
 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: KDIGO, Acute Kidney Injury, Biomarkers, Therapy
Acute kidney injury is a syndrome characterized by a sudden, sustained, and potentially reversible decrease in glomerular filtration rate and tubular function, which globally impacts renal function. It comprises of a series of events starting with the presence of risk factors, then evolving towards acute kidney injury progression, characterized by stress, injury, and renal failure, culminating with either the use of renal replacement therapy or death. Currently, the use of biomarkers that differentiate between the initial functional deterioration and late-onset structural damage of the kidney enables the clinician to perform an early diagnosis and indicate treatment before the stages of acute kidney injury progression are established, thus increasing survival rates.
Main messages
|
The natural progression of acute kidney injury (AKI) is defined by a continuum of events that begin with the presence of risk factors and continue with the phases of stress, kidney injury, and kidney failure[1]. The latter is characterized by a sudden, sustained, and potentially reversible decrease in glomerular filtration rate and tubular functions in less than 30 days, globally affecting renal function[2],[3]. Therefore, acute kidney injury can cause alterations in blood pressure homeostasis, platelet function, blood volume, fluid and electrolyte balance, acid-base balance, drug half-life, among others. Current studies show that the early presence of kidney damage can be sought-out through the use of biomarkers, introducing the concepts of stress and kidney injury as phases before failure.
The purpose of this review is to integrate the available scientific evidence that describes the previous phases of acute renal failure, reviewing in detail its possible causes, classifications, most current diagnostic methods, and generalities of the main current recommendations on its driving. For this, reviews were carried out in PubMed, ScienceDirect, and Google Scholar.
Acute renal failure has an incidence that varies between 15 and 35%, determined by the etiologic trigger[4]. These include shock and sepsis (50%), major surgery or trauma (between 25 and 35%), intraabdominal hypertension, nephrotoxic substances (less than 14%), among others[5],[6],[7]. A prospective multicentric study in Chile shows a similar frequency to that described in international literature, indicating sepsis (50.9%), ischemia (46.9%), surgery (26.3%), exogenous toxicity (24.5%), endogenous toxicity (11.4%), acute glomerular damage (6.1%) and obstructive uropathy (6.1%) as causes[8].
Acute kidney injury mortality varies between 35 and 60% in patients who require renal replacement therapy[4]. Therefore, even though a person can recover renal function after the kidney injury phase, there is still a higher risk of mortality or a greater risk of rehospitalization within two years[9], which is also a risk factor for chronic kidney disease. According to the classification criteria of the Acute Kidney Injury Network (AKIN), survival rates diminish depending on the state of severity and days of hospitalization. In patients without a diagnosis of acute renal failure, the survival rate is 95%, with AKIN 1 of 80%, AKIN 2 of 65%, and AKIN 3 of less than 60%[10]. Therefore, acute kidney injury is considered an indicator of severity and mortality that defines the prognostic of accompanying disease[11].
Currently, the classic etiologic classification for the differential diagnosis of acute kidney injury (table 1)[12], which is based on the location and frequency of presentation, is being replaced by observable patterns of kidney injury[13] based on the presence of biomarkers or the phenomena that overlap the pre-renal and parenchymal etiologies[14]. Evidence informing the use of blood urea nitrogen and creatine as an indicator of the etiology with a cut-off value of 20 has been questioned since it does not indicate with certainty the location of the structural damage[15], which therefore renders its use as ill-advised. Hence, before an increase in serum creatinine ensues, the availability of biomarkers has allowed the detection of subtle changes in renal function and evidence of damage[3].
Table 1. Etiologies according to anatomical location and overlapping causes.
Historically blood urea nitrogen and serum creatinine have been used as biomarkers of renal function. The latter increases once the glomerular filtration rate decreases more than 50%, with a kinetic that requires a period of 24 to 48 hours to evaluate changes[16], indicating the scarce correlation between serum creatinine and the progressive changes at the renal level, making it a late biomarker that underestimates the degree of renal failure at the beginning of the disease and overestimates the recovery[16].
Consequently, new biomarkers that screen for early onset of alterations to renal function have been investigated. Among these stand out NAG (N-Acetylglucosamine), GST (Glutathione-S-Transferase), L-FABP (Liver Fatty Acid Binding Protein), IL-18 (Interleukin 18), KIM-1 (Renal Injury Molecule), NGAL (Associated Lipocalin to Neutrophil Gelatinase, present in the renal tubule), C-C (Cystatin-C) y TIMP2-IGFBP7 (tissue inhibitor metalloproteinase-2, and insulin growth factor binding protein 7)[3],[17],[25]. These are classified as biomarkers of glomerular function (C-C) and tubular damage (NAG, GST, L-FABP, IL-18, KIM-1, NGAL), highlighting TIMP2-IGFBP7, because of their usefulness in the identification of renal stress (state prior to renal damage and highly susceptible to imminent damage)[25]. Although the pathophysiology and time of appearance of these biomarkers are still under study, it is estimated that IL-18 is released in the urine by proximal tubular cells four to six hours after a tubular injury (reflecting inflammatory activity). In comparison, KIM -1 is released between three to six hours after the onset of ischemia or nephrotoxicity, and NGAL is released three hours after the onset of damage (caused by neutrophils and renal tubular epithelial tissue)[3].
Through biomarkers of intrinsic damage, it is possible to detect kidney lesions before significant changes in the functional biomarkers occur(Figure 1); however, their use has not yet been standardized, given the limited development of guidelines, use, and monitoring[25]. Furthermore, there are no studies that determine the degree of sensitivity and specificity of these biomarkers for detecting kidney damage.
Figure 1. Classification according to biomarkers.
The biochemical analysis of urine provides information for the etiologic diagnosis that underlies acute kidney injury (table 1)[18]; however, its usefulness is controversial given the change in the pre-renal/renal paradigm[19] due to the appearance of biomarkers. Among the pathological findings that stand out are the following: presence of proteinuria and hematuria with red blood cell casts and dysmorphic erythrocytes (suggestive of glomerulonephritis), leukocyturia and aseptic pyuria (suggestive of interstitial nephritis), hemoglobinuria, and changes in urine color from black to pink (suggestive of kidney damage due to pigments or myoglobinuria) and tubular epithelial cell casts (suggestive of acute tubular necrosis).
Within the imaging modalities, renal ultrasound stands out for its easy accessibility, low cost, absence of adverse effects, and no exposure to radiation or IV contrast. Renal ultrasound provides useful information such as renal size (nine to 12 centimeters in length), cortex width (usually one centimeter), corticomedullary differentiation, echogenicity, pyelouretheral involvement, and vascularization[20]. Because recent-onset renal failure is potentially reversible, the purpose of using ultrasound is to identify patients in whom acute kidney failure could reverse or progress with further deterioration of the glomerular filtration rate.
Regarding renal size in acute renal failure, typically, it is normal; however, both acute tubular necrosis and interstitial edema can increase in dimensions. Although it is common to find a decreased renal size in chronic illness, there are chronic diseases in which there is a bilateral increase in renal size (diabetes, lymphomas, HIV nephropathies, multiple myeloma, and amyloidosis)[20].
Renal echogenicity is a subjective but useful ultrasound finding suggestive of underlying kidney disease. The normal echogenicity of the right kidney is equivalent or hypoechoic compared to the liver, while the left kidney is typically hypoechoic compared to the spleen. When cortical echogenicity is more pronounced than the liver, it is considered a reliable marker for renal dysfunction. Nonetheless, in acute kidney injury, the appearance varies depending on the etiology; for example, in pre-renal situations, cortical echogenicity is normal whereas in acute tubular necrosis, there is a more significant corticomedullary differentiation[20].
Assessment of the renal hilum may reveal hydronephrosis, often the consequence of obstructive (intrinsic or extrinsic) or non-obstructive uropathy (pregnancy, papillary necrosis). It is important to rule out any obstruction of the urinary tract in the presence of dilation of the renal pelvis (Table 1).
Depending on the classification system used, the diagnosis can be made according to the following: AKIN-KDIGO (Kidney Disease: Improving Global Outcomes) staging, etiology, temporality, or presence or absence of functional and kidney damage biomarkers (Table 2, Figure 1), which allows for an earlier diagnosis compared other classification systems. It is a dynamic process in which the patient can oscillate between one stage or another[3].
Table 2. Diagnosis of acute kidney injury.
The implementation of uniform management measurements has shown benefits in survival and hospitalization rates. Non-dialytic treatment and renal replacement therapy are among these management strategies.
1. Non-dialytic treatment
The treatment of underlying diseases and predisposing conditions is a vital pillar in the prevention and management of acute renal failure.
1.1. Volume expansion. A controlled resuscitation with crystalloids is recommended in case of volume depletion or as a prophylactic measure to prevent acute kidney failure associated with the use of drugs (see Table 1). By monitoring electrolyte levels and acid-base status, efforts should be made to avoid volume overload. Likewise, if intravascular contrast media is administered, the use of isotonic crystalloids is recommended. On the other hand, the use of serum albumin is reserved only for situations of septic shock, and the use of starch or dextrans is discouraged, given the evidence pointing to harmful effects[21].
1.2. Diuretics. Only recommended to control or avoid volume overload in patients who respond to diuretics[21].
1.3. Vasopressors. It is recommended to titrate vasopressors for a mean arterial pressure of 65 to 70 millimeters of hydrogen in septic shock, except if in the cases of previous chronic hypertension, where the target should be a mean arterial pressure (MAP) between 80 and 85. In case of hypotension, prefer the use of norepinephrine as the first choice or vasopressin in cases of vasoplegia[21].
1.4. Vasodilators: The use of dopamine, levosimendan, fenoldopam, or natriuretic peptides is not recommended for renal protection in critically ill patients as they may cause hypotension by counteracting compensatory vasoconstriction in occult hypovolemia[21].
1.5. Hormonal-metabolic. It is indicated to maintain glycemia between 110 and 149 micrograms per deciliter for the prevention of hyperglycemic kidney damage[22]. The use of selenium-IV, erythropoietin, or steroids is not recommended to prevent acute kidney injury, given its lack of benefit. The use of N-acetylcysteine in the prevention of contrast-associated acute kidney injury is also not recommended in critically ill patients due to conflicting results and possible adverse effects. The short-term use of atorvastatin or rosuvastatin to prevent contrast-associated acute renal failure is recommended in high-risk patients undergoing coronary angiography, as well as the perioperative use of high-dose statins to prevent postoperative acute kidney failure in cardiac surgery[21].
1.6. Nutritional. Nutrition should not be stopped in a patient with acute kidney injury, thus maintaining adequate nutritional support, preferably through the enteral route. A provision of 20 to 30 kilocalories per kilogram per day, and a protein contribution of 0.8 to one gram per kilogram per day (between 1 and 1.5 in renal replacement therapy, maximum 1.7 in hypercatabolic patients) is recommended. In cases of uremia, renal replacement therapy could be an option to restore nutrition as soon as possible, therefore preventing a uremic exacerbation[22].
2. Renal replacement therapy
Renal replacement therapy includes intermittent dialysis, continuous dialysis, or hybrid therapies. Each modality has its established protocols. However, there are still doubts about how to measure the quality of renal replacement therapy delivery, so the use of one is not preferred over the other[23].
The criteria for starting renal replacement therapy in acute kidney injury are oliguria (less than 200 milliliters in 12 hours) or anuria (less than 50 milliliters in 12 hours), hyperkalemia (greater than 6.5 microequivalents per liter), hypernatremia (greater than 155 microequivalents per liter) and hyponatremia (less than 120 microequivalents per liter) refractory to treatment; severe acidemia (pH less than 7), azotemia (blood urea nitrogen greater than 73 micrograms per deciliter or urea greater than 30 micrograms per deciliter), uremic complications (encephalopathy, neuropathy, myopathy, uremic pericarditis), hyperthermia and dialyzable drug overdose[24].
A Cochrane review including five low-quality randomized studies with 1084 patients compared standard initiation versus early initiation of renal replacement therapy[25]. The outcomes were reduced risk of death, increased recovery of kidney function, or an increased risk for adverse events in patients with severe acute kidney injury[25]. Although early initiation of renal replacement therapy has shown to reduce the risk of death and improve recovery of renal function, it also increases the risk of adverse events that worsen these results. Regarding mortality, the evidence did not show different patterns in the short or long term—given that there are results that show an increase and a decrease in mortality, both of little significance. Therefore, there is a need for additional studies with appropriate criteria to reduce the ambiguity of the results[26].
3. Follow-up
Follow-up with serial creatinine levels has the disadvantage of showing kidney function 48 hours before drawing the blood sample[16]. However, a declining creatinine curve could elucidate a pattern that warrants patient discharge, thus continuing with outpatient visits. In chronic dialysis patients, creatinine is monitored at least every three months, while urea nitrogen is monitored monthly.
The definition of acute kidney injury still tries to clarify concepts that allow an early diagnosis over an etiological diagnosis. Thus, the use of biomarkers is being introduced to establish the degree of renal involvement in conjunction with the current AKIN-KDIGO staging system. Despite this, more evidence is needed to expand its use in clinical practice. Consequently, management continues to focus on preventive measures and essential support to avoid the requirement for renal replacement therapy, which, although it may be beneficial in the recovery of kidney function and reducing the risk of death, has a negative impact by increasing the risk of adverse events. Likewise, it is unclear whether its use increases or reduces mortality.
Notes
Authorship contributions
BAR: investigation, conceptualization, writing of the article, critical review of its intellectual aspects and editing, general supervision, and final approval of the full version. MMR: investigation, writing of the article, critical review of its intellectual aspects. JWI, BLP, JVU: writing of the article and editing.
Funding
The authors declare that there were no external sources of funding.
Competing interests
The authors have completed the declaration of conflicts of interest form of the ICMJE, and declare not having received financing for the realization of the report; not having financial relationships with organizations that could have interests in the published article in the last three years; and not having other relationships or activities that could influence the published article. The forms can be requested by contacting the responsible author or the editorial direction of the Journal.
From the editors
The original version of this manuscript was submitted in Spanish and was the peer-reviewed version. This English translation was submitted by the authors and has been lightly copyedited by the Journal.

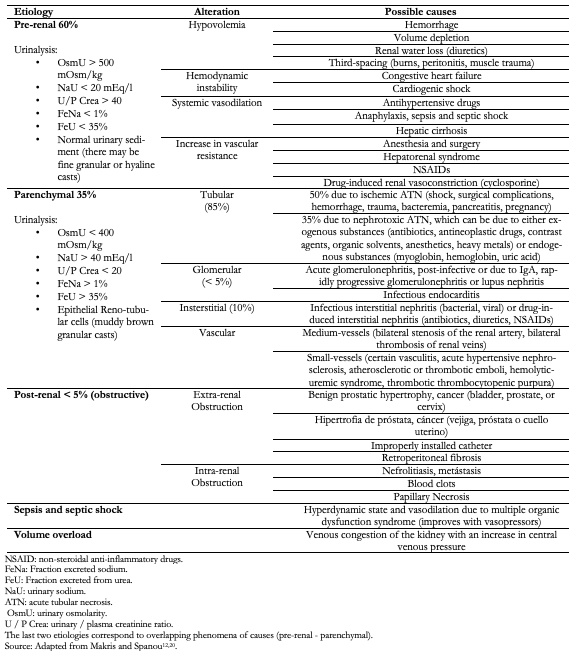 Table 1. Etiologies according to anatomical location and overlapping causes.
Table 1. Etiologies according to anatomical location and overlapping causes.

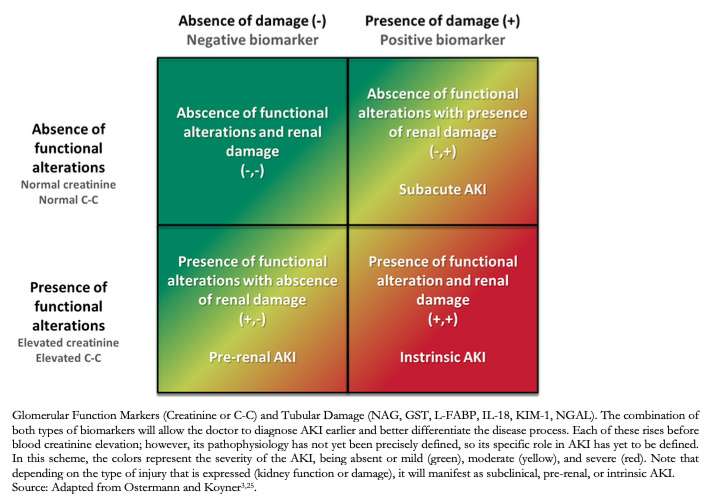 Figure 1. Classification according to biomarkers.
Figure 1. Classification according to biomarkers.

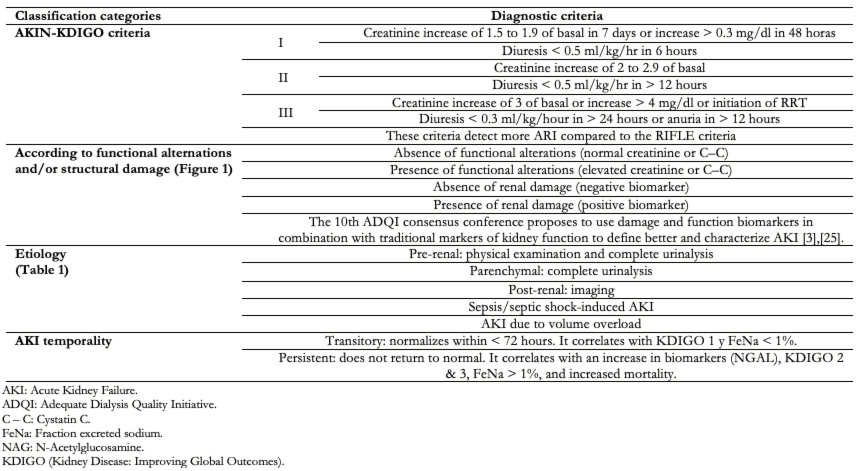 Table 2. Diagnosis of acute kidney injury.
Table 2. Diagnosis of acute kidney injury.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Acute kidney injury is a syndrome characterized by a sudden, sustained, and potentially reversible decrease in glomerular filtration rate and tubular function, which globally impacts renal function. It comprises of a series of events starting with the presence of risk factors, then evolving towards acute kidney injury progression, characterized by stress, injury, and renal failure, culminating with either the use of renal replacement therapy or death. Currently, the use of biomarkers that differentiate between the initial functional deterioration and late-onset structural damage of the kidney enables the clinician to perform an early diagnosis and indicate treatment before the stages of acute kidney injury progression are established, thus increasing survival rates.
 Autores:
Bastian Abarca Rozas[1], Manuel Mestas Rodríguez[2], Jorge Widerström Isea[1], Beatriz Lobos Pareja [3], Jocelyn Vargas Urra [4]
Autores:
Bastian Abarca Rozas[1], Manuel Mestas Rodríguez[2], Jorge Widerström Isea[1], Beatriz Lobos Pareja [3], Jocelyn Vargas Urra [4]

Citación: Abarca Rozas B, Mestas Rodríguez M, Widerström Isea J, Lobos Pareja B, Vargas Urra J. A current view on the early diagnosis and treatment of acute kidney failure. Medwave 2020;20(5):e7928 doi: 10.5867/medwave.2020.05.7928
Fecha de envío: 30/10/2019
Fecha de aceptación: 18/5/2020
Fecha de publicación: 9/6/2020
Origen: No solicitado.
Tipo de revisión: Con revisión por pares externa, por dos árbitros a doble ciego.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Murray PT, Devarajan P, Levey AS, Eckardt KU, Bonventre JV, Lombardi R,
et al. A framework and key research questions in AKI
diagnosis and staging in different environments. Clin J Am Soc Nephrol. 2008
May;3(3):864-8. | CrossRef | PubMed |
Murray PT, Devarajan P, Levey AS, Eckardt KU, Bonventre JV, Lombardi R,
et al. A framework and key research questions in AKI
diagnosis and staging in different environments. Clin J Am Soc Nephrol. 2008
May;3(3):864-8. | CrossRef | PubMed | Mehta RL, Chertow GM. Acute renal failure definitions and classification:
time for change? J Am Soc Nephrol. 2003 Aug;14(8):2178-87. | CrossRef | PubMed |
Mehta RL, Chertow GM. Acute renal failure definitions and classification:
time for change? J Am Soc Nephrol. 2003 Aug;14(8):2178-87. | CrossRef | PubMed | Ostermann M, Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic
workup. Crit Care. 2016 Sep 27;20(1):299. | CrossRef | PubMed |
Ostermann M, Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic
workup. Crit Care. 2016 Sep 27;20(1):299. | CrossRef | PubMed | Negi S, Koreeda D, Kobayashi S, Yano T, Tatsuta K, Mima T, et al. Acute kidney injury: Epidemiology, outcomes, complications, and therapeutic
strategies. Semin Dial. 2018 Sep;31(5):519-527. | CrossRef | PubMed |
Negi S, Koreeda D, Kobayashi S, Yano T, Tatsuta K, Mima T, et al. Acute kidney injury: Epidemiology, outcomes, complications, and therapeutic
strategies. Semin Dial. 2018 Sep;31(5):519-527. | CrossRef | PubMed | Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal
failure in critically ill patients: a multinational, multicenter study. JAMA.
2005 Aug 17;294(7):813-8. | CrossRef | PubMed |
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal
failure in critically ill patients: a multinational, multicenter study. JAMA.
2005 Aug 17;294(7):813-8. | CrossRef | PubMed | de Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M,
et al. Acute renal failure in the ICU: risk factors
and outcome evaluated by the SOFA score. Intensive Care Med. 2000
Jul;26(7):915-21. | CrossRef | PubMed |
de Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M,
et al. Acute renal failure in the ICU: risk factors
and outcome evaluated by the SOFA score. Intensive Care Med. 2000
Jul;26(7):915-21. | CrossRef | PubMed | Guerin C, Girard R, Selli JM, Perdrix JP, Ayzac L. Initial versus delayed
acute renal failure in the intensive care unit. A multicenter prospective
epidemiological study. Rhône-Alpes Area Study Group on Acute Renal Failure. Am J
Respir Crit Care Med. 2000 Mar;161(3 Pt 1):872-9. | CrossRef | PubMed |
Guerin C, Girard R, Selli JM, Perdrix JP, Ayzac L. Initial versus delayed
acute renal failure in the intensive care unit. A multicenter prospective
epidemiological study. Rhône-Alpes Area Study Group on Acute Renal Failure. Am J
Respir Crit Care Med. 2000 Mar;161(3 Pt 1):872-9. | CrossRef | PubMed | Vukusich A, Alvear F, Villanueva P, González C, Francisco O, Alvarado N,
et al. Epidemiología de la insuficiencia renal aguda grave. Un estudio
prospectivo multicéntrico en la Región Metropolitana [Epidemiology of severe
acute renal failure in Metropolitan Santiago]. Rev Med Chil. 2004
Nov;132(11):1355-61. | PubMed |
Vukusich A, Alvear F, Villanueva P, González C, Francisco O, Alvarado N,
et al. Epidemiología de la insuficiencia renal aguda grave. Un estudio
prospectivo multicéntrico en la Región Metropolitana [Epidemiology of severe
acute renal failure in Metropolitan Santiago]. Rev Med Chil. 2004
Nov;132(11):1355-61. | PubMed | Rydingsward JE, Horkan CM, Mogensen KM, Quraishi SA, Amrein K, Christopher
KB. Functional Status in ICU Survivors and Out of Hospital Outcomes: A Cohort
Study. Crit Care Med. 2016 May;44(5):869-79. | CrossRef | PubMed |
Rydingsward JE, Horkan CM, Mogensen KM, Quraishi SA, Amrein K, Christopher
KB. Functional Status in ICU Survivors and Out of Hospital Outcomes: A Cohort
Study. Crit Care Med. 2016 May;44(5):869-79. | CrossRef | PubMed | Wang HE, Jain G, Glassock RJ, Warnock DG. Comparison of absolute serum
creatinine changes versus Kidney Disease: Improving Global Outcomes consensus
definitions for characterizing stages of acute kidney injury. Nephrol Dial
Transplant. 2013 Jun;28(6):1447-54. | CrossRef | PubMed |
Wang HE, Jain G, Glassock RJ, Warnock DG. Comparison of absolute serum
creatinine changes versus Kidney Disease: Improving Global Outcomes consensus
definitions for characterizing stages of acute kidney injury. Nephrol Dial
Transplant. 2013 Jun;28(6):1447-54. | CrossRef | PubMed | Singbartl K, Joannidis M. Short-term Effects of Acute Kidney Injury. Crit
Care Clin. 2015 Oct;31(4):751-62. | CrossRef | PubMed |
Singbartl K, Joannidis M. Short-term Effects of Acute Kidney Injury. Crit
Care Clin. 2015 Oct;31(4):751-62. | CrossRef | PubMed | Makris K, Spanou L. Acute Kidney Injury: Definition, Pathophysiology and
Clinical Phenotypes. Clin Biochem Rev. 2016 May;37(2):85-98. | PubMed |
Makris K, Spanou L. Acute Kidney Injury: Definition, Pathophysiology and
Clinical Phenotypes. Clin Biochem Rev. 2016 May;37(2):85-98. | PubMed | Chen H, Busse LW. Novel Therapies for Acute Kidney Injury. Kidney Int Rep.
2017 Jun 28;2(5):785-799. | CrossRef | PubMed |
Chen H, Busse LW. Novel Therapies for Acute Kidney Injury. Kidney Int Rep.
2017 Jun 28;2(5):785-799. | CrossRef | PubMed | Endre ZH, Kellum JA, Di Somma S, Doi K, Goldstein SL, Koyner JL, et al. Differential diagnosis of AKI in clinical practice by
functional and damage biomarkers: workgroup statements from the tenth Acute
Dialysis Quality Initiative Consensus Conference. Contrib Nephrol.
2013;182:30-44. | CrossRef | PubMed |
Endre ZH, Kellum JA, Di Somma S, Doi K, Goldstein SL, Koyner JL, et al. Differential diagnosis of AKI in clinical practice by
functional and damage biomarkers: workgroup statements from the tenth Acute
Dialysis Quality Initiative Consensus Conference. Contrib Nephrol.
2013;182:30-44. | CrossRef | PubMed | Rachoin JS, Daher R, Moussallem C, Milcarek B, Hunter K, Schorr C, et al. The fallacy of the BUN:creatinine ratio in critically ill
patients. Nephrol Dial Transplant. 2012 Jun;27(6):2248-54. | CrossRef | PubMed |
Rachoin JS, Daher R, Moussallem C, Milcarek B, Hunter K, Schorr C, et al. The fallacy of the BUN:creatinine ratio in critically ill
patients. Nephrol Dial Transplant. 2012 Jun;27(6):2248-54. | CrossRef | PubMed | Huidobro E JP, Tagle R, Guzmán AM. Creatinina y su uso para la estimación de
la velocidad de filtración glomerular [Estimation of glomerular filtration rate
with creatinine]. Rev Med Chil. 2018 Mar;146(3):344-350. | CrossRef | PubMed |
Huidobro E JP, Tagle R, Guzmán AM. Creatinina y su uso para la estimación de
la velocidad de filtración glomerular [Estimation of glomerular filtration rate
with creatinine]. Rev Med Chil. 2018 Mar;146(3):344-350. | CrossRef | PubMed | Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising
biomarker for human acute kidney injury. Biomark Med. 2010 Apr;4(2):265-80. | CrossRef | PubMed |
Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising
biomarker for human acute kidney injury. Biomark Med. 2010 Apr;4(2):265-80. | CrossRef | PubMed | Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions,
diagnosis, pathogenesis, and therapy. J Clin Invest. 2004 Jul;114(1):5-14. | CrossRef | PubMed |
Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions,
diagnosis, pathogenesis, and therapy. J Clin Invest. 2004 Jul;114(1):5-14. | CrossRef | PubMed | Maciel AT, Vitorio D. Urine biochemistry assessment in critically ill
patients: controversies and future perspectives. J Clin Monit Comput. 2017
Jun;31(3):539-546. | CrossRef | PubMed |
Maciel AT, Vitorio D. Urine biochemistry assessment in critically ill
patients: controversies and future perspectives. J Clin Monit Comput. 2017
Jun;31(3):539-546. | CrossRef | PubMed | Kelahan LC, Desser TS, Troxell ML, Kamaya A. Ultrasound Assessment of Acute
Kidney Injury. Ultrasound Q. 2019 Jun;35(2):173-180. | CrossRef | PubMed |
Kelahan LC, Desser TS, Troxell ML, Kamaya A. Ultrasound Assessment of Acute
Kidney Injury. Ultrasound Q. 2019 Jun;35(2):173-180. | CrossRef | PubMed | Joannidis M, Druml W, Forni LG, Groeneveld ABJ, Honore PM, Hoste E,
et al. Prevention of acute kidney
injury and protection of renal function in the intensive care unit: update 2017
: Expert opinion of the Working Group on Prevention, AKI section, European
Society of Intensive Care Medicine. Intensive Care Med. 2017 Jun;43(6):730-749. | CrossRef | PubMed |
Joannidis M, Druml W, Forni LG, Groeneveld ABJ, Honore PM, Hoste E,
et al. Prevention of acute kidney
injury and protection of renal function in the intensive care unit: update 2017
: Expert opinion of the Working Group on Prevention, AKI section, European
Society of Intensive Care Medicine. Intensive Care Med. 2017 Jun;43(6):730-749. | CrossRef | PubMed | Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter. 2012;2:1–138. [Internet] | Link |
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter. 2012;2:1–138. [Internet] | Link | Rewa O, Mottes T, Bagshaw SM. Quality measures for acute kidney injury and
continuous renal replacement therapy. Curr Opin Crit Care. 2015 Dec;21(6):490-9. | CrossRef | PubMed |
Rewa O, Mottes T, Bagshaw SM. Quality measures for acute kidney injury and
continuous renal replacement therapy. Curr Opin Crit Care. 2015 Dec;21(6):490-9. | CrossRef | PubMed | Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005 Jan
29-Feb 4;365(9457):417-30. | CrossRef | PubMed |
Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005 Jan
29-Feb 4;365(9457):417-30. | CrossRef | PubMed |