Key Words: altitude, acute mountain sickness, acetazolamide, Epistemonikos, GRADE.
Abstract
INTRODUCTION
Acute mountain sickness is the most prevalent illness related to acute exposure to high altitude, secondary to the hypobaric hypoxia effects in our body. Acetazolamide has been traditionally used for its prevention and treatment, however, there is still controversy regarding the degree of usefulness of this medication as monotherapy.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified a systematic review that included two primary studies, both corresponding to randomized trials. We conclude that it is not possible to establish clearly whether treatment with acetazolamide reduces the symptoms of acute mountain disease or increases the risk of adverse effects, because the certainty of the existing evidence has been evaluated as very low.
Problem
When the organism is exposed to acute high-altitude hypobaric hypoxia, it develops a series of adaptive responses. However, if these responses are insufficient or abnormal, a spectrum of high-altitude illnesses that mainly include acute mountain sickness, high-altitude pulmonary edema and high-altitude cerebral edema, can occur. Of the three conditions previously indicated, acute mountain sickness is the most frequent, with its main symptoms being headache, fatigue, dizziness, nausea, vomiting, sleep disturbances and anorexia [1].
For the prevention and treatment of this syndrome, a series of non-pharmacological and pharmacological measures have been used. One of them is acetazolamide, which inhibits the carbonic anhydrase enzyme at the renal level and causes urinary bicarbonate excretion and metabolic acidosis. Its effect triggers compensatory hyperventilation and respiratory alkalosis, which promote the physiological response to the hypoxic stimulus. Despite being traditionally used, there is still controversy about the usefulness of acetazolamide as a treatment for acute mountain sickness[1].
Methods
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found one systematic review [1], which included two primary studies [2], [3], both corresponding to randomized trials. |
|
What types of patients were included* |
Both trials included patients with acute mountain sickness, that is, subjects acutely exposed to high-altitude, presenting symptoms of headache, fatigue, dizziness, nausea, vomiting, sleep disturbances and anorexia. |
|
What types of interventions were included* |
Both trials evaluated the use of acetazolamide orally. One trial [2] administered 250 mg at 0 and 8 hours and the other [3] 20 mg/kg at baseline and then 500 mg daily for 5 days. |
|
What types of outcomes |
The trials reported multiple outcomes, which were grouped by systematic review as follows:
Primary outcomes:
Secondary outcomes:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
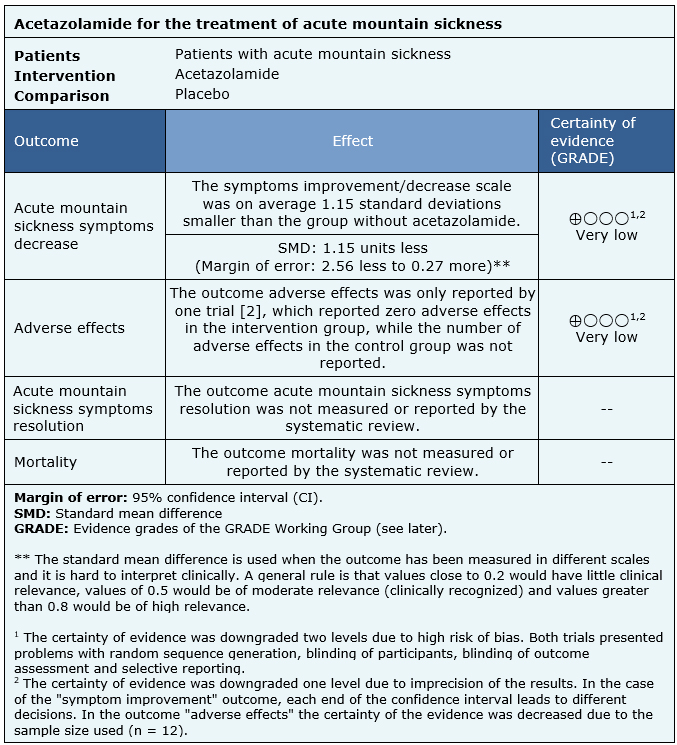
The information on the effects of acetazolamide for the treatment for acute mountain sickness is based on two randomized trials that included 25 patients.
Two trials reported acute mountain sickness symptoms decrease (25 patients) [2], [3], only one trial reported adverse effects, with incomplete results (12 patients) [2]. No trial reported the outcomes mortality or acute mountain sickness symptoms resolution.
The summary of findings is as follows:
- We are uncertain whether treatment with acetazolamide decreases acute mountain sickness symptoms as the certainty of the evidence has been assessed as very low.
- We are uncertain whether treatment with acetazolamide increases the risk of adverse effects as the certainty of the evidence has been assessed as very low.
- No studies were found to evaluate the reduction of mortality with treatment with acetazolamide.
- No studies were found to evaluate acute mountain sickness symptoms resolution with acetazolamide treatment.
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
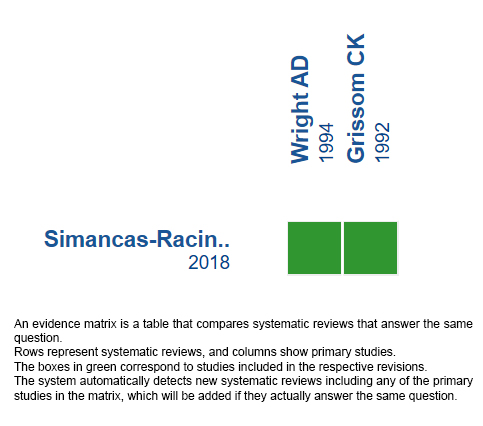
Follow the link to access the interactive version: Acetazolamide for the treatment of acute mountain syndrome.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
El mal agudo de montaña es la patología más prevalente relacionada con la exposición aguda a la altura, secundaria a los efectos de la hipoxia hipobárica en nuestro organismo. La acetazolamida se ha utilizado tradicionalmente para su prevención y tratamiento, sin embargo, aún existe controversia respecto al grado de utilidad que tiene este medicamento como monoterapia.
MÉTODOS
Realizamos una búsqueda en Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante el cribado de múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, analizamos los datos de los estudios primarios, realizamos un meta análisis y preparamos una tabla de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Identificamos una revisión sistemática que incluyó dos estudios primarios, ambos correspondientes a ensayos aleatorizados. Concluimos que no es posible establecer con claridad si el tratamiento con acetazolamida disminuye los síntomas del mal agudo de montaña ni si aumenta el riesgo de efectos adversos, debido a que la certeza de la evidencia existente ha sido evaluada como muy baja.
 Authors:
Luciano Tapia[1,2], Sebastián Irarrázaval[2,3]
Authors:
Luciano Tapia[1,2], Sebastián Irarrázaval[2,3]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Traumatología, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: sirarraz@med.puc.cl
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Diagonal Paraguay 476 Santiago Chile

Citation: Tapia L, Irarrázaval S. Acetazolamide for the treatment of acute mountain sickness. Medwave 2019;19(11):e7736 doi: 10.5867/medwave.2019.11.7736
Submission date: 3/7/2019
Acceptance date: 1/10/2019
Publication date: 9/12/2019
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Simancas‐Racines, Daniel, Arevalo‐Rodriguez, Ingrid, Osorio, Dimelza, Franco, Juan VA, Xu, Yihan, Hidalgo, Ricardo. Interventions for treating acute high altitude illness. Cochrane Database of Systematic Reviews. 2018;6:CD009567.
- Grissom CK, Roach RC, Sarnquist FH, Hackett PH. Acetazolamide in the treatment of acute mountain sickness: clinical efficacy and effect on gas exchange. Annals of internal medicine. 1992;116(6):461-5.
- Wright AD, Winterborn MH, Forster PJ, Delamere JP, Harrison GL, Bradwell AR. Carbonic anhydrase inhibition in the immediate therapy of acute mountain sickness. Journal of Wilderness Medicine. 1994;5(1):49-55.
- Netzer N, Strohl K, Faulhaber M, Gatterer H, Burtscher M. Hypoxia-related altitude illnesses. J Travel Med. 2013 Jul-Aug;20(4):247-55.
- Luks AM, McIntosh SE, Grissom CK, Auerbach PS, Rodway GW, Schoene RB, Zafren K, Hackett PH; Wilderness Medical Society. Wilderness Medical Society practice guidelines for the prevention and treatment of acute altitude illness: 2014 update. Wilderness Environ Med. 2014 Dec;25(4 Suppl):S4-14.
 Simancas‐Racines, Daniel, Arevalo‐Rodriguez, Ingrid, Osorio, Dimelza, Franco, Juan VA, Xu, Yihan, Hidalgo, Ricardo. Interventions for treating acute high altitude illness. Cochrane Database of Systematic Reviews. 2018;6:CD009567.
Simancas‐Racines, Daniel, Arevalo‐Rodriguez, Ingrid, Osorio, Dimelza, Franco, Juan VA, Xu, Yihan, Hidalgo, Ricardo. Interventions for treating acute high altitude illness. Cochrane Database of Systematic Reviews. 2018;6:CD009567.  Grissom CK, Roach RC, Sarnquist FH, Hackett PH. Acetazolamide in the treatment of acute mountain sickness: clinical efficacy and effect on gas exchange. Annals of internal medicine. 1992;116(6):461-5.
Grissom CK, Roach RC, Sarnquist FH, Hackett PH. Acetazolamide in the treatment of acute mountain sickness: clinical efficacy and effect on gas exchange. Annals of internal medicine. 1992;116(6):461-5.  Wright AD, Winterborn MH, Forster PJ, Delamere JP, Harrison GL, Bradwell AR. Carbonic anhydrase inhibition in the immediate therapy of acute mountain sickness. Journal of Wilderness Medicine. 1994;5(1):49-55.
Wright AD, Winterborn MH, Forster PJ, Delamere JP, Harrison GL, Bradwell AR. Carbonic anhydrase inhibition in the immediate therapy of acute mountain sickness. Journal of Wilderness Medicine. 1994;5(1):49-55.  Netzer N, Strohl K, Faulhaber M, Gatterer H, Burtscher M. Hypoxia-related altitude illnesses. J Travel Med. 2013 Jul-Aug;20(4):247-55.
Netzer N, Strohl K, Faulhaber M, Gatterer H, Burtscher M. Hypoxia-related altitude illnesses. J Travel Med. 2013 Jul-Aug;20(4):247-55.  Luks AM, McIntosh SE, Grissom CK, Auerbach PS, Rodway GW, Schoene RB, Zafren K, Hackett PH; Wilderness Medical Society. Wilderness Medical Society practice guidelines for the prevention and treatment of acute altitude illness: 2014 update. Wilderness Environ Med. 2014 Dec;25(4 Suppl):S4-14.
Luks AM, McIntosh SE, Grissom CK, Auerbach PS, Rodway GW, Schoene RB, Zafren K, Hackett PH; Wilderness Medical Society. Wilderness Medical Society practice guidelines for the prevention and treatment of acute altitude illness: 2014 update. Wilderness Environ Med. 2014 Dec;25(4 Suppl):S4-14. Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








