Key Words: COVID-19, SARS-CoV-2, Kawasaki disease, Child, COVID-19 related pediatric multisystem inflammatory disease
Abstract
Pediatric inflammatory multisystem syndrome temporally associated with COVID-19 is a potentially severe and rare condition that still needs a better understanding to guide its management. Reports worldwide, and especially in Latin America, are still scarce. This report presents ten cases of pediatric inflammatory multisystem syndrome temporally associated with COVID-19 in children between 2 and 12 years old treated in a Peruvian hospital, diagnosed using the Centers for Disease Control and Prevention criteria. Severe Acute Respiratory Syndrome Coronavirus 2 was detected through serological tests (immunoglobulin M or G). Most had gastrointestinal symptoms. Therapeutics consisted mainly of intravenous immunoglobulin, corticosteroids, ivermectin, hydroxychloroquine, digoxin, and antibiotic therapy. Three patients underwent mechanical ventilation; no mortality occurred in this case series. In conclusion, the manifestations presented here are similar to those reported in the literature. A timely diagnosis is necessary for proper management.
|
Main messages
|
Introduction
The COVID-19 pandemic has affected a great number of people throughout the world [1]. The presentations in children are usually mild. However, there have been severe COVID-19 cases reported in children with similar clinical characteristics to Kawasaki disease, a very infrequent childhood vasculitis that could cause aneurysms in coronary arteries [2],[3].
Pediatric inflammatory multisystem syndrome is described as a clinical presentation between four to six weeks after a SARS-CoV-2 infection, characterized by fever, gastrointestinal manifestations such as diarrhea, vomiting or abdominal pain, shock criteria, or Kawasaki disease. There have been reports of coronary alterations, hypercoagulation, and laboratory parameters at a severe inflammatory range, with and without an association with shock [4],[5]. Like Kawasaki disease, no pathognomonic findings or diagnostic testing exist for pediatric inflammatory multisystem syndrome.
The first reports suggest that the present syndrome is associated with a more frequent cardiovascular effect [6], which is why its management requires a multidisciplinary approach to avoid potentially fatal complications. Despite Peru being one of the countries with highest COVID-19 incidence rates, the pediatric inflammatory multisystem syndrome reports are rare [7]. For this reason, the objective of the present article is to describe the clinical and laboratory characteristics, and the progress of 10 pediatric patient cases admitted to the emergency room of a Peruvian national hospital.
Clinical cases
The present study was performed in the Pediatric Emergency Department of Hospital Nacional Edgardo Rebagliati Martins from Lima, Peru, between June 12 and August 23, 2020. For this work, we obtained approval from the ethics committee of the Health and Investigation Technology Evaluation Institute and written informed consent from a direct family member (father or mother).
Ten COVID-19 patients were included, confirmed through laboratory, diagnosed through quantitative reverse transcriptase with polymerase chain reaction (RT-PCR) and/or positive serology for SARS-CoV-2 infection, which also met the United States Centers for Disease Control and Prevention criteria for pediatric inflammatory multisystem syndrome diagnosis [4] (Table 1).
Table 1. CDC diagnostic criteria for inflammatory multisystem syndrome[4].
The detailed demographic and clinical characteristics are summarized in Table 2, while those of laboratory and supplementary exams are summarized in Table 3. The oxygen saturation was reported during admission or the first outcome. None of the patients died, and all patients were discharged to their homes. In all cases, drugs were administered intravenously, except for acetylsalicylic acid, hydroxychloroquine, digoxin, and ivermectin, which was given orally.
Clinical case 1
12-year-old grade school male without comorbidities, with a history of COVID-19 positive parents. He presented symptomatology characterized by fever, nausea, abdominal pain, and constipation. He received private medical attention with treatment involving symptomatic drugs, corticoids, and antibiotics. On his second day of illness, he went to a local hospital where he persisted febrile, with increased abdominal pain and diarrhea. The serologic test result for SARS-CoV-2 was immunoglobulin M negative and immunoglobulin G positive.
The abdominal computed tomography scan evidenced dilated colon loops with air-fluid levels, free fluid in the cavity, and inguinal lymphadenopathies. In the thorax, we observed ground glass opacity in the left lung base. He remained under observation with antibiotic treatment: meropenem (120 milligrams per kilogram per day) and vancomycin (60 milligrams per kilogram per day), with persistent symptomatology. On the fifth day of hospitalization, the patient presented dyspnea at rest, significant abdominal distension, bilious residue, and clinical signs of intestinal obstruction.
Exploratory laparotomy was performed. Free inflammatory fluid was found, fibrin between the bowel loops, and increased size of mesenteric lymph nodes. In the immediate post-operative period, the patient did not improve and was referred to our hospital. He was admitted in a poor general state, with respiratory distress using a reservoir mask and signs of shock.
In the analysis, there were elevated inflammatory markers and a pathological chest X-ray. He required advanced airway management, and we initiated conventional mechanical ventilation. We performed an echocardiogram which evidenced a dilated left ventricle and ejection fraction within normal limits. The patient was treated with inotropes. The diameter of coronary arteries was normal.
The patient met the criteria for pediatric inflammatory multisystem syndrome. He received treatment with one dose of human immunoglobulin (2 grams per kilogram), methylprednisolone (2 grams per kilogram per day), meropenem (120 milligrams per kilogram per day), vancomycin (60 milligrams per kilogram per day), and adrenaline (0.4 micrograms per kilogram per minute). The patient evolved favorably and was discharged after 11 days of hospitalization. There is no record of outpatient visits after discharge and no follow-up echocardiogram or thoracic computed tomography scan.
Clinical case 2
7-year-old grade school female, previously healthy, had contact with a COVID-19 positive person. She was admitted to the emergency room with four days of illness characterized by hypoxia, fever, odynophagia, vomiting, diarrhea, and respiratory distress. Upon admission, the vital signs registered were non-detectable blood pressure, heart rate 150 beats per minute, temperature 36 degrees Celsius, oxygen saturation at 80% (fraction of inspired oxygen at 21%). Upon physical exam: pale, distal coldness, hypoactive tendency, respiratory distress, and erythematous lesions in fingers and non-exudative conjunctival injection.
In the analysis, there was marked leukocytosis, low platelet count, and elevation of inflammatory markers. The serologic test result for SARS-CoV-2 was immunoglobulin M negative and immunoglobulin G positive. The patient presented cardiogenic shock, with moderate ventricular dysfunction and mild dilatation of coronary arteries, requiring treatment with multiple vasoactive drugs, epinephrine (0.4 micrograms per kilogram per minute), norepinephrine (0.6 micrograms per kilogram per minute), dopamine (8 micrograms per kilogram per minute), dobutamine (10 micrograms per kilogram per minute), vasopressin (0.003 international units per kilogram per hour), and levosimendan (0.1 micrograms per kilogram per minute). No computed tomography scans were performed during hospitalization.
The patient met the criteria for pediatric inflammatory multisystem syndrome. She required advanced airway management with conventional mechanical ventilation. She received two doses of human immunoglobulin (2 grams per kilogram), acetylsalicylic acid (50 milligrams per kilogram per day), dexamethasone (0.15 milligrams per kilogram per day), antibiotics (meropenem 120 milligrams per kilogram per day), and vancomycin (60 milligrams per kilogram per day), enoxaparin (1 milligram per kilogram per day) and digoxin (5 micrograms per kilogram per day).
The patient had a prolonged hospitalization with finger necrosis in her right hand and both feet related to distal peripheral arterial insufficiency. There is no record of outpatient visits after discharge and no follow-up echocardiogram or thoracic computed tomography scan.
Clinical case 3
A 7-year-old female patient without a history of prior disease reports having had a contact with a COVID-19 positive person. Two days before admission to the emergency room, she presented a fever (38.5 to 39 degrees Celsius) associated with intermittent abdominal pain. The following day, she presented odynophagia, nasal congestion, and lesions in her palms, for which she went to the emergency room. Laboratory tests revealed elevated C-reactive protein and positive serology for SARS-CoV-2. Chest X-ray was normal. The patient met the criteria for pediatric inflammatory multisystem syndrome.
The necessary treatment included a dose of human immunoglobulin (2 grams per kilogram), acetylsalicylic acid (50 milligrams per kilogram followed by 3 milligrams per kilogram per day), and ceftriaxone (100 milligrams per kilogram per day). No echocardiogram or computed tomography scan was performed during hospitalization. The patient evolved favorably and was discharged on the sixth day of hospitalization. There is no record of outpatient visits after discharge and no follow-up echocardiogram or thoracic computed tomography scan.
Clinical case 4
An 11-year-old male patient, without comorbidities, with a history of close home contact. The patient presented illness for five days, characterized by high fever associated with odynophagia, persistent abdominal pain, increasing daily, and non-pruritic skin rash and conjunctival injection. Exams were performed upon clinical suspicion of pediatric inflammatory multisystem syndrome, highlighting a blood count with low platelet count, C-reactive protein at 17.3 milligrams per deciliter, and ferritin 872 nanograms per milliliter, in addition to COVID-19 positive serology test.
Upon diagnostic confirmation, treatment was started with one dose of human immunoglobulin (2 grams per kilogram), methylprednisolone (2 milligrams per kilogram per day), and broad-spectrum antibiotics: meropenem (120 milligrams per kilogram per day) and vancomycin (60 milligrams per kilogram per day), later tapered down to ceftriaxone (100 milligrams per kilogram per day). An echocardiogram was performed on the first day of hospitalization, and the results were normal. The patient’s clinical and laboratory course was favorable, and he was discharged on the seventh day of hospitalization. There is no record of outpatient visits after discharge and no follow-up echocardiogram or thoracic computed tomography scan.
Clinical case 5
A 7-year-old female patient without comorbidities who did not refer contact with a COVID-19 positive person. She began illness with fever, headache, and abdominal pain for three days, contacting our hospital emergency room for the first time. The ultrasound diagnosis was mesenteric adenitis. She was discharged with analgesics. The following day, due to persisting symptoms in addition to diarrhea, she was re-admitted to the emergency room. In the laboratory exams, we found elevated C-reactive protein, D-dimer, and ferritin. Serology for COVID-19 was positive. Bilateral parenchymal infiltrates were observed in chest X-ray, mainly right side. No echocardiogram or thoracic computed tomography scan was performed during hospitalization.
The patient met the criteria for pediatric inflammatory multisystem syndrome. We decided to begin treatment with one human immunoglobulin dose (2 grams per kilogram) and acetylsalicylic acid (5 milligrams per kilogram per day). On the second day, she presented with respiratory decline and was admitted to the intensive care unit. She received oxygen support, additional treatment with ceftriaxone (100 milligrams per kilogram per day), metronidazole (30 milligrams per kilogram per day), hydroxychloroquine (7.4 milligrams per kilogram per day), ivermectin (0.3 milligrams per kilogram), and dexamethasone (1 milligram per kilogram per day). The patient had good clinical progress, with progressive weaning off oxygen. She completed treatment and was discharged on the 10th day of hospitalization. There is no record of outpatient visits after discharge and no follow-up echocardiogram or thoracic computed tomography scan.
Clinical case 6
An 8-year-old male patient, previously healthy, with a history of COVID-19 positive father. He began illness with a high fever (temperature 40 Celsius degrees) associated with abdominal pain that persisted for three days. He received home treatment with symptomatic. He was admitted to a local hospital diagnosed with acute abdomen with surgical indication and was then referred to our hospital two days later. During the emergency room evaluation, the patient was in a poor general condition, with respiratory distress, signs of hypoperfusion, disturbance of consciousness, conjunctival injection, and edema in both hands, and a painful, mildly depressible abdomen. In the analysis, we observed lymphopenia and elevated inflammatory markers, and myocardial dysfunction markers. The serologic test result for SARS-CoV-2 was immunoglobulin M negative and immunoglobulin G positive.
The chest and abdomen computed tomography shows a ground-glass pattern associated with bilateral basal parenchymal consolidation with bilateral laminar pleural effusion. In addition, there was evidence of loss of haustrations and edematous wall of ascending colon and in a lesser degree of the transverse and descending colon, associated with free fluid in the pelvic cavity and inflammatory alteration of mesenteric fat.
The patient met the criteria for pediatric inflammatory multisystem syndrome. He required advanced airway management with conventional mechanical ventilation. Eco vision was performed with poor left ventricle contractility, diminished ejection fraction, and coronaries without aneurysms. He required treatment with human immunoglobulin (2 grams per kilogram), antibiotic coverage with ceftriaxone (100 milligrams per kilogram per day). Later he was changed to vancomycin (60 milligrams per kilogram per day) plus meropenem (120 milligrams per kilogram per day), methylprednisolone (2 milligrams per kilogram per day), and acetylsalicylic acid (3 milligrams per kilogram per day). He evolved favorably and was extubated with posterior tapering of oxygen requirement. He completed medical treatment and was discharged to his home on the 10th day. There is no record of outpatient visits after discharge and no follow-up echocardiogram or thoracic computed tomography scan.
Clinical case 7
A 6-year-old male patient, without comorbidities, with a history of COVID-19 positive mother. He was admitted to the emergency room of our hospital with symptoms of three days that began with fever and odynophagia. One day before, he presented with progressively intense, diffuse abdominal pain and diarrhea. During hospitalization, we requested abdominal ultrasound, which revealed mesenteric enlarged lymph nodes and thickening of the ileum. We also performed a computed tomography scan that showed thickening of walls of intestinal loops in all four quadrants, without collections, free fluid in the pelvis, and scarce bilateral basal pleural effusion and bibasilar atelectasis in the thorax. He received an evaluation by surgery, who ruled out any abdominal surgical pathology.
Studies were performed which revealed leukocytosis without lymphocytosis and elevation of inflammatory and cardiac markers. SARS-CoV-2 serologic test was reactive. The patient met the criteria for pediatric inflammatory multisystem syndrome. Treatment was initiated with one dose of human immunoglobulin (2 grams per kilogram). In addition, he evolved with signs of hypoperfusion which required adrenaline (0.05 micrograms per kilogram per minute). An ultrasound was performed with conserved cardiac function and artery diameters. The patient had a favorable clinical course with progressive tapering of oxygen. He completed antibiotic treatment with ceftriaxone (100 milligrams per kilogram per day) plus metronidazole (30 milligrams per kilogram per day) and was discharged on the ninth day of hospitalization. There is no record of outpatient visits after discharge and no follow-up echocardiogram or thoracic computed tomography scan.
Clinical case 8
A 3-year-old male patient, with a diagnosis of stage 5 chronic kidney disease in peritoneal dialysis, and a history of hemolytic uremic syndrome with renal failure in 2017. He reported a COVID-19 contact, his mother. He was hospitalized for COVID-19 from May 7 to 12 in 2020, with SARS-CoV-2 serologic test on May 7, 2020 with positive immunoglobulin M and G. He had pneumonia from COVID-19 requiring supplemental oxygen for 48 hours.
He was admitted to the emergency room with an illness of four days that began with hypoactivity and hypoxia. Two days prior, he was febrile and had a three-minute seizure. He was treated in a private clinic and was later discharged to his home. In addition, on the last day, he presented loose stools in regular quantity without blood or mucous three times per day. At the examination in the emergency room, the patient was febrile, with erythema and lip fissure, conjunctival injection, and mild bi-palpebral edema. No echocardiogram or thoracic computed tomography scan was performed during hospitalization.
The analysis revealed lymphopenia, hypoalbuminemia, elevated creatinine due to chronic renal failure, elevated inflammatory markers, and myocardial dysfunction. SARS-CoV-2 serologic test results were immunoglobulin M negative and immunoglobulin G positive. The echocardiogram revealed aneurismatic dilatation of the left and right coronary trunk. The patient met the criteria for pediatric inflammatory multisystem syndrome. We initiated treatment with one dose of human immunoglobulin (2 grams per kilogram), acetylsalicylic acid (30 milligrams per kilogram per day), albumin infusion (0.5 grams per kilogram), furosemide (2 milligrams per kilogram per day), and antibiotics (ceftriaxone 100 milligrams per kilogram per day and vancomycin 60 milligrams per kilogram per day). The patient evolved favorably, with a drop in the febrile curve. He completed medical treatment and was discharged after six days of hospitalization. There is no record of outpatient visits after discharge and no follow-up echocardiogram or thoracic computed tomography scan.
Clinical case 9
A 2-year-old male patient, previously healthy, with a history of mother being COVID-19 positive. He started with a fever (38 degrees Celsius) and with general malaise. He went to the hospital, and due to his epidemiological history, they performed a SARS-CoV-2 serologic test, which resulted positive. He was given ambulatory treatment and indicated home isolation, and was monitored by phone. He persisted febrile for five days, plus generalized skin rash, conjunctival injection, and red lips, the reason why he went to the emergency room of our hospital, where we suspected pediatric inflammatory multisystem syndrome.
Within the supplementary exams performed, we found an elevated C-reactive protein, D-dimer, creatine kinase myocardial band, ferritin, fibrinogen, and positive serology for SARS-CoV-2. No echocardiogram or computed tomography scan was performed during hospitalization. He received treatment of one dose of human immunoglobulin (2 grams per kilogram), acetylsalicylic acid (30 milligrams per kilogram per day), and methylprednisolone (2 milligrams per kilogram per day). He did not receive antibiotics. The patient evolved favorably with a drop in the febrile curve and was discharged. The patient was seen in outpatient pediatric cardiology, the follow-up echocardiogram was normal. There were no follow-up computed tomography scans indicated.
Clinical case 10
A 4-year-old male patient, previously healthy, without a history of COVID-19 contact. He was admitted to our emergency department referred from a province hospital with suspicion of pediatric inflammatory multisystem syndrome, with 10 days of illness characterized initially by fever and pharyngeal pain. In the last two days, he had abdominal pain, edema of hands, conjunctival injection, skin rash in extremities and lab tests with lymphopenia, low platelet count, and elevated C-reactive protein. He had a serologic test taken at a local hospital five days before admission, which was reactive to immunoglobulin M and G for SARS-CoV-2.
He was admitted in a poor general state, edematous, pale, with a prolonged capillary refill, distal coldness, tachycardic heartbeats, mild tachypnea, mild abdominal distension, very painful diffusely at superficial palpation. He required fluid resuscitation, inotrope support with adrenaline (0.047 micrograms per kilogram per minute), one dose of human immunoglobulin (2 grams per kilogram), albumin (0.5 grams per kilogram), methylprednisolone (2 milligrams per kilogram per day), and ceftriaxone (100 milligrams per kilogram per day). No echocardiogram or computed tomography scan was performed during hospitalization. The patient evolved favorably and was discharged. There is no record of outpatient visits after discharge and no follow-up echocardiogram or thoracic computed tomography scan.
Discussion
Initially, when the pandemic due to SARS-CoV-2 infection was announced, pediatricians worldwide were moderately calm that most children were asymptomatic or developed a mild disease. However, after April 2020, reports of cases of children and adolescents who presented signs that we now know as part of pediatric inflammatory multisystem syndrome, there is a greater concern in the pediatric population, deriving many strategies and efforts from health organizations for early detection and treatment [2].
Even today, after almost half a year in which research and constant reports have not ceased, we cannot be completely sure about the clinical presentation of pediatric inflammatory multisystem syndrome, still being quite variable and unpredictable in many cases [2],[6],[8],[9],[10],[11],[12]. This is why there is a need to characterize the presentation of the said syndrome in our population.
The international literature reports that cases of this disease have presented from two to four weeks after its highest incidence of COVID-19 cases, within which we would consider a post-infectious stage [12].
Regarding the age of presentation, our cases included patients between 2 and 12 years old, where nine of the ten cases were over five years old. Similar results have been observed in international studies as one of the main differential characteristics with the classic Kawasaki disease [10],[11],[12]. However, in a series of reported cases in the United States of younger than 21 years old, the age group that predominated was between 1 and 4 years old [6].
Concerning comorbidities of the included patients as probable risk factors, most patients (nine out of ten) were previously healthy, in contrast to the cohort of 35 pediatric patients reported by Belhadier et al., where the main comorbidities described were asthma and obesity [4].
The study by Pouletty [9] et al. identified 16 patients with pediatric inflammatory multisystem syndrome, of which five had documented recent contact with a SARS-CoV-2 positive individual [3]. In comparison with our report, nine out of ten patients had identified having contact with a COVID-19 individual, mainly caretakers (at home contagium).
In the literature, it is noted that approximately 50 a 60% of patients had a positive serology with a negative reverse transcriptase-polymerase chain reaction, and approximately 25 to 30% are positive to both diagnostic tests. A minority of patients (10 to 15%) had negative results to both tests. In these cases, the diagnosis of pediatric inflammatory multisystem syndrome depends on the epidemiologic data [6],[11],[12],[13]. In the present series of cases, we observe that 100% of patients had positive SARS-CoV-2 serology tests, with a resulting immunoglobulin G positive result, coinciding with the given data in other series of cases in which we observe the presence of the syndrome during a late stage of infection [11],[12].
The “phenotypes” described in other reports have a febrile presentation from the second week of COVID-19 infection, up to severe presentations with hemodynamic compromise and gastrointestinal symptoms (vomiting, abdominal pain, diarrhea), which may or may not have components of Kawasaki disease, hyperinflammation, and hypercoagulability, generally posterior to an acute infection [14].
In accordance with other reports, the most frequently observed clinical presentation were gastrointestinal symptoms, of which abdominal pain was the most frequent and in second place, diarrhea [10],[12]. Abdominal pain was described in three cases leading to an initial diagnosis of acute abdomen. We even reported a patient who, in the hospital of origin, was in the operating room, with necessary imaging studies, such as ultrasound or computed tomography scan as a diagnostic aid, with common findings of mesenteric adenitis, edema of the intestinal wall and free fluid in the peritoneal cavity.
The dermatological alterations were also considered an important part of the present syndrome, given that its presence (among other clinical signs) reminds us of the similarity with Kawasaki disease [10],[12]. 50% of our patients presented variable levels of mucocutaneous involvement. Pulmonary involvement was present in four patients, two of which needed invasive respiratory support, and the other two were managed with non-invasive oxygen support, with subsequent tapering. There were no clinical presentations of neurological or renal involvement in the patients included.
Likewise, we observed that our laboratory findings coincide with a hyperinflammatory syndrome with an elevation of the parameters described in the diagnostic criteria, similar to other reports, whose objective was to evaluate the function and impact of the disease in the different parenchyma and determine which treatment to offer [2],[6],[8],[9],[10],[11],[12]. In the blood count, lymphopenia (7/10) and thrombocytopenia (4/10) were common findings. Very elevated C-reactive protein was the most frequent inflammatory marker. We found that over half of the patients had elevated ferritin. With respect to coagulopathy markers, D-dimer elevation was present in 100% of cases. In studies performed in adults, the D-dimer elevation was a criterion for severe COVID-19 [15].
Of cardiac markers, the creatine kinase myocardial band was elevated in 80% of cases. We had difficulty with the availability of brain natriuretic pro-peptide dose; however, we could obtain it in five patients, in whom it was markedly increased. In addition, in three of these patients, pathological findings of pediatric inflammatory multisystem syndrome were found by ultrasound.
The echocardiogram is important to keep in mind at the initial evaluation of all patients with suspicion of pediatric inflammatory multisystem syndrome, in which we have findings such as those described in various studies [10],[12],[16]. In our reported patients, two evolved towards cardiogenic shock, with evidence of alterations per echocardiogram: left ventricle dysfunction (three patients) and dilatation of coronary arteries were reported in only two patients. In three patients who did not develop laboratory alterations of cardiac function nor signs of shock and who evolved favorably, we proposed an outpatient echocardiogram after discharge.
With respect to treatment, it will depend in part on the clinical presentation, if it has to do with distributive shock versus cardiac dysfunction versus similar characteristics to Kawasaki disease. These presentations may superimpose, and it may be appropriate to offer interventions from more than one category [14]. Furthermore, some interventions, such as empiric antibiotics, intravenous immunoglobulin, and prophylactic antithrombotic therapy, are appropriate for most patients with moderate to severe manifestations, independently of the predominant presentation type [14]. In the present case report, ten patients received intravenous immunoglobulin, and seven also received corticosteroids. Only one patient received prophylactic anticoagulation.
Our report has the same tendency observed in reports from other countries [8],[16], with a recovery period of 10 to 15 days, with discharge directly to the home. This offers us the notion that we are facing pathology with a good response to treatment in most cases, when provided with the necessary early vital support, in accordance with the complications that may arise. Only one patient had a prolonged hospital stay that exceeded 30 days due to the complication derived from a high dose of inotropes to safeguard the myocardial alterations, which was difficult to manage.
Conclusion
The patient characteristics of our case series have many similarities with prior international reports regarding pediatric inflammatory multisystem syndrome.
This syndrome is a new entity in the current context of the COVID-19 pandemic, which requires a timely diagnosis to prevent complications during its course.
Notas
Authorship contributions
CL: conceptualization, methodology, investigation, resources, drafting (revision and editing), supervision and project administration. GR, MS, AS: conceptualization, formal analysis, investigation, data and drafting management (preparing the original draft). LEC: methodology, investigation, drafting (revision and editing), supervision and project administration. DMQ: conceptualization, methodology, formal analysis, investigation, data and drafting management (revision and editing).
Ethics
Approval to publish was obtained from the ethics committee of the Instituto de Evaluación de Tecnologías Sanitarias y de Investigación (IETSI), certified by letter dated September 19, 2020. Likewise, written informed consent was obtained from the direct family member (father or mother) of all ten cases.
Acknowledgments
We thank Lucía Aguirre Estela for her support in the style review of the English revision.
Competing interests
The authors declare no competing interests.
Funding
No funding declared.
From the editors
This article was originally submitted in Spanish, the language in which it was peer-reviewed. This translation was provided by the authors and has been lightly edited by the Journal.

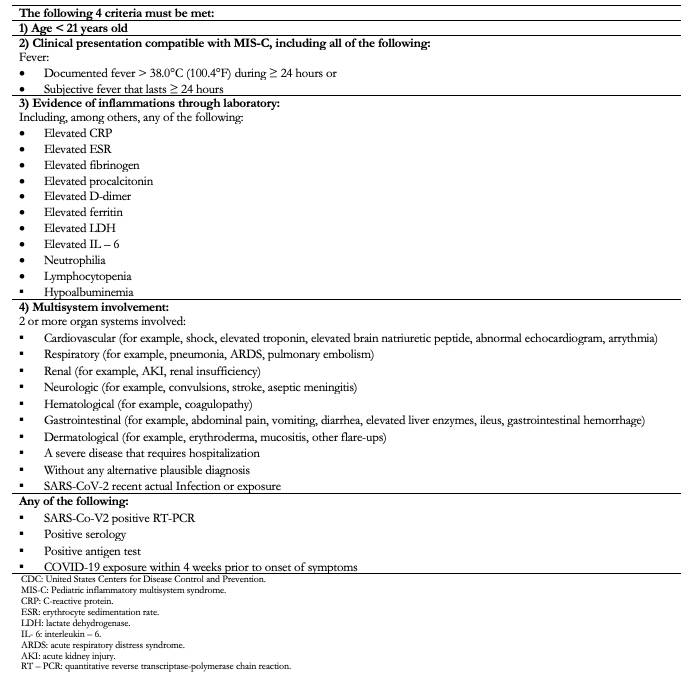 Table 1. CDC diagnostic criteria for inflammatory multisystem syndrome[4].
Table 1. CDC diagnostic criteria for inflammatory multisystem syndrome[4].

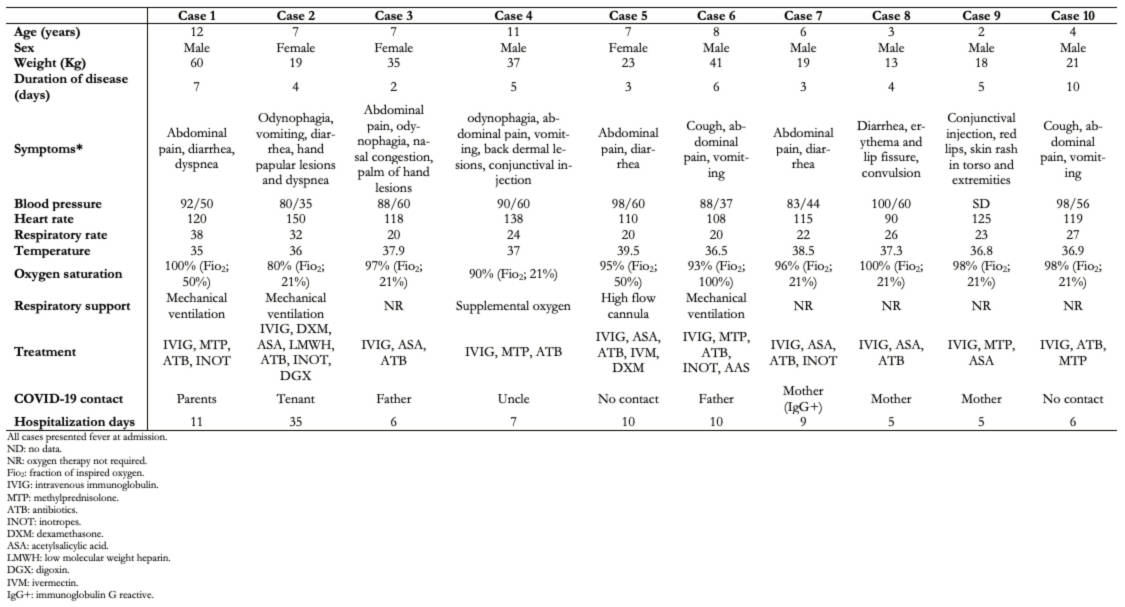 Table 2. . Demographic and clinical characteristics of patients with inflammatory multisystem syndrome admitted to the emergency room.
Table 2. . Demographic and clinical characteristics of patients with inflammatory multisystem syndrome admitted to the emergency room.

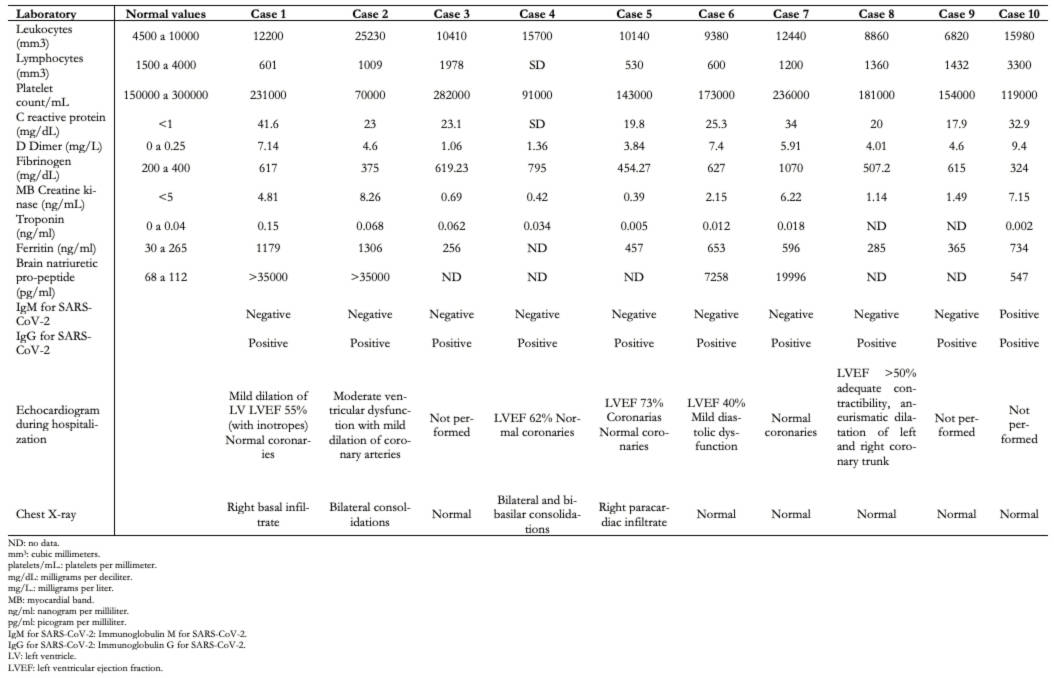 Table 3. Laboratory and imaging analysis of patients with inflammatory multisystem syndrome admitted to the emergency room.
Table 3. Laboratory and imaging analysis of patients with inflammatory multisystem syndrome admitted to the emergency room.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Pediatric inflammatory multisystem syndrome temporally associated with COVID-19 is a potentially severe and rare condition that still needs a better understanding to guide its management. Reports worldwide, and especially in Latin America, are still scarce. This report presents ten cases of pediatric inflammatory multisystem syndrome temporally associated with COVID-19 in children between 2 and 12 years old treated in a Peruvian hospital, diagnosed using the Centers for Disease Control and Prevention criteria. Severe Acute Respiratory Syndrome Coronavirus 2 was detected through serological tests (immunoglobulin M or G). Most had gastrointestinal symptoms. Therapeutics consisted mainly of intravenous immunoglobulin, corticosteroids, ivermectin, hydroxychloroquine, digoxin, and antibiotic therapy. Three patients underwent mechanical ventilation; no mortality occurred in this case series. In conclusion, the manifestations presented here are similar to those reported in the literature. A timely diagnosis is necessary for proper management.
 Authors:
Consuelo Luna-Muñoz[1,2], Giuliana Reyes-Florian[1], Martin Seminario-Aliaga[1], Angie Stapleton-Herbozo[1], Lucy E. Correa-López[2], Dante M. Quiñones-Laveriano[2]
Authors:
Consuelo Luna-Muñoz[1,2], Giuliana Reyes-Florian[1], Martin Seminario-Aliaga[1], Angie Stapleton-Herbozo[1], Lucy E. Correa-López[2], Dante M. Quiñones-Laveriano[2]
Affiliation:
[1] Servicio de Emergencia Pediátrica, Hospital Nacional Edgardo Rebagliati Martins, Lima, Perú
[2] Instituto de Investigación en Ciencias Biomédicas, Universidad Ricardo Palma, Lima, Perú
E-mail: lunaconsuelo21@gmail.com
Author address:
[1] Av. Edgardo Rebagliati #490
Jesús María 15072
Lima, Perú

Citation: Luna-Muñoz C, Reyes-Florian G, Seminario-Aliaga M, Stapleton-Herbozo A, Correa-López LE, Quiñones-Laveriano DM. Pediatric inflammatory multisystem syndrome associated with COVID-19: A report of 10 cases in a Peruvian hospital. Medwave 2021;21(02):e8142 doi: 10.5867/medwave.2021.02.8142
Publication date: 30/3/2021
Origin: Not commissioned.
Type of review: Externally peer-reviewed by three reviewers, double-blind.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Miranda-Novales MG, Vargas-Almanza I, Aragón-Nogales R. COVID-19 por SARS-CoV-2: la nueva emergencia de salud. Rev Mex Pediatr. 2020;86(6):213-8. | CrossRef |
- Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020 May 23;395(10237):1607-1608. | CrossRef | PubMed |
- Paediatric Critical Care Society. PICS Statement regarding novel presentation of multi-system inflammatory disease - Paediatric Critical Care Society. [citado 12 de septiembre de 2020]. [Internet] | Link |
- Centers for Disease Control and Prevention. Multisystem Inflammatory Syndrome in Children (MIS-C). Centers for Disease Control and Prevention. 2020 [citado 25 de enero de 2021]. [Internet] | Link |
- Yagnam F, Drago M, Izquierdo G, Piñera C, Leiva I, Rojas J, et al. Síndrome Inflamatorio Multisistémico Pediátrico asociado a COVID-19. Reporte preliminar de 6 casos en una Unidad de Paciente Crítico. Sociedad Chilena de Pediatría. 2020 [citado 25 de enero de 2021]. [Internet] | Link |
- Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020 Jul 23;383(4):334-346. | CrossRef | PubMed |
- Coll-Vela LE, Zamudio-Aquise MK, Nuñez-Paucar H, Bernal-Mancilla RR, Schult- Montoya SC, Ccorahua-De La Paz M, et al. Síndrome inflamatorio multisistémico asociado a COVID-19 en niños: serie de casos en un hospital pediátrico de Perú [COVID-19-associated multisystem inflammatory syndrome in children: case series at a pediatric hospital in Peru]. Rev Peru Med Exp Salud Publica. 2020 Dec 2;37(3):559-565. | CrossRef | PubMed |
- Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med. 2020 Jul 23;383(4):347-358. | CrossRef | PubMed |
- Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS- CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020 Aug;79(8):999-1006. | CrossRef | PubMed |
- Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation. 2020 Aug 4;142(5):429-436. | CrossRef | PubMed |
- Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020 Jun 6;395(10239):1771-1778. | CrossRef | PubMed |
- Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020 Jul 21;324(3):259-269. | CrossRef | PubMed |
- Organizacion Mundial de la Salud. Protocolo síndrome inflamatorio multisistémico en niños, niñas y adolescentes con SARS-CoV-2. 2020 [citado 25 de enero de 2021]. [Internet] | Link |
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. | CrossRef | PubMed |
- Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020 Jun 1;10(1):69. | CrossRef | PubMed |
- Kaushik S, Aydin SI, Derespina KR, Bansal PB, Kowalsky S, Trachtman R, et al. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection (MIS-C): A Multi-institutional Study from New York City. J Pediatr. 2020 Sep;224:24-29. | CrossRef | PubMed |
 Miranda-Novales MG, Vargas-Almanza I, Aragón-Nogales R. COVID-19 por SARS-CoV-2: la nueva emergencia de salud. Rev Mex Pediatr. 2020;86(6):213-8. | CrossRef |
Miranda-Novales MG, Vargas-Almanza I, Aragón-Nogales R. COVID-19 por SARS-CoV-2: la nueva emergencia de salud. Rev Mex Pediatr. 2020;86(6):213-8. | CrossRef | Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020 May 23;395(10237):1607-1608. | CrossRef | PubMed |
Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020 May 23;395(10237):1607-1608. | CrossRef | PubMed | Paediatric Critical Care Society. PICS Statement regarding novel presentation of multi-system inflammatory disease - Paediatric Critical Care Society. [citado 12 de septiembre de 2020]. [Internet] | Link |
Paediatric Critical Care Society. PICS Statement regarding novel presentation of multi-system inflammatory disease - Paediatric Critical Care Society. [citado 12 de septiembre de 2020]. [Internet] | Link | Centers for Disease Control and Prevention. Multisystem Inflammatory Syndrome in Children (MIS-C). Centers for Disease Control and Prevention. 2020 [citado 25 de enero de 2021]. [Internet] | Link |
Centers for Disease Control and Prevention. Multisystem Inflammatory Syndrome in Children (MIS-C). Centers for Disease Control and Prevention. 2020 [citado 25 de enero de 2021]. [Internet] | Link | Yagnam F, Drago M, Izquierdo G, Piñera C, Leiva I, Rojas J, et al. Síndrome Inflamatorio Multisistémico Pediátrico asociado a COVID-19. Reporte preliminar de 6 casos en una Unidad de Paciente Crítico. Sociedad Chilena de Pediatría. 2020 [citado 25 de enero de 2021]. [Internet] | Link |
Yagnam F, Drago M, Izquierdo G, Piñera C, Leiva I, Rojas J, et al. Síndrome Inflamatorio Multisistémico Pediátrico asociado a COVID-19. Reporte preliminar de 6 casos en una Unidad de Paciente Crítico. Sociedad Chilena de Pediatría. 2020 [citado 25 de enero de 2021]. [Internet] | Link | Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020 Jul 23;383(4):334-346. | CrossRef | PubMed |
Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020 Jul 23;383(4):334-346. | CrossRef | PubMed | Coll-Vela LE, Zamudio-Aquise MK, Nuñez-Paucar H, Bernal-Mancilla RR, Schult- Montoya SC, Ccorahua-De La Paz M, et al. Síndrome inflamatorio multisistémico asociado a COVID-19 en niños: serie de casos en un hospital pediátrico de Perú [COVID-19-associated multisystem inflammatory syndrome in children: case series at a pediatric hospital in Peru]. Rev Peru Med Exp Salud Publica. 2020 Dec 2;37(3):559-565. | CrossRef | PubMed |
Coll-Vela LE, Zamudio-Aquise MK, Nuñez-Paucar H, Bernal-Mancilla RR, Schult- Montoya SC, Ccorahua-De La Paz M, et al. Síndrome inflamatorio multisistémico asociado a COVID-19 en niños: serie de casos en un hospital pediátrico de Perú [COVID-19-associated multisystem inflammatory syndrome in children: case series at a pediatric hospital in Peru]. Rev Peru Med Exp Salud Publica. 2020 Dec 2;37(3):559-565. | CrossRef | PubMed | Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med. 2020 Jul 23;383(4):347-358. | CrossRef | PubMed |
Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med. 2020 Jul 23;383(4):347-358. | CrossRef | PubMed | Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS- CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020 Aug;79(8):999-1006. | CrossRef | PubMed |
Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS- CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020 Aug;79(8):999-1006. | CrossRef | PubMed | Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation. 2020 Aug 4;142(5):429-436. | CrossRef | PubMed |
Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation. 2020 Aug 4;142(5):429-436. | CrossRef | PubMed | Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020 Jun 6;395(10239):1771-1778. | CrossRef | PubMed |
Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020 Jun 6;395(10239):1771-1778. | CrossRef | PubMed | Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020 Jul 21;324(3):259-269. | CrossRef | PubMed |
Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020 Jul 21;324(3):259-269. | CrossRef | PubMed | Organizacion Mundial de la Salud. Protocolo síndrome inflamatorio multisistémico en niños, niñas y adolescentes con SARS-CoV-2. 2020 [citado 25 de enero de 2021]. [Internet] | Link |
Organizacion Mundial de la Salud. Protocolo síndrome inflamatorio multisistémico en niños, niñas y adolescentes con SARS-CoV-2. 2020 [citado 25 de enero de 2021]. [Internet] | Link | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. | CrossRef | PubMed |
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. | CrossRef | PubMed | Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020 Jun 1;10(1):69. | CrossRef | PubMed |
Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020 Jun 1;10(1):69. | CrossRef | PubMed | Kaushik S, Aydin SI, Derespina KR, Bansal PB, Kowalsky S, Trachtman R, et al. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection (MIS-C): A Multi-institutional Study from New York City. J Pediatr. 2020 Sep;224:24-29. | CrossRef | PubMed |
Kaushik S, Aydin SI, Derespina KR, Bansal PB, Kowalsky S, Trachtman R, et al. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection (MIS-C): A Multi-institutional Study from New York City. J Pediatr. 2020 Sep;224:24-29. | CrossRef | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








