Key Words: linear IgA bullous dermatosis, bullous disease
Abstract
We present the case of a sixty five year old woman with two months history of pruritus and hyperpigmented annular lesions on the trunk, buttocks and upper extremities. In addition, she presents vesicles with healthy skin on the basis, in the flexor aspect of wrists. No evidence of mucosal involvement. Histological study showed subepidermal vesicular dermatitis with inflammatory infiltrate of neutrophils and eosinophils. Direct immunofluorescence evidenced linear and continuous deposition of immunoglobulin A in basement membrane zone, compatible with linear immunoglobulin A disease.
Introduction
Linear Immunoglobulin A Dermatosis is a rare autoimmune disorder characterized by the presence of vesicle and blistering lesions. Its diagnosis has been defined by a unique immunopathology pattern consisting of linear deposition of Immunoglobulin A along the dermoepidermal junction [1]. Its incidence in South America is still unknown.
We present this case because of the low incidence of this pathology in our population and its broad differential diagnosis, both in the pediatric and adult population. Also, it exhibits a specific treatment compared to other autoimmune blistering dermatoses.
Clinical case
A 65-year-old female patient presented with a history of adrenal adenoma, high blood pressure, and chronic obstructive pulmonary disease secondary to smoking, on treatment with atenolol. The patient reported a history of 2 months characterized initially by intense itching and the subsequent appearance of erythematous lesions associated with vesicles, in the trunk and upper extremities, which increased in size, some of them acquired an annular distribution and later evolved to peripheral crusts.
Physical examination revealed an extensive hyperpigmented annular plaque on the trunk (Figure 1), irregularly shaped, with erythematous border and crustal erosions of peripheral predominance. She also presented lesions of similar characteristics of smaller size in the back, buttocks (Figure 2) and upper extremities. On flexor aspects of both wrists, the patient exhibited tight blisters and vesicles with a serous content on an erythematous base and unscathed skin (Figure 3). No mucosal involvement was observed.
Because of these clinical findings, the diagnosis of linear Immunoglobulin A dermatosis was proposed, a biopsy specimen was obtained for confirmation, and then 30 mg prednisone were prescribed.
The histological examination with hematoxylin-eosin staining showed subepidermal vesicular dermatitis with inflammatory infiltrate of neutrophils and eosinophils (Figure 4), consistent with subepidermal autoimmune blistering disease. Direct immunofluorescence showed linear and continuous deposit of immunoglobulin A in the basement membrane zone (Figure 5), compatible with linear immunoglobulin A dermatosis.
Treatment was started with dapsone 50 mg and 100 mg every other day. The patient evolved with asymmetric post-inflammatory hyperpigmentation on the back, flank, and right axilla, without showing new blistering lesions. Laboratory tests and glucose-6-phosphate dehydrogenase activity were normal. Due to this, oral prednisone was progressively lowered to the suspension, and the dose of dapsone was reduced to 50 mg daily, with favorable long-lasting clinical remission.
Figure 2. Hyperpigmented annular plaque with crusts on an erythematous background on buttocks.
Figure 4. Hematoxylin & eosin staining.
Figure 5. Direct immunofluorescence.
Discussion
Linear immunoglobulin A dermatosis is a chronic, acquired, autoimmune blistering disease [1]. It is characterized by the presence of subepidermal vesicles and linear deposits of immunoglobulin A autoantibodies against multiple antigens located in the basement membrane, visualized by direct immunofluorescence or indirect immunofluorescence [2]. It occurs in both pediatric and adult populations, with some semiological differences [3].
It can be subdivided into two types by immunoelectron microscopy findings:
- Deposits within the lamina lucida: the most common antigens are LAD-1 (120 kDa) and LABD97 (97 kDa) [4].
- Deposits within the sublamina densa: (also referred to as epidermolysis bullosa acquired by immunoglobulin A): the detachment is deeper; the target antigen is collagen VII [5].
Linear immunoglobulin A dermatosis was first recognized as a clinical entity in 1979 by Chorzelski and Jablonska [6]. It presents a variable incidence according to geographical location, being greater in China, Southeast Asia and Africa [1]. In Europe, the incidence is estimated in 0.5 new cases per million each year [7], while in South America is still unknown.
It can occur at any age, but classically shows two peaks: the first in children between 4 and 5 years, and the second, as in the case presented, in adults after 60 years, with a slight predominance in the female sex [8].
Most research on the disease has shown that there is a strong association between linear immunoglobulin A dermatosis and human leukocyte antigens B8, CW7 and DR3 [9], which would be related to an early onset of the disease. On the other hand, it has been shown an association with tumor necrosis factor-2, whose presence is related to poor prognosis [10].
The pathophysiological mechanism that triggers the autoimmune response in linear immunoglobulin A dermatosis is still unknown. Investigations over the past 30 years have identified different related antigens, which are located in the epithelial basement membrane zone [11]. Both the humoral and cellular responses appear to be involved in the pathogenesis of the disease [2].
The humoral response is determined by the pathological production of immunoglobulin A against antigens of the basement membrane zone. Most develop antibodies against more than one antigen, the most common being LAD-1 and LABD97, derived from BPAg2 or collagen XVII [12],[13]. A portion of the extracellular segment of BPAg2 is physiologically evolved and degraded by proteolytic action to produce fragments and new epitopes, a process known as "epitope diffusion" [14]. In in vitro models, binding of immunoglobulin A antibodies caused skin tissue damage in culture and neutrophil binding to the basement membrane [15]. Passive serum transference in mice with linear immunoglobulin A dermatosis to a mouse with severe combined immunodeficiency can promote neutrophil infiltration and basal membrane vesiculation [16].
Regarding the cellular immune response, immunohistochemical analyses have demonstrated the presence of neutrophils and eosinophils [6], whose activation would cause the recruitment and activation of a greater number of polymorphonuclear cells, which would be responsible for releasing cytokines related to the disruption of the basement membrane. The type of cytokine is varied, but an increased production of mediated T helper cell 1 interleukin 2 and mediated T helper cell 2 interleukin 5 have been identified [17].
Concerning clinical presentation, there are differences according to the age of the patient:
Linear Immunoglobulin A dermatosis in Pediatric Population or Chronic Childhood Blistering disease: It is the most common acquired blistering autoimmune disease of childhood. Classically it presents as an acute episode of vesicular eruption or blistering with facial involvement (usually perioral and periocular), and the anogenital zone, including the lower abdomen, vulva, thighs, perineum and buttocks [18]. There may be mucosal involvement with oral ulcers or erosions, nasal bleeding, and conjunctivitis, but less frequently than in the adult population [19].
They may present on the healthy skin or in erythematous plaques, which often adopt an annular or polycyclic pattern [20], with blisters around the edge producing the signs "crown of pearls" in circular lesions, and "string of pearls" in more serpiginous paths [7]. It is associated with variable symptoms ranging from pruritus to severe burning.
This presentation is usually self-limiting and remit in months or years, however, in some cases, may persist into adulthood, with the same clinical characteristics as the adult form [21].
Linear immunoglobulin A Dermatosis in adults: The classic clinical presentation in adults is similar to the clinical case we show; the disease begins abruptly, with variable burning sensation or intense pruritus associated with scratches. It evolves with clear and/or hemorrhagic vesicles or blisters, appearing on normal or erythematous or urticarial skin. They are usually tense, of different size and tend to form annular or polycyclic plates due to the coalescence of the lesions [9]. In adults, extensor, trunk, gluteal and face surfaces, especially the perioral area, are preferentially involved, and may or may not be symmetrical [1].
No mucosal involvement was observed in our patient. In the literature, 50% of the patients present mucosal involvement [22] having painful ulcers and erosions in the oral cavity, with the soft and hard palate being more affected, as well as the palatal arches and the oral mucosa. Less frequent is the involvement of the tongue, gums, oral vestibule and lips [19]. Nasal and ocular involvement may be unilateral or bilateral, with chronic conjunctivitis that can end in fibrosis and symblepharon, which may evolve to blindness without treatment [23]. Although uncommon, mucosal involvement may be the only clinical sign or precede skin lesions [20]. Genitals are less frequently affected than in the pediatric population.
Even though most cases of linear immunoglobulin A dermatosis are idiopathic, as presumed in the clinical case described since no predisposing agent was detected, some precipitating factors have been identified in the literature such as:
Drugs: linear immunoglobulin A dermatosis is usually self-limiting and usually resolves quickly and spontaneously after drug suspension. The temporal relationship between the drug and the rare presence of linear deposits of IgA in the basement membrane zone are often useful for diagnosis [2]. Vancomycin is the drug most commonly associated with the disease, followed by amiodarone, nonsteroidal anti-inflammatory drugs, captopril, ceftriaxone, and metronidazole [1]. The mechanism of drug-induced linear Immunoglobulin A dermatosis is unknown; drugs may cross-react with basement membrane autoantigens or unmask previously hidden antigens.
Systemic diseases: Some lymphoproliferative disorders have been associated with linear immunoglobulin A dermatosis, such as non-Hodgkin lymphoma and chronic lymphocytic leukemia [24].
Cutaneous trauma: Skin injuries such as burns or exposure to ultraviolet light have been associated with the onset of the disease [25].
Gastrointestinal diseases: Ulcerative colitis is the most commonly involved non-malignant disease, followed by systemic lupus erythematosus and Crohn's disease [26].
Finally, during pregnancy the clinical condition usually improves, probably due to an increased glycosylation of immunoglobulin A due to pregnancy hormones, altering the ability to bind to the antigen [27].
Given the difficulty of distinguishing linear immunoglobulin A dermatosis from other blistering diseases through clinical and histopathological findings, the diagnosis should be confirmed by direct immunofluorescence [2]. In all cases, drug-induced conditions should be ruled out [28].
Histopathology is characterized by the presence of subepithelial blisters with predominantly neutrophilic infiltrate in the upper dermis, forming papillary microabscesses [29]. Also, eosinophils and mononuclear cells are observed [30].
In the clinical case, direct immunofluorescence was performed on a perilesional skin specimen, which is considered the gold standard diagnosis [1]. This includes the presence of immunoglobulin A depositions along the basement membrane zone in a linear and continuous pattern, which is pathognomonic of the disease. Less common is the presence of immunoglobulin G, immunoglobulin M and complement C3 [2]. Positive indirect immunofluorescence is more frequently seen in the pediatric population (75%) than in adults (30%) [31]..
The "salt-split skin" technique can increase the sensitivity of serology, observing mainly deposits at the epidermal level (linear immunoglobulin A dermatosis lamina lucida type) or the dermal level (linear immunoglobulin A dermatosis sublamina densa type); however, in some cases a mixed distribution is presented [16],[32]. Curiously, in most cases of drug-induced linear immunoglobulin A dermatosis, the autoantibodies immunoglobulin A against the basement membrane zone are not detected [33].
Immunoelectron microscopy can detect the exact location of the immune deposits, either in lamina lucida, lamina densa or both. This immunopathological finding corresponds to a particular characteristic of patients with linear immunoglobulin A dermatosis, giving the appearance of mirror image [34]. If none of the laboratory tests are conclusive, the immunoblot may constitute an additional analysis to identify the antigen [35].
The differential diagnosis usually includes other blistering diseases, according to the age of presentation [1] (Table 1):
Table 1. Differential diagnosis of blistering diseases according to age of presentation
Linear immunoglobulin A dermatosis requires a complex and multidisciplinary management. Even though the patient started oral prednisone and dapsone was added later showing a good clinical response, in the literature dapsone is considered to be the first-line treatment, whereas corticosteroids are reserved for difficult cases [36].
The mechanism of action by which dapsone inhibits neutrophil chemotaxis at the immunoglobulin A deposition site, is not currently understood. It has been shown to inhibit lysosomal neutrophil activity and iodination mediated by myeloperoxidase but would have no effect on antibody or complement deposits [37]. On the other hand, dapsone inhibits the adhesion of neutrophils to the epithelial basement membrane area being a dose-dependent effect [37].
The initial dose is 25-50 mg daily in adults and 0.5 mg/kg/day in children, and it can be slowly increased to a recommended maximum of 2.5 to 3 mg/kg/day [2], with an average dose of 100 mg to control the disease. The response can be presented in 24 to 48 hours, with the resolution of the lesions within the subsequent days. Once the effect is achieved, a progressive and stepwise decrease in the dose is recommended (12.5 to 25 mg every 1 to 2 weeks). With the appearance of new small lesions, high-potency topical corticosteroids may be associated. Glucose-6-phosphate dehydrogenase levels should be evaluated before initiation of treatment, to avoid that the oxidative stress of dapsone does not induce a hemolytic crisis. Once the treatment is started, the patient should be monitored with a blood count every 2 to 4 weeks for the first three months to evaluate the presence of leukopenia or hemolysis.
The second line of treatment consists of sulfonamides such as sulfapyridine (16-60 mg/kg/day), sulfasalazine or sulfamethoxypyridazine, alone or in combination with dapsone. The combination delivers aggregate efficacy without further toxicity [38].
In cases of partial response, it may be associated with topical or systemic corticosteroids [39]. It has been shown that the use of topical corticosteroids in the skin or mucosa can be very effective as single agents in mild cases or as an adjuvant to systemic therapy in severe disease [2].
In those patients in whom the disease is not controlled, immunosuppressants such as mycophenolate, colchicine, cyclophosphamide, cyclosporine or topical tacrolimus may be used [40]. Some antimicrobial agents such as oxacillin, dicloxacillin, erythromycin, flucloxacillin and cotrimoxazole have shown to be useful in treatment [41]. The combined use of tetracycline and niacinamide has shown efficacy in the treatment of linear immunoglobulin A dermatosis in different series [42],[43],[44]. Intravenous immunoglobulin has been used successfully in refractory patients, such as patients with chronic renal failure or with chronic ocular involvement [45],[46],[47]. The use of rituximab in a refractory case with the favorable clinical response has recently been reported [48]. Most patients respond quickly to initial therapy, so these alternatives are reserved for refractory cases or with contraindications for their use.
Systemic therapy should be maintained until the patient enters complete clinical remission, with a maintenance dose according to the individual characteristics of each patient. If the disease recurs, systemic therapy should be restarted and maintained for weeks or months after the lesions are cleared [2].
Regarding prognosis, childhood linear immunoglobulin A dermatosis is self-limited, with an average duration of 1 to 5 years [18]. The adult form is chronic and refractory [2]. Mucosal involvement can generate significant functional consequences such as blindness [49]. In drug-induced cases, resolution begins in days to weeks after drug discontinuation [28].
Conclusion
Linear immunoglobulin A dermatosis is an uncommon immune-mediated disease characterized by the presence of immunoglobulin A deposits in the basal membrane zone, visible with direct immunofluorescence. Clinically it can be divided into an adult or pediatric presentation. Although its idiopathic form is the most frequent, the presence of triggering factors such as drugs or malignancies should always be ruled out.
The treatment of choice is dapsone, and there are numerous alternatives, including sulfa drugs, corticosteroids, and immunosuppressants. The prognosis is usually favorable.
We believe it is important to present this clinical case due to the low frequency of this pathology in our country, its presentation in different age groups and because it has a particular therapeutic approach.
Notes
From the editor
The authors originally submitted this article in Spanish and subsequently translated it into English. The Journal has not copyedited this version.
Acknowledgement
The authors thank the Dermatology Department, Hospital Clínico Universidad de Chile for the collaboration provided for the preparation of this manuscript.
Ethical aspects
The informed consent requested by Medwave, has been signed by the patient; a copy of this was sent to the editorial board of the magazine.
Conflicts of Interest
The authors have completed the ICMJE declaration of conflicts of interest form, and declare that they have not received funding for the report; not having financial relationships with organizations that might have interests in the published article in the past three years; and not having other relationships or activities that could influence the published article. Forms can be requested by contacting the responsible author or the editorial board of the Journal.
Financing
The authors state that there were no external sources of funding.

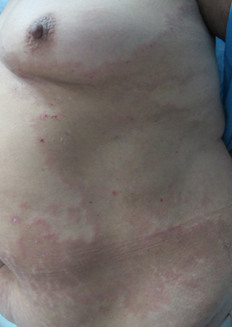 Figure 1. Extensive hyperpigmented annular plaque with crusts on erythematous background in the trunk.
Figure 1. Extensive hyperpigmented annular plaque with crusts on erythematous background in the trunk.

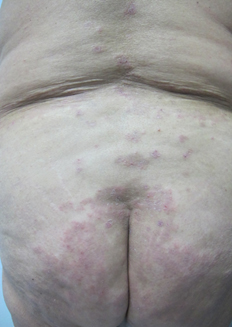 Figure 2. Hyperpigmented annular plaque with crusts on an erythematous background on buttocks.
Figure 2. Hyperpigmented annular plaque with crusts on an erythematous background on buttocks.

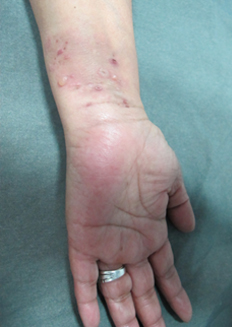 Figure 3. Vesicles, bullae, and crusts on an erythematous background and residual hyperpigmentation on wrists.
Figure 3. Vesicles, bullae, and crusts on an erythematous background and residual hyperpigmentation on wrists.

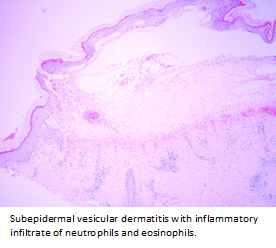 Figure 4. Hematoxylin & eosin staining.
Figure 4. Hematoxylin & eosin staining.

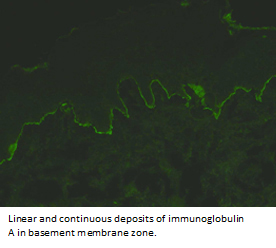 Figure 5. Direct immunofluorescence.
Figure 5. Direct immunofluorescence.

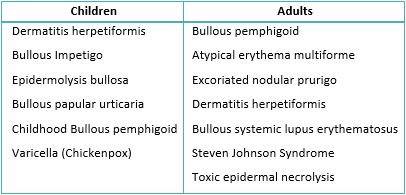 Table 1. Differential diagnosis of blistering diseases according to age of presentation
Table 1. Differential diagnosis of blistering diseases according to age of presentation
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

En este texto se presenta el caso de una paciente de sesenta y cinco años con sintomatología de dos meses de evolución consistente en prurito y lesiones hiperpigmentadas anulares en tronco, glúteos y extremidades superiores. En el área flexora de las muñecas presenta vesículas sobre base eritematosa y piel sana, sin evidencia de compromiso mucoso. Al estudio histológico se constata dermatitis vesicular subepidérmica con infiltrado inflamatorio de neutrófilos y eosinófilos. La inmunofluorescencia directa muestra depósito lineal y continuo de inmunoglobulina A en zona de membrana basal, compatible con dermatosis por inmunoglobulina A lineal.
 Autores:
Fernando Valenzuela Ahumada [1 ], Roberto Bustos Macaya [1 ], Gabriela Paz Romero Morgado [1 ], Margarita Sánchez Chacón [1 ]
Autores:
Fernando Valenzuela Ahumada [1 ], Roberto Bustos Macaya [1 ], Gabriela Paz Romero Morgado [1 ], Margarita Sánchez Chacón [1 ]
Affiliation:
[1] Facultad de Medicina, Universidad de Chile, Santiago, Chile
E-mail: robertobustos@med.uchile.cl
Author address:
[1] Departamento de Dermatología
Hospital Clínico Universidad de Chile
Santos Dumont 999
Independencia
Santiago
Chile

Citation: Valenzuela Ahumada F , Bustos Macaya R , Romero Morgado GP , Sánchez Chacón M . Linear immunoglobulin A dermatosis: A case report. Medwave 2017 Abr;17(3):e6901 doi: 10.5867/medwave.2017.03.6901
Submission date: 8/11/2016
Acceptance date: 26/1/2017
Publication date: 4/4/2017
Origin: not requested
Type of review: reviewed by three external peer reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Venning VA. Linear IgA disease: clinical presentation, diagnosis, and pathogenesis. Immunol Allergy Clin North Am. 2012 May;32(2):245-53, vi. | CrossRef | PubMed |
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012 Jan-Feb;30(1):38-50. | CrossRef | PubMed |
- Sandoval M, Farias MM, Gonzalez S. Linear IgA bullous dermatosis: report of five cases in Chile. Int J Dermatol. 2012 Nov;51(11):1303-6. | CrossRef | PubMed |
- Zone JJ, Taylor TB, Kadunce DP, Meyer LJ. Identification of the cutaneous basement membrane zone antigen and isolation of antibody in linear immunoglobulin A bullous dermatosis. J Clin Invest. 1990 Mar;85(3):812-20. | PubMed |
- Vodegel RM, de Jong MC, Pas HH, Jonkman MF. IgA-mediated epidermolysis bullosa acquisita: two cases and review of the literature. J Am Acad Dermatol. 2002 Dec;47(6):919-25. | PubMed |
- Conleth A, Zone J. Linear IgA bullous dermatosis. Int J Dermatol. 1999 Nov;38(11):818-27. | PubMed |
- Fuentelsaz V, Campos M. Dermatosis IgA lineal de la infancia. Rev Pediatr Aten Primaria. 2013;15:141-5. | Link |
- Chen S, Mattei P, Fischer M, Gay JD, Milner SM, Price LA. Linear IgA bullous dermatosis. Eplasty. 2013 Jul 2;13:ic49. | PubMed |
- Ingen-Housz-Oro S. [Linear IgA bullous dermatosis: a review]. Ann Dermatol Venereol. 2011 Mar;138(3):214-20. | CrossRef | PubMed |
- Collier PM, Wojnarowska F, Welsh K, McGuire W, Black MM. Adult linear IgA disease and chronic bullous disease of childhood: the association with human lymphocyte antigens Cw7, B8, DR3 and tumour necrosis factor influences disease expression. Br J Dermatol. 1999 Nov;141(5):867-75. | PubMed |
- Patsatsi A. Chronic Bullous Disease or Linear IgA Dermatosis of Childhood –Revisited. J Genet Syndr Gene Ther. 2013;4:6 | CrossRef |
- Hirako Y, Usukura J, Uematsu J, Hashimoto T, Kitajima Y, Owaribe K. Cleavage of BP180, a 180-kDa bullous pemphigoid antigen, yields a 120-kDa collagenous extracellular polypeptide. J Biol Chem. 1998 Apr 17;273(16):9711-7. | PubMed |
- Zone JJ, Taylor TB, Meyer LJ, Petersen MJ. The 97 kDa linear IgA bullous disease antigen is identical to a portion of the extracellular domain of the 180 kDa bullous pemphigoid antigen, BPAg2. J Invest Dermatol. 1998 Mar;110(3):207-10. | PubMed |
- Allen J, Wojnarowska F. Linear IgA disease: the IgA and IgG response to dermal antigens demonstrates a chiefly IgA response to LAD285 and a dermal 180-kDa protein. Br J Dermatol. 2003 Nov;149(5):1055-8. | PubMed |
- Hendrix JD, Mangum KL, Zone JJ, Gammon WR. Cutaneous IgA deposits in bullous diseases function as ligands to mediate adherence of activated neutrophils. J Invest Dermatol. 1990 May;94(5):667-72. | PubMed |
- Zone JJ, Egan CA, Taylor TB, Meyer LJ. IgA autoimmune disorders: development of a passive transfer mouse model. J Investig Dermatol Symp Proc. 2004 Jan;9(1):47-51. | PubMed |
- Caproni M, Rolfo S, Bernacchi E, Bianchi B, Brazzini B, Fabbri P. The role of lymphocytes, granulocytes, mast cells and their related cytokines in lesional skin of linear IgA bullous dermatosis. Br J Dermatol. 1999 Jun;140(6):1072-8. | PubMed |
- Mintz EM, Morel KD. Clinical features, diagnosis, and pathogenesis of chronic bullous disease of childhood. Dermatol Clin. 2011 Jul;29(3):459-62, ix. | CrossRef | PubMed |
- Weinberg MA, Insler MS, Campen RB. Mucocutaneous features of autoimmune blistering diseases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997 Nov;84(5):517-34. | PubMed |
- Shimizu S, Natsuga K, Shinkuma S, Yasui C, Tsuchiya K, Shimizu H. Localized linear IgA/IgG bullous dermatosis. Acta Derm Venereol. 2010 Nov;90(6):621-4. | CrossRef | PubMed |
- Burge S, Wojnarowska F, Marsden A. Chronic bullous dermatosis of childhood persisting into adulthood. Pediatr Dermatol. 1988 Nov;5(4):246-9. | PubMed |
- Verma R, Vasudevan B, Pragasam V, Dabbas D. Linear IgA disease in an adult with unusual clinical features. Indian Dermatol Online J. 2013 Apr;4(2):115-8. | CrossRef | PubMed |
- Aultbrinker EA, Starr MB, Donnenfeld ED. Linear IgA disease. The ocular manifestations. Ophthalmology. 1988 Mar;95(3):340-3. | PubMed |
- Godfrey K, Wojnarowska F, Leonard J. Linear IgA disease of adults: association with lymphoproliferative malignancy and possible role of other triggering factors. Br J Dermatol. 1990 Oct;123(4):447-52. | PubMed |
- Girão L, Fiadeiro T, Rodrigues JC. Burn-induced linear IgA dermatosis. J Eur Acad Dermatol Venereol. 2000 Nov;14(6):507-10. | PubMed |
- Vargas TJ, Fialho M, Santos LT, Rodrigues PA, Vargas AL, Sousa MA. Linear IgA dermatosis associated with ulcerative colitis: complete and sustained remission after total colectomy. An Bras Dermatol. 2013 Jul-Aug;88(4):600-3. | CrossRef | PubMed |
- Collier PM, Kelly SE, Wojnarowska F. Linear IgA disease and pregnancy. J Am Acad Dermatol. 1994 Mar;30(3):407-11. | PubMed |
- Camilleri M, Pace JL. Drug-induced linear immunoglobulin-A bullous dermatosis. Clin Dermatol. 1998 May-Jun;16(3):389-91. | PubMed |
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001 Nov-Dec;19(6):719-27. | PubMed |
- Reyes-Baraona F, Andino R, Carrasco JE, Arriagada C, Guerrero S. [Linear IgA bullous dermatosis of childhood: case report]. Arch Argent Pediatr. 2014 Apr;112(2):e57-60. | CrossRef | PubMed |
- Wojnarowska F, Marsden RA, Bhogal B, Black MM. Chronic bullous disease of childhood, childhood cicatricial pemphigoid, and linear IgA disease of adults. A comparative study demonstrating clinical and immunopathologic overlap. J Am Acad Dermatol. 1988 Nov;19(5 Pt 1):792-805. | PubMed |
- Willsteed E, Bhogal BS, Black MM, McKee P, Wojnarowska F. Use of 1M NaCl split skin in the indirect immunofluorescence of the linear IgA bullous dermatoses. J Cutan Pathol. 1990 Jun;17(3):144-8. | PubMed |
- Kuechle MK, Stegemeir E, Maynard B, Gibson LE, Leiferman KM, Peters MS. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994 Feb;30(2 Pt 1):187-92. | PubMed |
- Bhogal B, Wojnarowska F, Marsden RA, Das A, Black MM, McKee PH. Linear IgA bullous dermatosis of adults and children: an immunoelectron microscopic study. Br J Dermatol. 1987 Sep;117(3):289-96. | PubMed |
- Allen J, Wojnarowska F. Linear IgA disease: the IgA and IgG response to the epidermal antigens demonstrates that intermolecular epitope spreading is associated with IgA rather than IgG antibodies, and is more common in adults. Br J Dermatol. 2003 Nov;149(5):977-85. | PubMed |
- Mintz EM, Morel KD. Treatment of chronic bullous disease of childhood. Dermatol Clin. 2011 Oct;29(4):699-700. | CrossRef | PubMed |
- Wojnarowska F. Linear IgA dapsone responsive bullous dermatosis. J R Soc Med. 1980 May;73(5):371-3. | PubMed |
- McFadden JP, Leonard JN, Powles AV, Rutman AJ, Fry L. Sulphamethoxypyridazine for dermatitis herpetiformis, linear IgA disease and cicatricial pemphigoid. Br J Dermatol. 1989 Dec;121(6):759-62. | PubMed |
- Mervic L, Dragos V, Pavlović MD. Linear IgA bullous dermatosis of childhood: successful treatment with miocamycin and topical corticosteroid. Clin Exp Dermatol. 2009 Oct;34(7):e391-2. | CrossRef | PubMed |
- Kasperkiewicz M, Schmidt E. Current treatment of autoimmune blistering diseases. Curr Drug Discov Technol. 2009 Dec;6(4):270-80. | PubMed |
- Alajlan A, Al-Khawajah M, Al-Sheikh O, Al-Saif F, Al-Rasheed S, Al-Hoqail I, et al. Treatment of linear IgA bullous dermatosis of childhood with flucloxacillin. J Am Acad Dermatol. 2006 Apr;54(4):652-6. | PubMed |
- Chaffins ML, Collison D, Fivenson DP. Treatment of pemphigus and linear IgA dermatosis with nicotinamide and tetracycline: a review of 13 cases. J Am Acad Dermatol. 1993 Jun;28(6):998-1000. | PubMed |
- Peoples D, Fivenson DP. Linear IgA bullous dermatosis: successful treatment with tetracycline and nicotinamide. J Am Acad Dermatol. 1992 Mar;26(3 Pt2):498-9. | PubMed |
- Yomada M, Komai A, Hashimato T. Sublamina densa-type linear IgA bullous dermatosis successfully treated with oral tetracycline and niacianamide. Br J Dermatol. 1999 Sep;141(3):608-9. | PubMed |
- Khan IU, Bhol KC, Ahmed AR. Linear IgA bullous dermatosis in a patient with chronic renal failure: response to intravenous immunoglobulin therapy. J Am Acad Dermatol. 1999 Mar;40(3):485-8. | PubMed |
- Kroiss MM, Vogt T, Landthaler M, Stolz W. High-dose intravenous immune globulin is also effective in linear IgA disease. Br J Dermatol. 2000 Mar;142(3):582. Erratum in: Br J Dermatol 2000 Jun;142(6):1268. | PubMed |
- Letko E, Bhol K, Foster CS, Ahmed AR. Linear IgA bullous disease limited to the eye: a diagnostic dilemma: response to intravenous immunoglobulin therapy. Ophthalmology. 2000 Aug;107(8):1524-8. | PubMed |
- Lozinski A, Baum S, Sagi L, Volkov A, Trau H, Barzilai A. [Rituximab (Mabthera) for treatment of rare autoimmune bullous skin disorders]. Harefuah. 2012 Oct;151(10):562-5, 606. | PubMed |
- Talhari C, Althaus C, Megahed M. Ocular linear IgA disease resulting in blindness. Arch Dermatol. 2006 Jun;142(6):786-7. | PubMed |
 Venning VA. Linear IgA disease: clinical presentation, diagnosis, and pathogenesis. Immunol Allergy Clin North Am. 2012 May;32(2):245-53, vi. | CrossRef | PubMed |
Venning VA. Linear IgA disease: clinical presentation, diagnosis, and pathogenesis. Immunol Allergy Clin North Am. 2012 May;32(2):245-53, vi. | CrossRef | PubMed | Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012 Jan-Feb;30(1):38-50. | CrossRef | PubMed |
Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012 Jan-Feb;30(1):38-50. | CrossRef | PubMed | Sandoval M, Farias MM, Gonzalez S. Linear IgA bullous dermatosis: report of five cases in Chile. Int J Dermatol. 2012 Nov;51(11):1303-6. | CrossRef | PubMed |
Sandoval M, Farias MM, Gonzalez S. Linear IgA bullous dermatosis: report of five cases in Chile. Int J Dermatol. 2012 Nov;51(11):1303-6. | CrossRef | PubMed | Zone JJ, Taylor TB, Kadunce DP, Meyer LJ. Identification of the cutaneous basement membrane zone antigen and isolation of antibody in linear immunoglobulin A bullous dermatosis. J Clin Invest. 1990 Mar;85(3):812-20. | PubMed |
Zone JJ, Taylor TB, Kadunce DP, Meyer LJ. Identification of the cutaneous basement membrane zone antigen and isolation of antibody in linear immunoglobulin A bullous dermatosis. J Clin Invest. 1990 Mar;85(3):812-20. | PubMed | Vodegel RM, de Jong MC, Pas HH, Jonkman MF. IgA-mediated epidermolysis bullosa acquisita: two cases and review of the literature. J Am Acad Dermatol. 2002 Dec;47(6):919-25. | PubMed |
Vodegel RM, de Jong MC, Pas HH, Jonkman MF. IgA-mediated epidermolysis bullosa acquisita: two cases and review of the literature. J Am Acad Dermatol. 2002 Dec;47(6):919-25. | PubMed | Conleth A, Zone J. Linear IgA bullous dermatosis. Int J Dermatol. 1999 Nov;38(11):818-27. | PubMed |
Conleth A, Zone J. Linear IgA bullous dermatosis. Int J Dermatol. 1999 Nov;38(11):818-27. | PubMed | Fuentelsaz V, Campos M. Dermatosis IgA lineal de la infancia. Rev Pediatr Aten Primaria. 2013;15:141-5. | Link |
Fuentelsaz V, Campos M. Dermatosis IgA lineal de la infancia. Rev Pediatr Aten Primaria. 2013;15:141-5. | Link | Chen S, Mattei P, Fischer M, Gay JD, Milner SM, Price LA. Linear IgA bullous dermatosis. Eplasty. 2013 Jul 2;13:ic49. | PubMed |
Chen S, Mattei P, Fischer M, Gay JD, Milner SM, Price LA. Linear IgA bullous dermatosis. Eplasty. 2013 Jul 2;13:ic49. | PubMed | Ingen-Housz-Oro S. [Linear IgA bullous dermatosis: a review]. Ann Dermatol Venereol. 2011 Mar;138(3):214-20. | CrossRef | PubMed |
Ingen-Housz-Oro S. [Linear IgA bullous dermatosis: a review]. Ann Dermatol Venereol. 2011 Mar;138(3):214-20. | CrossRef | PubMed | Collier PM, Wojnarowska F, Welsh K, McGuire W, Black MM. Adult linear IgA disease and chronic bullous disease of childhood: the association with human lymphocyte antigens Cw7, B8, DR3 and tumour necrosis factor influences disease expression. Br J Dermatol. 1999 Nov;141(5):867-75. | PubMed |
Collier PM, Wojnarowska F, Welsh K, McGuire W, Black MM. Adult linear IgA disease and chronic bullous disease of childhood: the association with human lymphocyte antigens Cw7, B8, DR3 and tumour necrosis factor influences disease expression. Br J Dermatol. 1999 Nov;141(5):867-75. | PubMed | Patsatsi A. Chronic Bullous Disease or Linear IgA Dermatosis of Childhood –Revisited. J Genet Syndr Gene Ther. 2013;4:6 | CrossRef |
Patsatsi A. Chronic Bullous Disease or Linear IgA Dermatosis of Childhood –Revisited. J Genet Syndr Gene Ther. 2013;4:6 | CrossRef | Hirako Y, Usukura J, Uematsu J, Hashimoto T, Kitajima Y, Owaribe K. Cleavage of BP180, a 180-kDa bullous pemphigoid antigen, yields a 120-kDa collagenous extracellular polypeptide. J Biol Chem. 1998 Apr 17;273(16):9711-7. | PubMed |
Hirako Y, Usukura J, Uematsu J, Hashimoto T, Kitajima Y, Owaribe K. Cleavage of BP180, a 180-kDa bullous pemphigoid antigen, yields a 120-kDa collagenous extracellular polypeptide. J Biol Chem. 1998 Apr 17;273(16):9711-7. | PubMed | Zone JJ, Taylor TB, Meyer LJ, Petersen MJ. The 97 kDa linear IgA bullous disease antigen is identical to a portion of the extracellular domain of the 180 kDa bullous pemphigoid antigen, BPAg2. J Invest Dermatol. 1998 Mar;110(3):207-10. | PubMed |
Zone JJ, Taylor TB, Meyer LJ, Petersen MJ. The 97 kDa linear IgA bullous disease antigen is identical to a portion of the extracellular domain of the 180 kDa bullous pemphigoid antigen, BPAg2. J Invest Dermatol. 1998 Mar;110(3):207-10. | PubMed | Allen J, Wojnarowska F. Linear IgA disease: the IgA and IgG response to dermal antigens demonstrates a chiefly IgA response to LAD285 and a dermal 180-kDa protein. Br J Dermatol. 2003 Nov;149(5):1055-8. | PubMed |
Allen J, Wojnarowska F. Linear IgA disease: the IgA and IgG response to dermal antigens demonstrates a chiefly IgA response to LAD285 and a dermal 180-kDa protein. Br J Dermatol. 2003 Nov;149(5):1055-8. | PubMed | Hendrix JD, Mangum KL, Zone JJ, Gammon WR. Cutaneous IgA deposits in bullous diseases function as ligands to mediate adherence of activated neutrophils. J Invest Dermatol. 1990 May;94(5):667-72. | PubMed |
Hendrix JD, Mangum KL, Zone JJ, Gammon WR. Cutaneous IgA deposits in bullous diseases function as ligands to mediate adherence of activated neutrophils. J Invest Dermatol. 1990 May;94(5):667-72. | PubMed | Zone JJ, Egan CA, Taylor TB, Meyer LJ. IgA autoimmune disorders: development of a passive transfer mouse model. J Investig Dermatol Symp Proc. 2004 Jan;9(1):47-51. | PubMed |
Zone JJ, Egan CA, Taylor TB, Meyer LJ. IgA autoimmune disorders: development of a passive transfer mouse model. J Investig Dermatol Symp Proc. 2004 Jan;9(1):47-51. | PubMed | Caproni M, Rolfo S, Bernacchi E, Bianchi B, Brazzini B, Fabbri P. The role of lymphocytes, granulocytes, mast cells and their related cytokines in lesional skin of linear IgA bullous dermatosis. Br J Dermatol. 1999 Jun;140(6):1072-8. | PubMed |
Caproni M, Rolfo S, Bernacchi E, Bianchi B, Brazzini B, Fabbri P. The role of lymphocytes, granulocytes, mast cells and their related cytokines in lesional skin of linear IgA bullous dermatosis. Br J Dermatol. 1999 Jun;140(6):1072-8. | PubMed | Mintz EM, Morel KD. Clinical features, diagnosis, and pathogenesis of chronic bullous disease of childhood. Dermatol Clin. 2011 Jul;29(3):459-62, ix. | CrossRef | PubMed |
Mintz EM, Morel KD. Clinical features, diagnosis, and pathogenesis of chronic bullous disease of childhood. Dermatol Clin. 2011 Jul;29(3):459-62, ix. | CrossRef | PubMed | Weinberg MA, Insler MS, Campen RB. Mucocutaneous features of autoimmune blistering diseases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997 Nov;84(5):517-34. | PubMed |
Weinberg MA, Insler MS, Campen RB. Mucocutaneous features of autoimmune blistering diseases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997 Nov;84(5):517-34. | PubMed | Shimizu S, Natsuga K, Shinkuma S, Yasui C, Tsuchiya K, Shimizu H. Localized linear IgA/IgG bullous dermatosis. Acta Derm Venereol. 2010 Nov;90(6):621-4. | CrossRef | PubMed |
Shimizu S, Natsuga K, Shinkuma S, Yasui C, Tsuchiya K, Shimizu H. Localized linear IgA/IgG bullous dermatosis. Acta Derm Venereol. 2010 Nov;90(6):621-4. | CrossRef | PubMed | Burge S, Wojnarowska F, Marsden A. Chronic bullous dermatosis of childhood persisting into adulthood. Pediatr Dermatol. 1988 Nov;5(4):246-9. | PubMed |
Burge S, Wojnarowska F, Marsden A. Chronic bullous dermatosis of childhood persisting into adulthood. Pediatr Dermatol. 1988 Nov;5(4):246-9. | PubMed | Verma R, Vasudevan B, Pragasam V, Dabbas D. Linear IgA disease in an adult with unusual clinical features. Indian Dermatol Online J. 2013 Apr;4(2):115-8. | CrossRef | PubMed |
Verma R, Vasudevan B, Pragasam V, Dabbas D. Linear IgA disease in an adult with unusual clinical features. Indian Dermatol Online J. 2013 Apr;4(2):115-8. | CrossRef | PubMed | Aultbrinker EA, Starr MB, Donnenfeld ED. Linear IgA disease. The ocular manifestations. Ophthalmology. 1988 Mar;95(3):340-3. | PubMed |
Aultbrinker EA, Starr MB, Donnenfeld ED. Linear IgA disease. The ocular manifestations. Ophthalmology. 1988 Mar;95(3):340-3. | PubMed | Godfrey K, Wojnarowska F, Leonard J. Linear IgA disease of adults: association with lymphoproliferative malignancy and possible role of other triggering factors. Br J Dermatol. 1990 Oct;123(4):447-52. | PubMed |
Godfrey K, Wojnarowska F, Leonard J. Linear IgA disease of adults: association with lymphoproliferative malignancy and possible role of other triggering factors. Br J Dermatol. 1990 Oct;123(4):447-52. | PubMed | Girão L, Fiadeiro T, Rodrigues JC. Burn-induced linear IgA dermatosis. J Eur Acad Dermatol Venereol. 2000 Nov;14(6):507-10. | PubMed |
Girão L, Fiadeiro T, Rodrigues JC. Burn-induced linear IgA dermatosis. J Eur Acad Dermatol Venereol. 2000 Nov;14(6):507-10. | PubMed | Vargas TJ, Fialho M, Santos LT, Rodrigues PA, Vargas AL, Sousa MA. Linear IgA dermatosis associated with ulcerative colitis: complete and sustained remission after total colectomy. An Bras Dermatol. 2013 Jul-Aug;88(4):600-3. | CrossRef | PubMed |
Vargas TJ, Fialho M, Santos LT, Rodrigues PA, Vargas AL, Sousa MA. Linear IgA dermatosis associated with ulcerative colitis: complete and sustained remission after total colectomy. An Bras Dermatol. 2013 Jul-Aug;88(4):600-3. | CrossRef | PubMed | Collier PM, Kelly SE, Wojnarowska F. Linear IgA disease and pregnancy. J Am Acad Dermatol. 1994 Mar;30(3):407-11. | PubMed |
Collier PM, Kelly SE, Wojnarowska F. Linear IgA disease and pregnancy. J Am Acad Dermatol. 1994 Mar;30(3):407-11. | PubMed | Camilleri M, Pace JL. Drug-induced linear immunoglobulin-A bullous dermatosis. Clin Dermatol. 1998 May-Jun;16(3):389-91. | PubMed |
Camilleri M, Pace JL. Drug-induced linear immunoglobulin-A bullous dermatosis. Clin Dermatol. 1998 May-Jun;16(3):389-91. | PubMed | Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001 Nov-Dec;19(6):719-27. | PubMed |
Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001 Nov-Dec;19(6):719-27. | PubMed | Reyes-Baraona F, Andino R, Carrasco JE, Arriagada C, Guerrero S. [Linear IgA bullous dermatosis of childhood: case report]. Arch Argent Pediatr. 2014 Apr;112(2):e57-60. | CrossRef | PubMed |
Reyes-Baraona F, Andino R, Carrasco JE, Arriagada C, Guerrero S. [Linear IgA bullous dermatosis of childhood: case report]. Arch Argent Pediatr. 2014 Apr;112(2):e57-60. | CrossRef | PubMed | Wojnarowska F, Marsden RA, Bhogal B, Black MM. Chronic bullous disease of childhood, childhood cicatricial pemphigoid, and linear IgA disease of adults. A comparative study demonstrating clinical and immunopathologic overlap. J Am Acad Dermatol. 1988 Nov;19(5 Pt 1):792-805. | PubMed |
Wojnarowska F, Marsden RA, Bhogal B, Black MM. Chronic bullous disease of childhood, childhood cicatricial pemphigoid, and linear IgA disease of adults. A comparative study demonstrating clinical and immunopathologic overlap. J Am Acad Dermatol. 1988 Nov;19(5 Pt 1):792-805. | PubMed | Willsteed E, Bhogal BS, Black MM, McKee P, Wojnarowska F. Use of 1M NaCl split skin in the indirect immunofluorescence of the linear IgA bullous dermatoses. J Cutan Pathol. 1990 Jun;17(3):144-8. | PubMed |
Willsteed E, Bhogal BS, Black MM, McKee P, Wojnarowska F. Use of 1M NaCl split skin in the indirect immunofluorescence of the linear IgA bullous dermatoses. J Cutan Pathol. 1990 Jun;17(3):144-8. | PubMed | Kuechle MK, Stegemeir E, Maynard B, Gibson LE, Leiferman KM, Peters MS. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994 Feb;30(2 Pt 1):187-92. | PubMed |
Kuechle MK, Stegemeir E, Maynard B, Gibson LE, Leiferman KM, Peters MS. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994 Feb;30(2 Pt 1):187-92. | PubMed | Bhogal B, Wojnarowska F, Marsden RA, Das A, Black MM, McKee PH. Linear IgA bullous dermatosis of adults and children: an immunoelectron microscopic study. Br J Dermatol. 1987 Sep;117(3):289-96. | PubMed |
Bhogal B, Wojnarowska F, Marsden RA, Das A, Black MM, McKee PH. Linear IgA bullous dermatosis of adults and children: an immunoelectron microscopic study. Br J Dermatol. 1987 Sep;117(3):289-96. | PubMed | Allen J, Wojnarowska F. Linear IgA disease: the IgA and IgG response to the epidermal antigens demonstrates that intermolecular epitope spreading is associated with IgA rather than IgG antibodies, and is more common in adults. Br J Dermatol. 2003 Nov;149(5):977-85. | PubMed |
Allen J, Wojnarowska F. Linear IgA disease: the IgA and IgG response to the epidermal antigens demonstrates that intermolecular epitope spreading is associated with IgA rather than IgG antibodies, and is more common in adults. Br J Dermatol. 2003 Nov;149(5):977-85. | PubMed | Mintz EM, Morel KD. Treatment of chronic bullous disease of childhood. Dermatol Clin. 2011 Oct;29(4):699-700. | CrossRef | PubMed |
Mintz EM, Morel KD. Treatment of chronic bullous disease of childhood. Dermatol Clin. 2011 Oct;29(4):699-700. | CrossRef | PubMed | Wojnarowska F. Linear IgA dapsone responsive bullous dermatosis. J R Soc Med. 1980 May;73(5):371-3. | PubMed |
Wojnarowska F. Linear IgA dapsone responsive bullous dermatosis. J R Soc Med. 1980 May;73(5):371-3. | PubMed | McFadden JP, Leonard JN, Powles AV, Rutman AJ, Fry L. Sulphamethoxypyridazine for dermatitis herpetiformis, linear IgA disease and cicatricial pemphigoid. Br J Dermatol. 1989 Dec;121(6):759-62. | PubMed |
McFadden JP, Leonard JN, Powles AV, Rutman AJ, Fry L. Sulphamethoxypyridazine for dermatitis herpetiformis, linear IgA disease and cicatricial pemphigoid. Br J Dermatol. 1989 Dec;121(6):759-62. | PubMed | Mervic L, Dragos V, Pavlović MD. Linear IgA bullous dermatosis of childhood: successful treatment with miocamycin and topical corticosteroid. Clin Exp Dermatol. 2009 Oct;34(7):e391-2. | CrossRef | PubMed |
Mervic L, Dragos V, Pavlović MD. Linear IgA bullous dermatosis of childhood: successful treatment with miocamycin and topical corticosteroid. Clin Exp Dermatol. 2009 Oct;34(7):e391-2. | CrossRef | PubMed | Kasperkiewicz M, Schmidt E. Current treatment of autoimmune blistering diseases. Curr Drug Discov Technol. 2009 Dec;6(4):270-80. | PubMed |
Kasperkiewicz M, Schmidt E. Current treatment of autoimmune blistering diseases. Curr Drug Discov Technol. 2009 Dec;6(4):270-80. | PubMed | Alajlan A, Al-Khawajah M, Al-Sheikh O, Al-Saif F, Al-Rasheed S, Al-Hoqail I, et al. Treatment of linear IgA bullous dermatosis of childhood with flucloxacillin. J Am Acad Dermatol. 2006 Apr;54(4):652-6. | PubMed |
Alajlan A, Al-Khawajah M, Al-Sheikh O, Al-Saif F, Al-Rasheed S, Al-Hoqail I, et al. Treatment of linear IgA bullous dermatosis of childhood with flucloxacillin. J Am Acad Dermatol. 2006 Apr;54(4):652-6. | PubMed | Chaffins ML, Collison D, Fivenson DP. Treatment of pemphigus and linear IgA dermatosis with nicotinamide and tetracycline: a review of 13 cases. J Am Acad Dermatol. 1993 Jun;28(6):998-1000. | PubMed |
Chaffins ML, Collison D, Fivenson DP. Treatment of pemphigus and linear IgA dermatosis with nicotinamide and tetracycline: a review of 13 cases. J Am Acad Dermatol. 1993 Jun;28(6):998-1000. | PubMed | Peoples D, Fivenson DP. Linear IgA bullous dermatosis: successful treatment with tetracycline and nicotinamide. J Am Acad Dermatol. 1992 Mar;26(3 Pt2):498-9. | PubMed |
Peoples D, Fivenson DP. Linear IgA bullous dermatosis: successful treatment with tetracycline and nicotinamide. J Am Acad Dermatol. 1992 Mar;26(3 Pt2):498-9. | PubMed | Yomada M, Komai A, Hashimato T. Sublamina densa-type linear IgA bullous dermatosis successfully treated with oral tetracycline and niacianamide. Br J Dermatol. 1999 Sep;141(3):608-9. | PubMed |
Yomada M, Komai A, Hashimato T. Sublamina densa-type linear IgA bullous dermatosis successfully treated with oral tetracycline and niacianamide. Br J Dermatol. 1999 Sep;141(3):608-9. | PubMed | Khan IU, Bhol KC, Ahmed AR. Linear IgA bullous dermatosis in a patient with chronic renal failure: response to intravenous immunoglobulin therapy. J Am Acad Dermatol. 1999 Mar;40(3):485-8. | PubMed |
Khan IU, Bhol KC, Ahmed AR. Linear IgA bullous dermatosis in a patient with chronic renal failure: response to intravenous immunoglobulin therapy. J Am Acad Dermatol. 1999 Mar;40(3):485-8. | PubMed | Kroiss MM, Vogt T, Landthaler M, Stolz W. High-dose intravenous immune globulin is also effective in linear IgA disease. Br J Dermatol. 2000 Mar;142(3):582. Erratum in: Br J Dermatol 2000 Jun;142(6):1268. | PubMed |
Kroiss MM, Vogt T, Landthaler M, Stolz W. High-dose intravenous immune globulin is also effective in linear IgA disease. Br J Dermatol. 2000 Mar;142(3):582. Erratum in: Br J Dermatol 2000 Jun;142(6):1268. | PubMed | Letko E, Bhol K, Foster CS, Ahmed AR. Linear IgA bullous disease limited to the eye: a diagnostic dilemma: response to intravenous immunoglobulin therapy. Ophthalmology. 2000 Aug;107(8):1524-8. | PubMed |
Letko E, Bhol K, Foster CS, Ahmed AR. Linear IgA bullous disease limited to the eye: a diagnostic dilemma: response to intravenous immunoglobulin therapy. Ophthalmology. 2000 Aug;107(8):1524-8. | PubMed | Lozinski A, Baum S, Sagi L, Volkov A, Trau H, Barzilai A. [Rituximab (Mabthera) for treatment of rare autoimmune bullous skin disorders]. Harefuah. 2012 Oct;151(10):562-5, 606. | PubMed |
Lozinski A, Baum S, Sagi L, Volkov A, Trau H, Barzilai A. [Rituximab (Mabthera) for treatment of rare autoimmune bullous skin disorders]. Harefuah. 2012 Oct;151(10):562-5, 606. | PubMed | Talhari C, Althaus C, Megahed M. Ocular linear IgA disease resulting in blindness. Arch Dermatol. 2006 Jun;142(6):786-7. | PubMed |
Talhari C, Althaus C, Megahed M. Ocular linear IgA disease resulting in blindness. Arch Dermatol. 2006 Jun;142(6):786-7. | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








