or due to lack of efficacy, and the quality-adjusted life years expected in each strategy. We analyzed direct medical costs (which are expressed in Bolívares Fuertes, BsF) comprising drug acquisition besides additional care devoted to treatment of adverse events and poor pain relief. We conducted both deterministic and probabilistic sensitivity analyses.
RESULTS
Total expected costs per 1000 patients were BsF 1 046 146 (26%) lower with duloxetine than with pregabalin. Most of these savings (91%) corresponds to the difference in the acquisition’s cost of each medication. duloxetine also provided 23 more patients achieving good pain relief and a gain of about two quality-adjusted life years per 1000 treated. Model was robust to plausible changes in main parameters. Duloxetine remained the preferred option in 93.9% of the second-order Monte Carlo simulations.
CONCLUSIONS
This study suggests duloxetine dominates (i.e., is more effective and lead to gains in quality-adjusted life years), remaining less costly than pregabalin for treatment of painful diabetic peripheral neuropathy.
Introduction
Painful diabetic peripheral neuropathy (PDPN) is a chronic neuropathic pain condition that affects patients with diabetes mellitus and its main symptoms are aching, burning, stabbing or tingling sensations which generally begin in the feet and are often worse at night [1],[2]. The reported prevalence of chronic pain with neuropathic characteristics is 10% to 20% in overall diabetic population, and ranges from 40% to 50% in those with diabetic neuropathy [3].
Painful diabetic peripheral neuropathy is a major public health problem as it is associated with sleep disturbances, anxiety, depression, and interference with daily activities; all of them cause lower physical and mental functioning as well as impaired quality of life [3],[4],[5]. In addition, painful diabetic peripheral neuropathy imposes a substantial economic burden in terms of both direct medical costs and indirect costs related with work productivity losses due to absenteeism and/or poor work performances (presenteeism) [6],[7],[8].
Pharmacological management for painful diabetic peripheral neuropathy includes treatment with tricyclic antidepressants, other types of antidepressants, anticonvulsants, and opioids and opioid-like drugs [9]. However, only duloxetine (a re-uptake inhibitor of both serotonin and norepinephrine) and pregabalin (an anticonvulsant) have evidence-based support for controlling painful diabetic peripheral neuropathy [10] and are formally approved for the management of this condition both in Europe [11] and United States of America (US) [12]. The Venezuelan Consensus on Neuropathic Pain recommends the use of these two agents [2].
Examining the pharmacoeconomic profile of newer drugs is necessary to make an optimal allocation of available resources and to maximize the clinical and economic benefits to society [13]. We aimed to evaluate the costs and outcomes of duloxetine and pregabalin when used as a first-line treatment of painful diabetic peripheral neuropathy from the perspective of the Instituto Venezolano de los Seguros Sociales (IVSS).
Methods
We performed both a cost-effectiveness and a cost-utility analysis. We defined the target population as those adults diagnosed with painful diabetic peripheral neuropathy whom first-line therapy with duloxetine or pregabalin was prescribed to for addressing moderate to severe pain.
We evaluated the following competing interventions: Duloxetine (DUL) 60 mg once daily and pregabalin (PGB) 150 mg twice daily (i.e., 300 mg per day); all given orally. These doses are generally recommended for the management of painful diabetic peripheral neuropathy [1],[2],[14],[15] and have also been used in some recently published studies [16],[17]. Moreover, these schemes were also validated by three specialists from the Instituto Venezolano de los Seguros Sociales.
As in other economic analysis of pharmacological treatments for painful diabetic peripheral neuropathy [16],[18], we considered a time horizon of 12 weeks, which is consistent with the duration of the blinded phase of the randomized placebo-controlled clinical trials of duloxetine in painful diabetic peripheral neuropathy [19],[20],[21] and reflects the usual time devoted to evaluate a first-line therapy for this condition [18]. Therefore, costs and benefits were not discounted at any rate since all of them occur within the 1-year time-frame [22].
Decision model
We used a decision tree with a very similar structure as that published by Carlos et al [16] (Figure 1). Briefly, the model consists of seven different pathways defined according to the magnitude of pain relief, the presence of adverse events, and the possibility of withdrawal owing to intolerable adverse events or due to lack of efficacy whether combined with tolerable adverse events or not. Poor pain relief and adverse events lead to additional costs and disutility (i.e., loss of utility). We assumed that all health states resulting from treatment are present for the entire horizon and that pain relief is related to a reduction in the symptoms, and not the duration of pain [16],[18].
Resource use and costs
We analyzed three different categories of direct medical costs:
- Cost acquisition of the medication considered in each competing intervention
- Additional costs derived from treating tolerable and intolerable adverse events
- Additional costs due to poor pain relief.
Since the analysis was conducted from the perspective of the Instituto Venezolano de los Seguros Sociales, we excluded indirect costs related to productivity losses as well as the intangible costs.
We calculated the cost of medication in each competing intervention as the product of three factors: the duration of therapy (expressed in days), the adherence rate, and the daily cost of that medication. We assumed that patients achieving good pain relief completed the 12-week treatment either if they experienced no adverse events or a tolerable adverse events. We also assumed that a proportion of patients with poor pain relief may remain in the assigned therapy for the whole period because of the perception of having achieved certain degree of pain relief. In contrast, other patients with poor pain relief may stop the assigned therapy prematurely. This drop-out can be attributable to lack of efficacy alone or to the coexistence of tolerable adverse events and lack of efficacy. By definition, all of the patients experiencing intolerable adverse events withdraw the treatment early. We set the mean duration of treatment for those patients stopping therapy owing to intolerable adverse events and due to lack of efficacy at 7 and 14 days, respectively. Patients with both lack of efficacy and tolerable adverse events also spend 14 days on therapy. These assumptions were all validated by three specialists from the Instituto Venezolano de los Seguros Sociales.
Figure 1. Structure of the model.
Treatment adherence depends on the daily frequency that each agent must be taken and estimates on adherence were used to calculate the expected costs of medication.
We derived the estimates of adherence from a systematic review performed by Saini et al [23]. According to an analysis of that source, the proportion of correct number of doses taken (i.e., the proportion of total correct openings) is higher for once daily dosing schemes (mean adherence rate of 93%) than for schemes that need two administrations (mean adherence rates of 87%) [16]. We obtained the average prices for medications to the Instituto Venezolano de los Seguros Sociales from personal communication of wholesalers.
We carried out an interview to three experts from the Instituto Venezolano de los Seguros Sociales in order to define the typical resource use given additionally to painful diabetic peripheral neuropathy patients experiencing tolerable and intolerable adverse events or failing to achieve good pain relief. For instance, in comparison to patients achieving good pain relief who remained free of adverse events, those with good pain relief but tolerable adverse events had one extra visit (20% with a general practitioner and 80% with a specialist). We estimated the unit cost for a visit to the general practitioner or to the specialist by dividing the average monthly income in each case [24] by the frequency of outpatient visits they give during a typical month. The unit cost per visit to the Emergency Department was calculated similarly.
We estimated the cost per day of hospitalization as the sum of monthly payroll staff and charges owing to acquisition of goods and services devoted to inpatient care divided by the number of days of active beds held by the Instituto Venezolano de los Seguros Sociales in a typical month. Table 1 shows the resource use, the unit cost per resource, and the additional expected cost per type of patient. All costs were calculated and are expressed in 2014 Bolívares Fuertes (BsF; current exchanges rates: 6.30 BsF per 1 US dollar and 7.89 BsF per 1 euro) [25]. The expected cost per resource is calculated as the product of three factors: the probability of use, the frequency if used, and the unit cost. In the end, all the expected costs per resource in each type of event are summed down for the grand total.
Table 1. Resource use and expect cost associated to adverse events and lack of efficacy.
Health outcomes
We selected the achievement of good pain relief (here and after good pain relief) as the effectiveness measure. Carlos et al [16] describe in detail the methods followed by them to define this outcome. Briefly, those authors calculated the pooled proportion of patients achieving good pain relief in all the placebo arms in 14 clinical trials (including three studies for duloxetine [19],[20],[21] and seven for pregabalin [26],[27],[28],[29],[30],[31],[32]) identified through systematic review. That figure represents the “baseline risk”. Then, they conducted an indirect treatment comparison for estimating the specific intervention risk ratio (RR) of achieving good pain relief compared to placebo, so the probability of achieving good pain relief in each competing intervention is the product of the baseline risk and the correspondent risk ratio. A similar approach was followed by them to estimate the probabilities of experiencing any adverse events and withdrawal owing to intolerable adverse events [16]. We used all of those published values in the present model. We gathered the probability of stopping any therapy because of poor pain relief from O´Connor et al [18].
We selected the quality-adjusted life years (QALY) as the main health outcome for the cost-utility analysis and used the same pain-state utility weights than Carlos et al [16] did. Therefore, patients with poor pain relief (the baseline pain state, which includes moderate and severe pain states) had a utility value of 0.38 while those achieving good pain relief (i.e., improving to mild pain) had a utility value of 0.64. Both utilities values are located in a scale from 0 to 1 where 0 is equivalent to be dead and 1 represents the perfect health state. The 0.38 value is a weighted average of the mean EuroQol 5 Dimensions (EQ-5D®) utility values reported in three studies [4],[33],[34] for moderate (0.48) and severe (0.22) health-pain states by using a 63%/37% ratio for the probabilities of being experiencing moderate/severe pain, which is based on the report of a cross-sectional survey of 255 patients with painful diabetic peripheral neuropathy performed in the US [4]. The 0.64 is the simple average of the utility values for mild pain reported in the aforementioned studies [4],[33],[34]. As in other papers [16],[17],[18], we used RR of 0.95 and 0.90 for indicating that tolerable and intolerable adverse events are associated with a relative loss in health utility of 5% and 10%, respectively.
Statistical analysis
We performed all the analyses in TreeAge software (TreeAge Pro Suite 2013, TreeAge Software, Inc., Williamstown, MA). Specifically, we calculated expected cost and effectiveness (utilities) for a hypothetical cohort of 2000 patients divided into two groups of 1000 patients each, assigned to any of the two competitive strategies. We supplemented the base-case estimates with both a deterministic and probabilistic sensitivity analysis. We summarized the results of the deterministic sensitivity analysis by using a tornado diagram for the net monetary benefit (NMB) of duloxetine, calculated as it follows: NMB = U * WTP – C; where U is number of expected QALY with duloxetine, WTP is the willingness to pay (i.e., the decision maker´s threshold) for each additional QALY, and C is the expected cost of duloxetine. We set the willingness to pay at BsF 106 615, which is the value of the gross domestic product (GDP) per capita projected for Venezuela in year 2014 [35]. As part of the probabilistic sensitivity analysis, we conducted a second-order Monte Carlo simulation with 1000 repetitions, which results are displayed graphically in terms of an incremental cost-utility scatter plot. Table 2 shows the model parameters. Pain-state utility values were multiplied by the disutility factor adjustment to create the utility of the combined health state (presence of adverse events along with the extent of pain).
Table 2. Model parameters values: base-case, ranges and distributions.
Results
Table 3 shows the summary of the economic evaluation. The acquisition of either duloxetine or pregabalin was the main cost driver, representing 44.9% and 56.6% of the total expected cost in each competing intervention. The additional care given to patients with poor pain relief was the second category in terms of costs, accounting for 43.0% in the duloxetine group and for 33.6% in the pregabalin group. Total expected costs per 1000 patients were BsF 1 046 146 (26%) lower with duloxetine than with pregabalin. Most of these savings (91%) corresponds to the difference in the acquisition cost of each medication, followed by the differences in the costs due to poor pain relief and managing adverse events, which accounted for 6% and 3% of the net savings in favor to duloxetine.
Duloxetine was not only less costly than pregabalin but it was also more effective, providing 23 additional patients with good pain relief (per 1000 treated). The slightly better efficacy and safety profiles of duloxetine versus pregabalin yielded a gain of about 2 QALY per each 1000 treated. Therefore, duloxetine dominated pregabalin.
Table 3. Outcomes from the base-case analysis (per 1000 patients).
Figure 2 displays the tornado diagram for the net monetary benefit of duloxetine under the cost-utility analysis. For simplicity, only the ten parameters that influenced the most on the net monetary benefit are shown. The length of each bar indicates the effect of the respective variable on the calculated net monetary benefit. The net monetary benefit is expressed in BsF and was calculated for a willingness to pay of BsF 106 615 which corresponds to the gross domestic product per capita projected for Venezuela in year 2014.
Figure 2. Tornado diagram for the net monetary benefit of duloxetine.
The incremental cost-utility scatter plot is depicted in figure 3, with each point representing a simulated economic evaluation of duloxetine versus pregabalin. We computed that 635 out of the 1000 simulations were located in the quadrant IV, indicating duloxetine was both less costly and associated with QALY gains when compared to pregabalin. According to a maximum willingness to pay equal to the gross domestic product per capita in Venezuela for each additional QALY, duloxetine was also the preferred option in other 304 simulations.
Figure 3. Incremental cost-utility scatter plot (DUL Vs. PGB).
Discussion
The current prevalence of diabetes mellitus in Venezuela is 6.61% [36]. Both the prevalence and the economic burden due to diabetes mellitus and its complications are projected to grow considerably during next decades imposing a need for a more rational use of available resources [35],[36]. In this study, we evaluated the expected costs and benefits of duloxetine and pregabalin for managing painful diabetic peripheral neuropathy from the Instituto Venezolano de los Seguros Sociales perspective and found that the former is associated with 26% lower total costs. This relative decrease is comparable to the proportional overall savings favoring duloxetine reported with similar models in Mexico (22%) [16] and US (20%) [18]. duloxetine exhibited net saving in all the three cost categories we analyzed: BsF 947 (41%) in drug´s acquisition, BsF 65 (5%) in additional care due to poor pain relief, and BsF 34 (8%) in treatment of emergent adverse event, per patient.
We estimated 23 additional patients achieving good pain relief plus a gain of 2 QALY per 1000 treated with duloxetine instead of with pregabalin. These small differences are consistent with published literature showing the efficacy and safety profiles of these two agents are quite comparable [9],[16],[17],[18],[38]. Hence, even adopting a more conservative cost-minimization approach duloxetine would be selected as the preferred option.
We found the model is robust to plausible changes in parameters. The acquisition of pharmacological agents was the main cost driver, while the probability of achieving good pain relief with duloxetine and the utility values assigned to good pain relief and poor pain relief were among the most influential parameters in the deterministic sensitivity analysis. The probability of duloxetine being dominant or at least cost-effective given a threshold value of 1 times the gross domestic product per capita in Venezuela was 93.9%.
Patients may prefer medications with once-daily regimens instead of having multiple takings per day since these regimens are more comfortable and lead to higher compliance compared with twice-daily or thrice-daily treatment regimens [23]. Zhao et al reported that painful diabetic peripheral neuropathy patients initiating duloxetine had significantly higher medication adherence and lower healthcare costs than those initiating pregabalin [39]. In another observational study, mean median daily dosage actually taken over 6 months with pregabalin was only 173.5 mg (which is far from the recommended 300 mg), whereas, the daily dosage for duloxetine was close to the 60 mg. This may explain the lower effectiveness shown by pregabalin in that study [40].
We believe good standards of quality were met when performing this study. However, there are some limitations that merit discussion. First, one can reasonably argue that a longer timeframe would better represent the chronic nature of painful diabetic peripheral neuropathy. Adopting a short timeframe avoids the need to make assumptions about titration schemes, the composition of the sequential treatments and sustainability of the effects [16]. Pharmacoeconomic evaluations that explored longer time frames and included sequential therapies also favored duloxetine over pregabalin [14],[17],[41]. Unit cost of services considered into the model rely on own calculations, so the specific amount of cost-savings might be taken with some caution. However, this not affects the dominant condition of duloxetine since more than 90% of the overall savings is attributable to the differences in the cost acquisition of the compared agents. The same applies for the resource use data derived from the panel of experts. We assigned utility values from published literature, which is common in Latin America because of the scarcity of data reflecting local preferences for health states [16]. Given the short time horizon and the small difference in expected QALY, the effect of this limitation on the results is negligible.
The present study suggests that duloxetine 60 mg per day dominates (i.e., is more effective and lead to QALY gains, remaining less costly than) pregabalin 300 mg daily for the treatment of individuals with painful diabetic peripheral neuropathy affiliated to Instituto Venezolano de los Seguros Sociales. This is in line with previous studies based on modeling and with the evidence derived from recent real-world observational studies.
Notes
From the editor
This article was originally submitted in Spanish and English by the authors. The Journal has not copyedited the English version.
Financing
This study was funded by an unrestricted grant from Eli Lilly y Compañía de México, S.A. de C.V.
Conflicts of interests
The authors have completed the ICMJE Conflicts of Interests form. Fernando Carlos reports grants from Eli Lilly y Compañía de México, S.A. de C.V., during the conduct of the study; he also reports grants from Eli Lilly y Compañía de México, S.A. de C.V. and grants from Pfizer, S.A. de C.V., outside the submitted work. Luis Espejel reports grants from R A C Salud Consultores, S.A. de C.V. outside the submitted work. Diego Novick reports other financial relationships with Eli Lilly and Company Limited, outside the submitted work. Rubén López is a Lilly employee. Daniel Flores reports other financial relationships with Eli Lilly Interamerica, outside the submitted work. No other conflicts of interests were reported from the authors. The forms are available upon request to the authors or the editors.

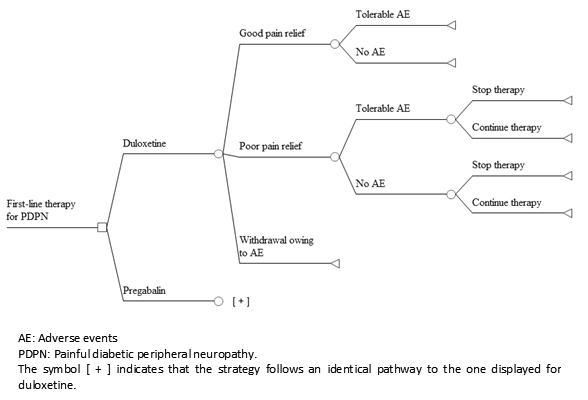 Figure 1. Structure of the model.
Figure 1. Structure of the model.

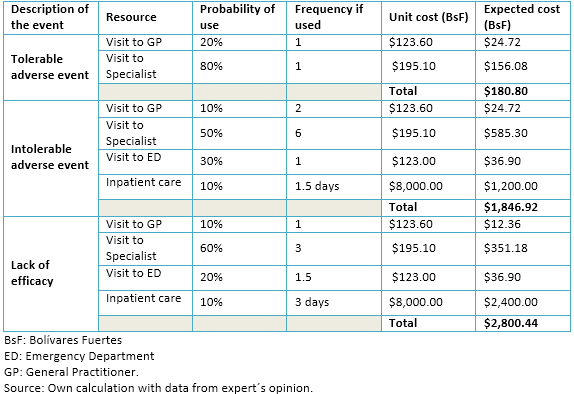 Table 1. Resource use and expect cost associated to adverse events and lack of efficacy.
Table 1. Resource use and expect cost associated to adverse events and lack of efficacy.

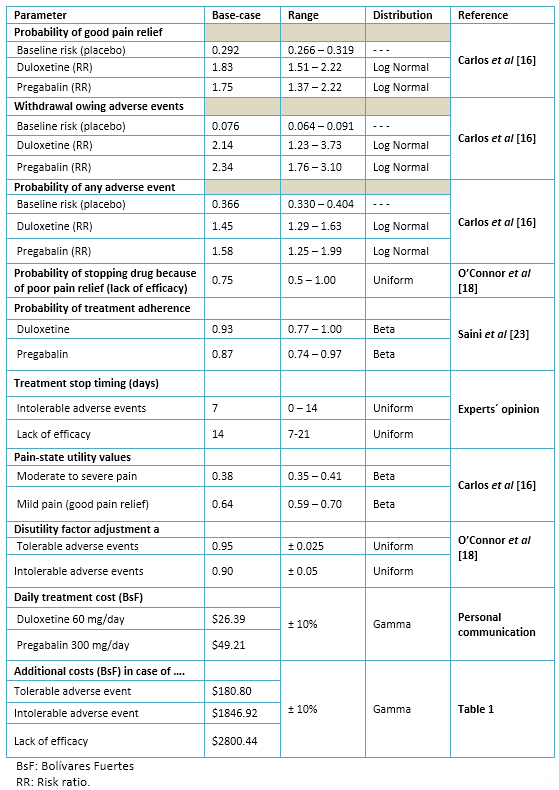 Table 2. Model parameters values: base-case, ranges and distributions.
Table 2. Model parameters values: base-case, ranges and distributions.

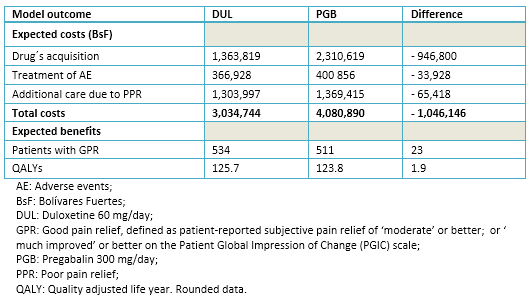 Table 3. Outcomes from the base-case analysis (per 1000 patients).
Table 3. Outcomes from the base-case analysis (per 1000 patients).

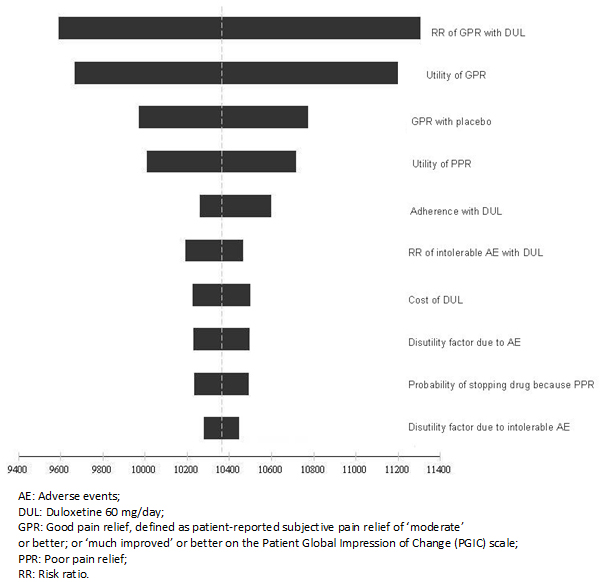 Figure 2. Tornado diagram for the net monetary benefit of duloxetine.
Figure 2. Tornado diagram for the net monetary benefit of duloxetine.

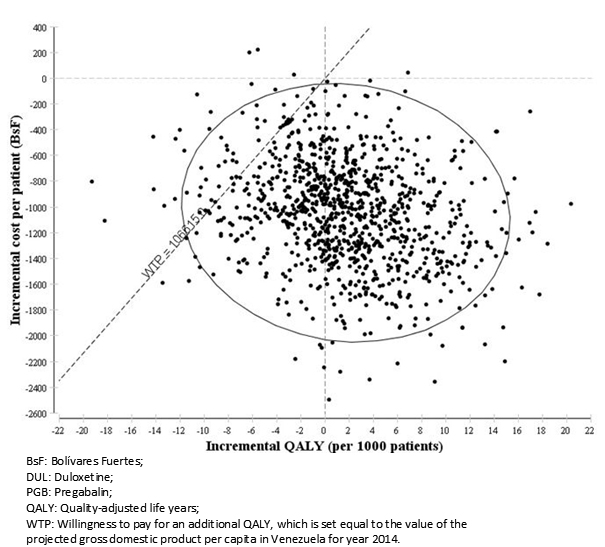 Figure 3. Incremental cost-utility scatter plot (DUL Vs. PGB).
Figure 3. Incremental cost-utility scatter plot (DUL Vs. PGB).
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
La neuropatía diabética periférica dolorosa (NDPD) afecta a 40-50% de los pacientes con neuropatía diabética y se asocia con un deterioro significativo de la calidad de vida y con costos de magnitud considerable. Tanto duloxetina (DUL) como pregabalina (PGB) cuentan con sustento científico basado en evidencias y han sido formalmente aprobados para controlar la NDPD.
MÉTODOS
Se utilizó un modelo de decisión a 12 semanas para examinar el tratamiento de primera línea para la neuropatía diabética periférica dolorosa, con dosis diarias de duloxetina 60 mg o con pregabalina 300 mg, bajo la perspectiva del Instituto Venezolano de los Seguros Sociales. Los parámetros del modelo proceden de literatura publicada y opinión de expertos, enfocándose en la magnitud del alivio del dolor, la presencia de eventos adversos, la posibilidad de abandono debido a eventos adversos intolerables o por falta de eficacia y en los años de vida ajustados por calidad esperados con cada estrategia. Se analizaron los costos médicos directos (expresados en bolívares fuertes), integrados por la adquisición de medicamentos, además del cuidado adicional que se origina por el tratamiento de los eventos adversos y como consecuencia de un pobre alivio del dolor. Se llevaron a cabo análisis de sensibilidad de tipo determinístico y probabilístico.
RESULTADOS
Los costos totales esperados por cada 1000 pacientes fueron de 1 046 146 bolívares fuertes (26%) más bajos con duloxetina en comparación con la pregabalina. La mayor parte de estos ahorros (91%), corresponde a la diferencia en el costo de adquisición entre ambos medicamentos. La duloxetina también se asoció con ganancias de 23 pacientes que lograron un buen alivio del dolor y de dos años de vida ajustados por calidad por cada 1000 tratados. El modelo se mantuvo robusto ante cambios plausibles en sus parámetros principales. La duloxetina continuó siendo la opción preferida en 93,9% de las simulaciones de Monte Carlo de segundo orden generadas.
CONCLUSIONES
El presente estudio sugiere que la duloxetina domina a (es más efectiva, conduce a ganancias en años de vida ajustados por calidad y es menos costosa que) la pregabalina, para el tratamiento de la neuropatía diabética periférica dolorosa.
 Authors:
Fernando Carlos[1], Luis Espejel[2], Diego Novick[3], Rubén López[4], Daniel Flores[5]
Authors:
Fernando Carlos[1], Luis Espejel[2], Diego Novick[3], Rubén López[4], Daniel Flores[5]
Affiliation:
[1] R A C Salud Consultores, S.A. de C.V.
[2] Consultor independiente
[3] Eli Lilly and Company, Windlesham, Surrey, UK
[4] Eli Lilly and Company, Caracas, Venezuela
[5] Eli Lilly and Company, Buenos Aires, Argentina
E-mail: fernando.carlos@racsalud.com
Author address:
[1] Insurgentes Sur 598 P2-204 Mza.
Col. Del Valle, Deleg. Benito Juárez
Ciudad de México, D.F.
México
C.P. 03100

Citation: Carlos F, Espejel L, Novick D, López R, Flores D. Duloxetine for the treatment of painful diabetic peripheral neuropathy in Venezuela: economic evaluation. Medwave 2015 Sep;15(8):e6265 doi: 10.5867/medwave.2015.08.6265
Submission date: 30/6/2015
Acceptance date: 20/8/2015
Publication date: 2/10/2015
Origin: not requested
Type of review: reviewed by two external peer reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Gobierno Federal, Estados Unidos Mexicanos. Diagnóstico y tratamiento médico del dolor por neuropatía diabética en adultos en el primer nivel de atención. México: Secretaría de Salud; 2009. | Link |
- Asociación Venezolana para el Estudio del Dolor. Consenso Venezolano sobre Dolor Neuropático, Caracas: AVED; 2007.
- Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008 Sep;9(6):660-74. | PubMed |
- Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005 Oct;30(4):374-85. | PubMed |
- Hoffman DL, Sadosky A, Alvir J. Cross-national burden of painful diabetic peripheral neuropathy in Asia, Latin America, and the Middle East. Pain Pract.2009 Jan-Feb;9(1):35-42. | CrossRef | PubMed |
- Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients' perspectives. J Pain. 2006 Dec;7(12):892-900. | PubMed |
- Ritzwoller DP, Ellis JL, Korner EJ, Hartsfield CL, Sadosky A. Comorbidities, healthcare service utilization and costs for patients identified with painful DPN in a managed-care setting. Curr Med Res Opin. 2009 Jun;25(6):1319-28. | CrossRef | PubMed |
- Mehra M, Merchant S, Gupta S, Potluri RC. Diabetic peripheral neuropathy: resource utilization and burden of illness. J Med Econ. 2014 Sep;17(9):637-45. | CrossRef | PubMed |
- Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Mehta S, Botteman M. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Pract. 2014 Feb;14(2):167-84. | CrossRef | PubMed |
- Iyer S, Tanenberg RJ. Pharmacologic management of diabetic peripheral neuropathic pain. Expert Opin Pharmacother. 2013 Sep;14(13):1765-75. | CrossRef | PubMed |
- European Medicines Agency. CHMP summary of positive opinion for Duloxetine Lilly. London ,UK: EMA; 2014 [on line]. | Link |
- Silver Spring MD. Highlights of prescribing information, Cymbalta. United States: FDA; 2014. | Link |
- Kobelt G. Health economics: an introduction to economic evaluation. 2nd ed. London, UK: Office for Health Economics; 2002.
- National Institute for Health and Clinical Excellence. Neuropathic pain: the pharmacological management of neuropathic pain in adults in nonspecialist settings of neuropathic pain in adults in non-specialist settings. London: NICE clinical guideline 96; 2010. | Link |
- Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011 May 17;76(20):1758-65. | CrossRef | PubMed |
- Carlos F, Ramírez-Gámez J, Dueñas H, Galindo-Suárez RM, Ramos E. Economic evaluation of duloxetine as a first-line treatment for painful diabetic peripheral neuropathy in Mexico. J Med Econ. 2012;15(2):233-44. | CrossRef | PubMed |
- Bellows BK, Dahal A, Jiao T, Biskupiak J. A cost-utility analysis of pregabalin versus duloxetine for the treatment of painful diabetic neuropathy. J Pain Palliat Care Pharmacother. 2012;26(2):153-64. | CrossRef |
- O'Connor AB, Noyes K, Holloway RG. A cost-utility comparison of four first-line medications in painful diabetic neuropathy. Pharmacoeconomics. 2008;26(12):1045-64. | CrossRef | PubMed |
- Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005 Jul;116(1-2):109-18. | PubMed |
- Raskin J, Pritchett YL, Wang F, D'Souza DN, Waninger AL, Iyengar S, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005 Sep-Oct;6(5):346-56. | PubMed |
- Wernicke JF, Pritchett YL, D'Souza DN, Waninger A, Tran P, Iyengar S, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006 Oct 24;67(8):1411-20. | PubMed |
- Drummond MF, Sculpher MJ, Torrance G, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford, UK: Oxford University Press; 2005.
- Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009 Jun 1;15(6):e22-33. | PubMed |
- República Bolivariana de Venezuela, Tribunal Supremo de Justicia. Gaceta Oficial Número 40.253. tsj.gov.ve [on line]. | Link |
- Banco Central de Venezuela. Tipos de Cambio de Referencia. bcv.org.ve [on line]. | Link |
- Arezzo JC, Rosenstock J, Lamoreaux L, Pauer L. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol. 2008 Sep 16;8:33. | CrossRef | PubMed |
- Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP Jr. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008 Feb;12(2):203-13. Epub 2007 Jul 16. | PubMed |
- Silver Spring MD. Center for Drug Evaluation and Research. Lyrica (pregabalin) capsules. Company: Pfizer Global Research & Development. Application No.: 021446. Approval date: 12/30/2004. United States: FDA; 2005. | Link |
- Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005 Jun;115(3):254-63. | PubMed |
- Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005 Apr;6(4):253-60. | PubMed |
- Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004 Dec 14;63(11):2104-10. | PubMed |
- Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004 Aug;110(3):628-38. | PubMed |
- Tölle T, Xu X, Sadosky AB. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J Diabetes Complications. 2006 Jan-Feb;20(1):26-33. | PubMed |
- Currie CJ, Poole CD, Woehl A, Morgan CL, Cawley S, Rousculp MD, et al. The health-related utility and health-related quality of life of hospital-treated subjects with type 1 or type 2 diabetes with particular reference to differing severity of peripheral neuropathy. Diabetologia. 2006 Oct;49(10):2272-80. | PubMed |
- International Monetary Fund. World Economic Outlook Database, October 2014 edition Washington, D.C: IMF; 2014[on line]. | Link |
- International Diabetes Federation. IDF Diabetes atlas, 6th ed. Brussels, Belgium: IDF; 2013. | PubMed |
- Giannini C, Mohn A, Chiarelli F. Technology and the issue of cost/benefit in diabetes. Diabetes Metab Res Rev. 2009 Sep;25 Suppl 1:S34-44. | CrossRef | PubMed |
- Quilici S, Chancellor J, Löthgren M, Simon D, Said G, Le TK, et al. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009 Feb 10;9:6. | CrossRef | PubMed |
- Zhao Y, Sun P, Watson P. Medication adherence and healthcare costs among patients with diabetic peripheral neuropathic pain initiating duloxetine versus pregabalin. Curr Med Res Opin. 2011 Apr;27(4):785-92. | CrossRef | PubMed |
- Happich M, Schneider E, Boess FG, Wilhelm S, Schacht A, Birklein F, et al. Effectiveness of duloxetine compared with pregabalin and gabapentin in diabetic peripheral neuropathic pain: results from a German observational study. Clin J Pain. 2014 Oct;30(10):875-85. | CrossRef | PubMed |
- Beard SM, McCrink L, Le TK, Garcia-Cebrian A, Monz B, Malik RA. Cost effectiveness of duloxetine in the treatment of diabetic peripheral neuropathic pain in the UK. Curr Med Res Opin. 2008 Feb;24(2):385-99. | PubMed |
 Gobierno Federal, Estados Unidos Mexicanos. Diagnóstico y tratamiento médico del dolor por neuropatía diabética en adultos en el primer nivel de atención. México: Secretaría de Salud; 2009. | Link |
Gobierno Federal, Estados Unidos Mexicanos. Diagnóstico y tratamiento médico del dolor por neuropatía diabética en adultos en el primer nivel de atención. México: Secretaría de Salud; 2009. | Link | Asociación Venezolana para el Estudio del Dolor. Consenso Venezolano sobre Dolor Neuropático, Caracas: AVED; 2007.
Asociación Venezolana para el Estudio del Dolor. Consenso Venezolano sobre Dolor Neuropático, Caracas: AVED; 2007.  Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008 Sep;9(6):660-74. | PubMed |
Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008 Sep;9(6):660-74. | PubMed | Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005 Oct;30(4):374-85. | PubMed |
Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005 Oct;30(4):374-85. | PubMed | Hoffman DL, Sadosky A, Alvir J. Cross-national burden of painful diabetic peripheral neuropathy in Asia, Latin America, and the Middle East. Pain Pract.2009 Jan-Feb;9(1):35-42. | CrossRef | PubMed |
Hoffman DL, Sadosky A, Alvir J. Cross-national burden of painful diabetic peripheral neuropathy in Asia, Latin America, and the Middle East. Pain Pract.2009 Jan-Feb;9(1):35-42. | CrossRef | PubMed | Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients' perspectives. J Pain. 2006 Dec;7(12):892-900. | PubMed |
Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients' perspectives. J Pain. 2006 Dec;7(12):892-900. | PubMed | Ritzwoller DP, Ellis JL, Korner EJ, Hartsfield CL, Sadosky A. Comorbidities, healthcare service utilization and costs for patients identified with painful DPN in a managed-care setting. Curr Med Res Opin. 2009 Jun;25(6):1319-28. | CrossRef | PubMed |
Ritzwoller DP, Ellis JL, Korner EJ, Hartsfield CL, Sadosky A. Comorbidities, healthcare service utilization and costs for patients identified with painful DPN in a managed-care setting. Curr Med Res Opin. 2009 Jun;25(6):1319-28. | CrossRef | PubMed | Mehra M, Merchant S, Gupta S, Potluri RC. Diabetic peripheral neuropathy: resource utilization and burden of illness. J Med Econ. 2014 Sep;17(9):637-45. | CrossRef | PubMed |
Mehra M, Merchant S, Gupta S, Potluri RC. Diabetic peripheral neuropathy: resource utilization and burden of illness. J Med Econ. 2014 Sep;17(9):637-45. | CrossRef | PubMed | Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Mehta S, Botteman M. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Pract. 2014 Feb;14(2):167-84. | CrossRef | PubMed |
Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Mehta S, Botteman M. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Pract. 2014 Feb;14(2):167-84. | CrossRef | PubMed | Iyer S, Tanenberg RJ. Pharmacologic management of diabetic peripheral neuropathic pain. Expert Opin Pharmacother. 2013 Sep;14(13):1765-75. | CrossRef | PubMed |
Iyer S, Tanenberg RJ. Pharmacologic management of diabetic peripheral neuropathic pain. Expert Opin Pharmacother. 2013 Sep;14(13):1765-75. | CrossRef | PubMed | European Medicines Agency. CHMP summary of positive opinion for Duloxetine Lilly. London ,UK: EMA; 2014 [on line]. | Link |
European Medicines Agency. CHMP summary of positive opinion for Duloxetine Lilly. London ,UK: EMA; 2014 [on line]. | Link | Silver Spring MD. Highlights of prescribing information, Cymbalta. United States: FDA; 2014. | Link |
Silver Spring MD. Highlights of prescribing information, Cymbalta. United States: FDA; 2014. | Link | Kobelt G. Health economics: an introduction to economic evaluation. 2nd ed. London, UK: Office for Health Economics; 2002.
Kobelt G. Health economics: an introduction to economic evaluation. 2nd ed. London, UK: Office for Health Economics; 2002.  National Institute for Health and Clinical Excellence. Neuropathic pain: the pharmacological management of neuropathic pain in adults in nonspecialist settings of neuropathic pain in adults in non-specialist settings. London: NICE clinical guideline 96; 2010. | Link |
National Institute for Health and Clinical Excellence. Neuropathic pain: the pharmacological management of neuropathic pain in adults in nonspecialist settings of neuropathic pain in adults in non-specialist settings. London: NICE clinical guideline 96; 2010. | Link | Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011 May 17;76(20):1758-65. | CrossRef | PubMed |
Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011 May 17;76(20):1758-65. | CrossRef | PubMed | Carlos F, Ramírez-Gámez J, Dueñas H, Galindo-Suárez RM, Ramos E. Economic evaluation of duloxetine as a first-line treatment for painful diabetic peripheral neuropathy in Mexico. J Med Econ. 2012;15(2):233-44.
| CrossRef | PubMed |
Carlos F, Ramírez-Gámez J, Dueñas H, Galindo-Suárez RM, Ramos E. Economic evaluation of duloxetine as a first-line treatment for painful diabetic peripheral neuropathy in Mexico. J Med Econ. 2012;15(2):233-44.
| CrossRef | PubMed | Bellows BK, Dahal A, Jiao T, Biskupiak J. A cost-utility analysis of pregabalin versus duloxetine for the treatment of painful diabetic neuropathy. J Pain Palliat Care Pharmacother. 2012;26(2):153-64. | CrossRef |
Bellows BK, Dahal A, Jiao T, Biskupiak J. A cost-utility analysis of pregabalin versus duloxetine for the treatment of painful diabetic neuropathy. J Pain Palliat Care Pharmacother. 2012;26(2):153-64. | CrossRef | O'Connor AB, Noyes K, Holloway RG. A cost-utility comparison of four first-line medications in painful diabetic neuropathy. Pharmacoeconomics. 2008;26(12):1045-64.
| CrossRef | PubMed |
O'Connor AB, Noyes K, Holloway RG. A cost-utility comparison of four first-line medications in painful diabetic neuropathy. Pharmacoeconomics. 2008;26(12):1045-64.
| CrossRef | PubMed | Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005 Jul;116(1-2):109-18. | PubMed |
Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005 Jul;116(1-2):109-18. | PubMed | Raskin J, Pritchett YL, Wang F, D'Souza DN, Waninger AL, Iyengar S, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005 Sep-Oct;6(5):346-56. | PubMed |
Raskin J, Pritchett YL, Wang F, D'Souza DN, Waninger AL, Iyengar S, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005 Sep-Oct;6(5):346-56. | PubMed | Wernicke JF, Pritchett YL, D'Souza DN, Waninger A, Tran P, Iyengar S, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006 Oct 24;67(8):1411-20. | PubMed |
Wernicke JF, Pritchett YL, D'Souza DN, Waninger A, Tran P, Iyengar S, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006 Oct 24;67(8):1411-20. | PubMed | Drummond MF, Sculpher MJ, Torrance G, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford, UK: Oxford University Press; 2005.
Drummond MF, Sculpher MJ, Torrance G, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford, UK: Oxford University Press; 2005.  Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009 Jun 1;15(6):e22-33. | PubMed |
Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009 Jun 1;15(6):e22-33. | PubMed | República Bolivariana de Venezuela, Tribunal Supremo de Justicia. Gaceta Oficial Número 40.253. tsj.gov.ve [on line]. | Link |
República Bolivariana de Venezuela, Tribunal Supremo de Justicia. Gaceta Oficial Número 40.253. tsj.gov.ve [on line]. | Link | Arezzo JC, Rosenstock J, Lamoreaux L, Pauer L. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol. 2008 Sep 16;8:33. | CrossRef | PubMed |
Arezzo JC, Rosenstock J, Lamoreaux L, Pauer L. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol. 2008 Sep 16;8:33. | CrossRef | PubMed | Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP Jr. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008 Feb;12(2):203-13. Epub 2007 Jul 16. | PubMed |
Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP Jr. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008 Feb;12(2):203-13. Epub 2007 Jul 16. | PubMed | Silver Spring MD. Center for Drug Evaluation and Research. Lyrica (pregabalin) capsules. Company: Pfizer Global Research & Development. Application No.: 021446. Approval date: 12/30/2004. United States: FDA; 2005. | Link |
Silver Spring MD. Center for Drug Evaluation and Research. Lyrica (pregabalin) capsules. Company: Pfizer Global Research & Development. Application No.: 021446. Approval date: 12/30/2004. United States: FDA; 2005. | Link | Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005 Jun;115(3):254-63. | PubMed |
Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005 Jun;115(3):254-63. | PubMed | Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005 Apr;6(4):253-60. | PubMed |
Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005 Apr;6(4):253-60. | PubMed | Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004 Dec 14;63(11):2104-10. | PubMed |
Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004 Dec 14;63(11):2104-10. | PubMed | Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004 Aug;110(3):628-38. | PubMed |
Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004 Aug;110(3):628-38. | PubMed | Tölle T, Xu X, Sadosky AB. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J Diabetes Complications. 2006 Jan-Feb;20(1):26-33. | PubMed |
Tölle T, Xu X, Sadosky AB. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J Diabetes Complications. 2006 Jan-Feb;20(1):26-33. | PubMed | Currie CJ, Poole CD, Woehl A, Morgan CL, Cawley S, Rousculp MD, et al. The health-related utility and health-related quality of life of hospital-treated subjects with type 1 or type 2 diabetes with particular reference to differing severity of peripheral neuropathy. Diabetologia. 2006 Oct;49(10):2272-80. | PubMed |
Currie CJ, Poole CD, Woehl A, Morgan CL, Cawley S, Rousculp MD, et al. The health-related utility and health-related quality of life of hospital-treated subjects with type 1 or type 2 diabetes with particular reference to differing severity of peripheral neuropathy. Diabetologia. 2006 Oct;49(10):2272-80. | PubMed | International Monetary Fund. World Economic Outlook Database, October 2014 edition Washington, D.C: IMF; 2014[on line]. | Link |
International Monetary Fund. World Economic Outlook Database, October 2014 edition Washington, D.C: IMF; 2014[on line]. | Link | International Diabetes Federation. IDF Diabetes atlas, 6th ed. Brussels, Belgium: IDF; 2013. | PubMed |
International Diabetes Federation. IDF Diabetes atlas, 6th ed. Brussels, Belgium: IDF; 2013. | PubMed | Giannini C, Mohn A, Chiarelli F. Technology and the issue of cost/benefit in diabetes. Diabetes Metab Res Rev. 2009 Sep;25 Suppl 1:S34-44. | CrossRef | PubMed |
Giannini C, Mohn A, Chiarelli F. Technology and the issue of cost/benefit in diabetes. Diabetes Metab Res Rev. 2009 Sep;25 Suppl 1:S34-44. | CrossRef | PubMed | Quilici S, Chancellor J, Löthgren M, Simon D, Said G, Le TK, et al. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the
treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009 Feb 10;9:6. | CrossRef | PubMed |
Quilici S, Chancellor J, Löthgren M, Simon D, Said G, Le TK, et al. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the
treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009 Feb 10;9:6. | CrossRef | PubMed | Zhao Y, Sun P, Watson P. Medication adherence and healthcare costs among patients with diabetic peripheral neuropathic pain initiating duloxetine versus pregabalin. Curr Med Res Opin. 2011 Apr;27(4):785-92. | CrossRef | PubMed |
Zhao Y, Sun P, Watson P. Medication adherence and healthcare costs among patients with diabetic peripheral neuropathic pain initiating duloxetine versus pregabalin. Curr Med Res Opin. 2011 Apr;27(4):785-92. | CrossRef | PubMed | Happich M, Schneider E, Boess FG, Wilhelm S, Schacht A, Birklein F, et al. Effectiveness of duloxetine compared with pregabalin and gabapentin in diabetic peripheral neuropathic pain: results from a German observational study. Clin J Pain. 2014 Oct;30(10):875-85. | CrossRef | PubMed |
Happich M, Schneider E, Boess FG, Wilhelm S, Schacht A, Birklein F, et al. Effectiveness of duloxetine compared with pregabalin and gabapentin in diabetic peripheral neuropathic pain: results from a German observational study. Clin J Pain. 2014 Oct;30(10):875-85. | CrossRef | PubMed | Beard SM, McCrink L, Le TK, Garcia-Cebrian A, Monz B, Malik RA. Cost effectiveness of duloxetine in the treatment of diabetic peripheral neuropathic pain in the UK. Curr Med Res Opin. 2008 Feb;24(2):385-99.
| PubMed |
Beard SM, McCrink L, Le TK, Garcia-Cebrian A, Monz B, Malik RA. Cost effectiveness of duloxetine in the treatment of diabetic peripheral neuropathic pain in the UK. Curr Med Res Opin. 2008 Feb;24(2):385-99.
| PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








