
 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
INTRODUCTION
Aqueous shunt has emerged as an alternative technique to trabeculectomy, considered the standard for glaucoma surgery. Currently, it is mainly indicated after failure of trabeculectomy or in glaucoma with high risk of failure. The Ahmed valve and the Baerveldt implant are the most commonly used aqueous shunts. However, it is not clear whether there are differences between them.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified five systematic reviews including 10 studies overall, of which two were randomized trials. We concluded the Ahmed valve probably achieves a lower decrease in intraocular pressure, might lead to less qualified success and probably needs more reinterventions than the Baerveldt implant. Regarding safety profile, the Ahmed valve is not clearly superior or inferior to the Baerveldt implant.
According to the World Health Organization, glaucoma is the third cause of blindness worldwide [1]. Among the known risk factors for the development of this disease, the intraocular pressure is the only one that can be modified.
Since late 20th century, the introduction of aqueous shunts has emerged as an alternative surgery to trabeculectomy for patients with glaucoma and surgery indication. These devices are made up of a silicone tube with a lumen attached to an explant plate. The main indication of this technique is in those who, having an indication for surgical resolution, have failed to trabeculectomy or have subtypes of glaucoma in which this surgery has a high risk of failure. Currently, the most used techniques are the Ahmed valve and the Baerveldt implant. They differ in that the Ahmed valve has a restricting flow mechanism for preventing postoperative hypotony. However, it is not clear which one is the best alternative.
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found five systematic reviews [2],[3],[4],[5],[6] including 10 primary studies reported in 16 references [7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20], [21],[22] of which two corresponded to randomized trials, reported in eight references [7],[8],[10],[11],[12],[14],[15],[16]. |
|
What types of patients were included* |
Regarding type of glaucoma, one trial included patients with primary open-angle glaucoma, primary chronic closed-angle glaucoma, neovascular glaucoma and uveitic glaucoma [10] and one trial included patients with primary open-angle glaucoma, primary chronic closed-angle glaucoma, neovascular glaucoma, uveitic glaucoma, post-traumatic glaucoma, combined mechanism glaucoma, congenital glaucoma and penetrating keratoplasty associated glaucoma [14]. |
|
What types of interventions were included* |
Both trials compared the Ahmed FP7 valve versus the Baerveldt 350 mm2 implant and placed them in the superotemporal quadrant [10],[14]. |
|
What types of outcomes |
The trials measured multiple outcomes, which were grouped by the systematic reviews as follows:
The average follow-up of the trials was 33 months, with a range between 12 and 60 months. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
The information on the effects of the use of Ahmed valve compared to the use of Baerveldt implant is based on two randomized trials involving 514 eyes [10],[14].
Both trials reported mean intraocular pressure at the end of the follow-up (365 eyes) [10],[14], qualified success rate (443 eyes), change of visual acuity (364 eyes), need of reintervention (514 eyes), corneal edema (514 eyes), risk of narrow anterior chamber (514 eyes) and risk of choroidal effusion (514 eyes). One trial evaluated hypotonic maculopathy (276 eyes) [10].
The summary of findings is the following:
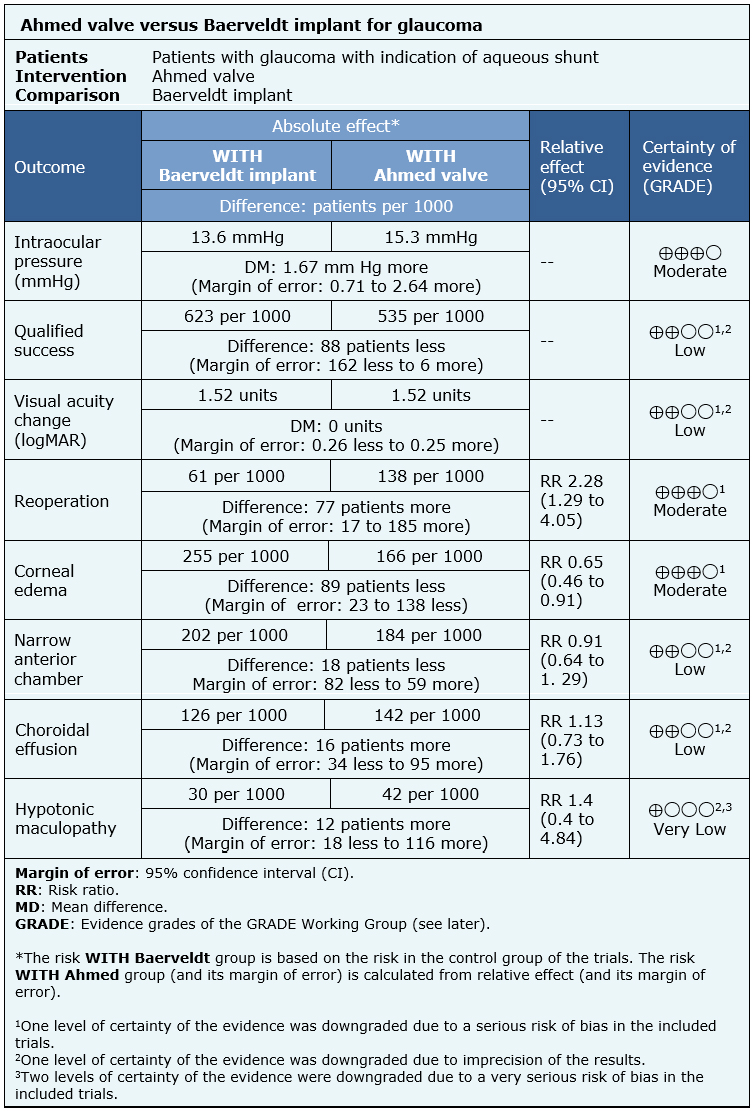
| Following the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
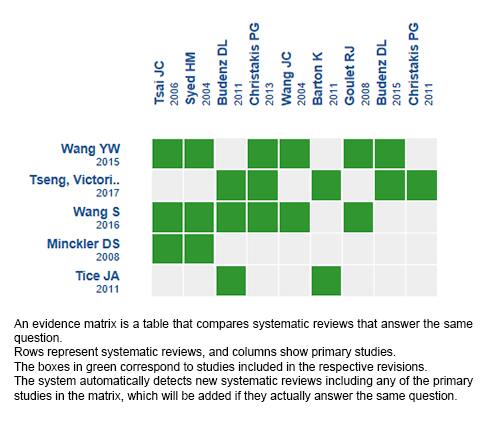
Follow the link to access the interactive version: Ahmed valve versus Baerveldt implant for glaucoma
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
Aqueous shunt has emerged as an alternative technique to trabeculectomy, considered the standard for glaucoma surgery. Currently, it is mainly indicated after failure of trabeculectomy or in glaucoma with high risk of failure. The Ahmed valve and the Baerveldt implant are the most commonly used aqueous shunts. However, it is not clear whether there are differences between them.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified five systematic reviews including 10 studies overall, of which two were randomized trials. We concluded the Ahmed valve probably achieves a lower decrease in intraocular pressure, might lead to less qualified success and probably needs more reinterventions than the Baerveldt implant. Regarding safety profile, the Ahmed valve is not clearly superior or inferior to the Baerveldt implant.
 Autores:
Eduardo Pimentel[1,2], Jimena Schmidt[2,3]
Autores:
Eduardo Pimentel[1,2], Jimena Schmidt[2,3]

Citación: Pimentel E, Schmidt J. Is Ahmed valve superior to Baerveldt implant as an aqueous shunt for the treatment of glaucoma?. Medwave 2018;18(5):e7238 doi: 10.5867/medwave.2018.05.7238
Fecha de envío: 29/11/2017
Fecha de aceptación: 29/12/2017
Fecha de publicación: 7/9/2018
Origen: Este artículo es producto del Epistemonikos Evidence Synthesis Project de la Fundación Epistemonikos, en colaboración con Medwave para su publicación.
Tipo de revisión: Con revisión por pares sin ciego por parte del equipo metodológico del Epistemonikos Evidence Synthesis Project.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Minckler DS, Francis BA, Hodapp EA, Jampel HD, Lin SC, Samples JR, Smith SD, Singh K. Aqueous shunts in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2008 Jun;115(6):1089-98. | CrossRef | PubMed |
Minckler DS, Francis BA, Hodapp EA, Jampel HD, Lin SC, Samples JR, Smith SD, Singh K. Aqueous shunts in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2008 Jun;115(6):1089-98. | CrossRef | PubMed | Tice JA. Aqueous shunts for the treatment of glaucoma. California Technology Assessment Forum (CTAF). 2011 Jun. | Link |
Tice JA. Aqueous shunts for the treatment of glaucoma. California Technology Assessment Forum (CTAF). 2011 Jun. | Link | Tseng VL, Coleman AL, Chang MY, Caprioli J. Aqueous shunts for glaucoma. Cochrane Database Syst Rev. 2017 Jul 28;7:CD004918. | CrossRef | PubMed | PMC |
Tseng VL, Coleman AL, Chang MY, Caprioli J. Aqueous shunts for glaucoma. Cochrane Database Syst Rev. 2017 Jul 28;7:CD004918. | CrossRef | PubMed | PMC | Wang YW, Wang PB, Zeng C, Xia XB. Comparison of the Ahmed glaucoma valve with the Baerveldt glaucoma implant: a meta-analysis. BMC Ophthalmol. 2015 Oct 13;15:132. | CrossRef | PubMed | PMC |
Wang YW, Wang PB, Zeng C, Xia XB. Comparison of the Ahmed glaucoma valve with the Baerveldt glaucoma implant: a meta-analysis. BMC Ophthalmol. 2015 Oct 13;15:132. | CrossRef | PubMed | PMC | Wang S, Gao X, Qian N. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma: a meta-analysis. BMC Ophthalmol. 2016 Jun 8;16:83. | CrossRef | PubMed | PMC |
Wang S, Gao X, Qian N. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma: a meta-analysis. BMC Ophthalmol. 2016 Jun 8;16:83. | CrossRef | PubMed | PMC | Barton K, Gedde SJ, Budenz DL, Feuer WJ, Schiffman J; Ahmed Baerveldt Comparison Study Group. The Ahmed Baerveldt Comparison Study methodology, baseline patient characteristics, and intraoperative complications. Ophthalmology. 2011 Mar;118(3):435-42. | CrossRef | PubMed | PMC |
Barton K, Gedde SJ, Budenz DL, Feuer WJ, Schiffman J; Ahmed Baerveldt Comparison Study Group. The Ahmed Baerveldt Comparison Study methodology, baseline patient characteristics, and intraoperative complications. Ophthalmology. 2011 Mar;118(3):435-42. | CrossRef | PubMed | PMC | Barton K, Feuer WJ, Budenz DL, Schiffman J, Costa VP, Godfrey DG, Buys YM; Ahmed Baerveldt Comparison Study Group. Three-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2014 Aug;121(8):1547-57.e1. | CrossRef | PubMed | PMC |
Barton K, Feuer WJ, Budenz DL, Schiffman J, Costa VP, Godfrey DG, Buys YM; Ahmed Baerveldt Comparison Study Group. Three-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2014 Aug;121(8):1547-57.e1. | CrossRef | PubMed | PMC | Beck AD, Freedman S, Kammer J, Jin J. Aqueous shunt devices compared with trabeculectomy with Mitomycin-C for children in the first two years of life. Am J Ophthalmol. 2003 Dec;136(6):994-1000. | PubMed |
Beck AD, Freedman S, Kammer J, Jin J. Aqueous shunt devices compared with trabeculectomy with Mitomycin-C for children in the first two years of life. Am J Ophthalmol. 2003 Dec;136(6):994-1000. | PubMed | Budenz DL, Barton K, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, Buys YM; Ahmed Baerveldt Comparison Study Group. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology. 2011 Mar;118(3):443-52. | CrossRef | PubMed | PMC |
Budenz DL, Barton K, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, Buys YM; Ahmed Baerveldt Comparison Study Group. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology. 2011 Mar;118(3):443-52. | CrossRef | PubMed | PMC | Budenz DL, Barton K, Gedde SJ, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, Buys YM; Ahmed Baerveldt Comparison Study Group. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015 Feb;122(2):308-16. | CrossRef | PubMed | PMC |
Budenz DL, Barton K, Gedde SJ, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, Buys YM; Ahmed Baerveldt Comparison Study Group. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015 Feb;122(2):308-16. | CrossRef | PubMed | PMC | Budenz DL, Feuer WJ, Barton K, Schiffman J, Costa VP, Godfrey DG, Buys YM; Ahmed Baerveldt Comparison Study Group. Postoperative Complications in the Ahmed Baerveldt Comparison Study During Five Years of Follow-up. Am J Ophthalmol. 2016 Mar;163:75-82.e3. | CrossRef | PubMed | PMC |
Budenz DL, Feuer WJ, Barton K, Schiffman J, Costa VP, Godfrey DG, Buys YM; Ahmed Baerveldt Comparison Study Group. Postoperative Complications in the Ahmed Baerveldt Comparison Study During Five Years of Follow-up. Am J Ophthalmol. 2016 Mar;163:75-82.e3. | CrossRef | PubMed | PMC | Chung AN, Aung T, Wang JC, Chew PT. Surgical outcomes of combined phacoemulsification and glaucoma drainage implant surgery for Asian patients with refractory glaucoma with cataract. Am J Ophthalmol. 2004 Feb;137(2):294-300. | PubMed |
Chung AN, Aung T, Wang JC, Chew PT. Surgical outcomes of combined phacoemulsification and glaucoma drainage implant surgery for Asian patients with refractory glaucoma with cataract. Am J Ophthalmol. 2004 Feb;137(2):294-300. | PubMed | Christakis PG, Kalenak JW, Zurakowski D, Tsai JC, Kammer JA, Harasymowycz PJ, Ahmed II. The Ahmed Versus Baerveldt study: one-year treatment outcomes. Ophthalmology. 2011 Nov;118(11):2180-9. | CrossRef | PubMed |
Christakis PG, Kalenak JW, Zurakowski D, Tsai JC, Kammer JA, Harasymowycz PJ, Ahmed II. The Ahmed Versus Baerveldt study: one-year treatment outcomes. Ophthalmology. 2011 Nov;118(11):2180-9. | CrossRef | PubMed | Christakis PG, Tsai JC, Zurakowski D, Kalenak JW, Cantor LB, Ahmed II. The Ahmed Versus Baerveldt study: design, baseline patient characteristics, and intraoperative complications. Ophthalmology. 2011 Nov;118(11):2172-9. | CrossRef | PubMed |
Christakis PG, Tsai JC, Zurakowski D, Kalenak JW, Cantor LB, Ahmed II. The Ahmed Versus Baerveldt study: design, baseline patient characteristics, and intraoperative complications. Ophthalmology. 2011 Nov;118(11):2172-9. | CrossRef | PubMed | Christakis PG, Tsai JC, Kalenak JW, Zurakowski D, Cantor LB, Kammer JA, Ahmed II. The Ahmed versus Baerveldt study: three-year treatment outcomes. Ophthalmology. 2013 Nov;120(11):2232-40. | CrossRef | PubMed |
Christakis PG, Tsai JC, Kalenak JW, Zurakowski D, Cantor LB, Kammer JA, Ahmed II. The Ahmed versus Baerveldt study: three-year treatment outcomes. Ophthalmology. 2013 Nov;120(11):2232-40. | CrossRef | PubMed | El Gendy NM, Song JC. Long term comparison between single stage Baerveldt and Ahmed glaucoma implants in pediatric glaucoma. Saudi J Ophthalmol. 2012 Jul;26(3):323-6. | CrossRef | PubMed | PMC |
El Gendy NM, Song JC. Long term comparison between single stage Baerveldt and Ahmed glaucoma implants in pediatric glaucoma. Saudi J Ophthalmol. 2012 Jul;26(3):323-6. | CrossRef | PubMed | PMC | Goulet RJ 3rd, Phan AD, Cantor LB, WuDunn D. Efficacy of the Ahmed S2 glaucoma valve compared with the Baerveldt 250-mm2 glaucoma implant. Ophthalmology. 2008 Jul;115(7):1141-7. | CrossRef | PubMed |
Goulet RJ 3rd, Phan AD, Cantor LB, WuDunn D. Efficacy of the Ahmed S2 glaucoma valve compared with the Baerveldt 250-mm2 glaucoma implant. Ophthalmology. 2008 Jul;115(7):1141-7. | CrossRef | PubMed | Syed HM, Law SK, Nam SH, Li G, Caprioli J, Coleman A. Baerveldt-350 implant versus Ahmed valve for refractory glaucoma: a case-controlled comparison. J Glaucoma. 2004;13:38-45. | Link |
Syed HM, Law SK, Nam SH, Li G, Caprioli J, Coleman A. Baerveldt-350 implant versus Ahmed valve for refractory glaucoma: a case-controlled comparison. J Glaucoma. 2004;13:38-45. | Link | Tesser R, Hess DB, Freedman SF. Combined intraocular lens implantation and glaucoma implant (tube shunt) surgery in pediatric patients: a case series. J AAPOS. 2005 Aug;9(4):330-5. | PubMed |
Tesser R, Hess DB, Freedman SF. Combined intraocular lens implantation and glaucoma implant (tube shunt) surgery in pediatric patients: a case series. J AAPOS. 2005 Aug;9(4):330-5. | PubMed | Tsai JC, Johnson CC, Kammer JA, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma II: longer-term outcomes from a single surgeon. Ophthalmology. 2006 Jun;113(6):913-7. | PubMed |
Tsai JC, Johnson CC, Kammer JA, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma II: longer-term outcomes from a single surgeon. Ophthalmology. 2006 Jun;113(6):913-7. | PubMed | Wang JC, See JL, Chew PT. Experience with the use of Baerveldt and Ahmed glaucoma drainage implants in an Asian population. Ophthalmology. 2004 Jul;111(7):1383-8. | PubMed |
Wang JC, See JL, Chew PT. Experience with the use of Baerveldt and Ahmed glaucoma drainage implants in an Asian population. Ophthalmology. 2004 Jul;111(7):1383-8. | PubMed | AOA. Care of the patient with open angle glaucoma. Optometric clinical practice guideline. 2011. | Link |
AOA. Care of the patient with open angle glaucoma. Optometric clinical practice guideline. 2011. | Link | ICO. Guidelines for glaucoma eye care. International council of ophthalmology guidelines. 2016. | Link |
ICO. Guidelines for glaucoma eye care. International council of ophthalmology guidelines. 2016. | Link |