Key Words: vasovagal syncope, orthostatic intolerance, autonomic nervous system, beta-adrenergic blockers, norepinephrine transporter antagonist, Mexico
Abstract
Vasovagal or neurocardiogenic syncope is a common clinical situation and, as with other entities associated with orthostatic intolerance, the underlying condition is a dysfunction of the autonomic nervous system. This article reviews various aspects of vasovagal syncope, including its relationship with orthostatic intolerance and the role of the autonomic nervous system in it. A brief history of the problem is given, as well as a description of how the names and associated concepts have evolved. The response of the sympathetic system to orthostatic stress, the physiology of the baroreflex system and the neurohumoral changes that occur with standing are analyzed. Evidence is presented of the involvement of the autonomic nervous system, including studies of heart rate variability, microneurography, cardiac innervation, and molecular genetic studies. Finally, we describe different studies on the use of beta-blockers and norepinephrine transporter inhibitors (sibutramine, reboxetine) and the rationality of their use to prevent this type of syncope.
Introduction
Orthostatic intolerance is defined as the inability to tolerate standing position manifested by the emergence or exacerbation of signs and symptoms secondary to cerebral hypoperfusion, which improves by returning to supine position [1]. When supine position is not acquired immediately, or sudden arterial hypotension is installed, a feeling of fainting (pre-syncope) may occur or even fainting itself. In both events, a typical fast recovery of the alert state occurs after lying down. In some individuals, chronic orthostatic intolerance can also develop, manifested as dizziness, blurred vision, memory loss, difficulty in reasoning or concentrating, headache, fatigue, weakness, heat sensation, nausea, abdominal pain, sweating, coldness of hands and feet, tremors, and even exercise intolerance. Orthostatic intolerance can also be manifested as palpitations when adopting standing position, due to sympathetic hyperactivity, which constitutes postural orthostatic tachycardia syndrome. Vasovagal syncope is a type of orthostatic intolerance. This term is used to refer to a syncope that results from reflex mechanisms associated with inappropriate vasodilation and variable degrees of bradycardia. It is also named reflex syncope, neurocardiogenic syncope or neurally mediated syncope.
Vasovagal syncope
Syncope is defined as a sudden temporary loss of consciousness and postural tone, as a result of a transitory decrease in global cerebral perfusion, with sudden onset, short duration and complete recovery [2]. Syncope is a significant clinical problem, responsible for 1% of hospital admissions and 3% of the visits to the emergency departments, with a morbidity and mortality superior to 7% [3],[4]. Syncope is a manifestation of different processes and therefore it should be regarded as a symptom, not a disease, and it should be classified according to the underlying cause. It is always important, especially in elderly patients, to rule out other causes of syncope, such as carotid occlusive disease, carotid sinus hypersensitivity or neurogenic orthostatic hypotension (autonomic neuropathy) [5].
Almost 40% of the general population suffers from at least one episode of syncope during lifetime [6]. Vasovagal syncope is the most common type of syncope. Its peak incidence is during adolescence. The majority of patients with vasovagal syncope are young women between 15 and 45 years old. It can be the result of a stressful emotion (which acts as a trigger) or a change of posture, like the sudden adoption of a standing position. A sensation of dizziness, weakness, nausea, sweating, and visual disturbances usually precede vasovagal syncope. It is interesting to note that vasovagal syncope, postural orthostatic tachycardia syndrome, chronic fatigue syndrome, and fibromyalgia, have a common underlying cause: a dysfunction of the autonomic nervous system, and therefore they are grouped in one term: “dysautonomia” [7],[8]. The autonomic nervous system regulates physiological functions that do not depend on consciousness (digestion, heart rate, blood pressure, and etcetera). It consists of two subsystems: the sympathetic and the parasympathetic system (or vagal). The first one stimulates neurovegetative functions while the second one depresses them. They are traditionally regarded as antagonistic systems and they are normally in equilibrium: if one is active, the other one is inhibited to compensate. When this balance is lost, different symptoms could appear including dyspnea, tachycardia, fatigue, paresthesia, dizziness, muscle pain, irritable bowel syndrome, chest pain, anxiety, depression and/or syncope.
The recurrence of syncope and the presence of clinical manifestations of orthostatic intolerance (fatigue, dizziness, and others) are of great importance and magnitude because they can lead to disability [9]; therefore any attempt to diminish or avoid them must be carefully taken into consideration.
Historical background
The first citation of vagotony as a clinical entity corresponds to the Viennese doctors Eppinger and Hess, who published the book “Die Vagotonie” [10] in 1910. In their book, they mentioned that sinus bradycardia could be controlled, albeit temporarily, through the administration of a vagolytic substance such as atropine. Later, many researchers continued the efforts to define vagotony in a more precise way. For an excellent historical overview on this topic, including an exhaustive analysis of the medical thesis of Prof. Salvador Zubirán ("Vagotonia", published in Mexico City in 1923) we refer the reader to the article by doctors Delgado and Estañol [11].
The term "vasovagal syncope" was introduced by Sir Thomas Lewis [12] but Da Costa had previously observed similar clinical manifestations in soldiers who fought in the Civil War of the United States of America [13] and he called it "irritable heart". Since then, medically unexplained symptoms arising in soldiers during times of conflict have been given many generic terms, and even as recent as the 1990’s, they have been grouped as “war syndromes” or “post-combat disorders”. For a complete review of these disorders we refer the reader to the work of Jones [14]. In short, during World War I, British doctors found similar clinical manifestations in soldiers and called them "Disorderly action of the heart"; Lewis called them "Heart of the soldier" or "Effort syndrome" and Levine gave them a more scientific name: "Neurocirculatory asthenia" [15]. Professor Ignacio Chávez, in 1933, in his book "Five Lessons of Clinical Cardiology", after having studied a patient with syncope, reported: "since she is complaining of many anatomical sites at the same time and with great intensity, it is very likely that she does not have lesions in any of them, and the disease comes from the neurovegetative system" [16]. During World War II, it was noted again that in response to stress during combat, somatic symptoms appeared in soldiers, including fatigue, palpitations, headaches, diarrhea, difficulty in concentration and sleep disturbances. Initially named “war neurosis”, the use of such term was later discouraged as it implied that the soldiers were sick, which put pressure on military to take responsibility of them as patients [12]. In 1941 Wood [17] attributed the symptoms to a form of psychoneurosis, and in the third edition (1968) of his book he included it among the cardiovascular manifestations of psychopathies. The interrelation of psychological aspects and vasovagal syncope is now well documented [18],[19]. The most recent findings related to post-combat disorders are those from the Gulf War (1990-1991). Some reports confirm that an autonomic nervous system dysfunction may be present in Gulf War veterans with chronic fatigue [20],[21],[22].
Pathophysiology of vasovagal syncope
Baseline autonomic tone in vasovagal syncope
Controversial results exist regarding data differences in baseline parameters of autonomic tone between syncopal patients and controls (healthy individuals). Table 1 [23],[24],[25],[26],[27] shows a list of studies which include time- and frequency-domain, heart rate variability and microneurography (muscle sympathetic nerve activity). The majority have failed to demonstrate a difference in the mean values of heart rate variability indexes between normal subjects and patients with vasovagal syncope. Only Shim et al. [27] have found evidence for an increased baseline vagal tone in children with vasovagal syncope. Guzmán et al. [24] reported an interesting observation: they found a decreased root-mean-square deviation of R-R intervals (RMSD) and a higher low frequency/high frequency ratio (LF/HF ratio) in subjects with a positive head-up tilt test with a vasodepressor response in comparison with those with a cardioinhibitory or mixed response. This finding could imply an increased sympathetic tone in the vasodepressor group although other researchers have not replicated it. Recently, Efremov et al. [26] reported that heart rate variability analysis within the first 20 minutes of passive tilting, demonstrated that a progressive decrement of parasympathetic activity characterizes patients with nitroglycerine-induced syncope, a fact that does not occur in patients with a negative response to nitroglycerine. In patients who were positive to the head-up tilt test, two heart rate variability spectral parameters: heart frequency and total power had a significant decrement from passive tilt to nitroglycerine tilt, while in negative subjects the average heart frequency and total power values did not change. We are in complete agreement with Efremov et al. that “further research is needed to evaluate whether or not appropriately timed heart rate variability analysis might improve the differentiation among different mechanisms of syncope”.
Table 1. Studies on baseline autonomic tone in vasovagal syncope
Neurohumoral response to standing
In humans, maintaining an adequate blood supply to vital organs when standing defying gravity constitutes an important physiological challenge. Standing should be considered an effort for our circulation regulatory capacities; it is required that the structure and good function of the heart, blood vessels, and the autonomic nervous system are intact. An adequate blood volume and the ability of the skeletal muscles of the legs to compress veins and facilitate blood return to the heart are also relevant. The change from supine to orthostatic position results in the accumulation of 300 to 800 mL of blood in the lower extremities and splanchnic circulation causing central hypovolemia, due to a decrease of the venous return to the heart with concomitant reduction of left ventricular filling (preload), with transient fall of cardiac output and blood pressure. For a better understanding of the baroreceptor reflex, we refer the reader to an excellent article in Spanish published by Estañol et al. [28] in 2011. In brief, in first place mechanoreceptors (or baroreceptors, which detect changes in blood pressure), and to a lesser extent chemoreceptors, regulate the activity of the sympathetic nervous system. Arterial baroreceptors (high-pressure receptors) are located in the carotid sinus and aortic arch, and cardiopulmonary baroreceptors (low-pressure receptors) in the large veins, auricles (receptors A and B, Bainbridge reflex [an increase in pressure or an increased distension of the right atrium causes acceleration of the heartbeat, also called “Bainbridge effect”]), and ventricles (Bezold-Jarisch reflex). In these mechanoreceptors, ion channels are activated by stretching and they modulate the efferent sympathetic activity sending afferent information to the nucleus of the solitary tract (tractus solitarius) [29].
Baroreceptors respond to the decrease in venous return and blood pressure that occur when assuming an orthostatic position, with an increase of efferent sympathetic activity and a decrease in parasympathetic tone, resulting in an increase in the heart rate and inotropism, and peripheral arterial vasoconstriction. Therefore, the transient central hypovolemia with concomitant transient fall of cardiac output results in lower distension of the baroreceptors of the carotid sinus and aortic arch, with reduction of afferent baroreflex activity to the brain stem. Consequently, an efferent sympathetic activation and parasympathetic inhibition occur that finally increase heart rate and peripheral vascular resistance, in an attempt, generally successful, to restore cardiac output and blood pressure. To help minimize the orthostatic blood pressure reduction, an increase in reabsorption of fluids associated with the increase in total peripheral resistances has been described [30],[31]. After recovery from the initial fall of blood pressure during orthostatism, blood volume decreases slowly due to microvascular filtration.
In normal conditions, orthostatic stress evokes compensatory vasoconstriction in multiple vascular beds including skeletal muscles. Muscle sympathetic nerve activity (MSNA) could be registered in humans by microneurography. This is a direct assessment of sympathetic nerve activity in conscious human beings [32] tightly linked to blood pressure via the baroreflex mechanism of each person. In response to a progressive tilt, in healthy individuals this sympathetic activity increases and correlates with the degree of the tilt [33].
Jacob et al. [34], studied neurohumoral changes in response to orthostatism in healthy individuals and noted a rapid and progressive increase in the release of norepinephrine, resulting from an increase in the activity of sympathetic nerves, and a decrease in serum noradrenaline [35]. The close correlation between heart rate and plasma adrenaline concentrations described by Jacob [34] suggests that this may be responsible for the increase in heart rate in the first minutes of the orthostatic challenge. The increase in plasma concentrations of norepinephrine stimulates alpha-1 adrenergic receptors and induces vasoconstriction. These authors also showed sustained elevation of aldosterone and increased activity of the renin-angiotensin-aldosterone system. These responses were correlated with the transient hypovolemia associated with the displacement of the intravascular fluid to the veins of different compartments including abdomen, pelvic region and extremities. The increase in renin and aldosterone activity exert multiple compensatory effects in order to maintain the posture: increased tubular reabsorption of sodium by the kidney, direct vasoconstriction, and facilitation of the release of norepinephrine by acting on presynaptic noradrenergic neurons, and possibly, in the central nervous system.
Sympathetic response to orthostatic challenge in head-up tilt test and its suppression preceding vasovagal syncope
Given the importance of the sympathetic nervous system in regulating the response to postural changes, it has been considered that various degrees of sympathetic dysfunction are involved in orthostatic intolerance. The first studies to investigate the pathophysiology of the vasovagal syncope showed that a paradoxical and compensatory reflex was involved. The so-called "ventricular theory" postulates that the baroreceptors react to the decrease of blood pressure with an activation of the sympathetic nervous system, causing a greater inotropic and chronotropic response as well as peripheral vasoconstriction [36]. This theory suggests that vigorous contractions of a left ventricle depleted in volume cause activation due to the stretching of the C fibers of the heart (mechanoreceptors formed by demyelinated fibers found in the atria, ventricles, and pulmonary artery). This afferent C fibers stimulation produces a "paradoxical" suppression of peripheral sympathetic tone and an increase in vagal tone, causing vasodilation and bradycardia [37]. Observations that are more recent disproved this theory. Novak et al. [38] using echocardiographic measurements of the left ventricle and cardiac output found no evidence of a progressive cardiac emptying before syncope; and Liu et al. [39], using measurements of effort and segmental left ventricular wall thickening, found no evidence of activation of the mechanoreceptors in this chamber.
The abrupt interruption of sympathetic nerve activity to the vasculature of skeletal muscles, representing a sympathetic “withdrawal”, has been considered an important step for the vasodilation that leads to hypotension. Early studies showed that before vasovagal syncope, the efferent sympathetic activity, measured with microneurography, decreased progressively or was suddenly interrupted [40]. This reduction in sympathetic activity directly correlated with the mean arterial blood pressure, while the parasympathetic activity, measured by spectral analysis, of the heart rate variability remained below the baseline, suggesting that the sympathetic control of total peripheral resistance was the predominant mechanism responsible for vasovagal syncope. Morillo et al. [23] demonstrated that during ≈100 seconds before the onset of presyncope, systolic and diastolic pressures and muscle sympathetic nerve activity tended to decline (P=0.015, 0.054 and 0.19). At the onset of presyncope, average microneurography and arterial pressure declined abruptly. This landmark study established the concept of “sympathetic withdrawal” as the main mechanism preceding vasovagal syncope. Mosqueda-Garcia et al. [33] found that the initial increase in sympathetic activity (as measured by microneurography) in response to circulating volume changes in orthostatism, was different between patients with vasovagal syncope and controls: it was reduced in the first group. These patients also showed a reduction in the maximum increase in microneurography and plasma norepinephrine that were inadequate to compensate the significant decrease in blood pressure. Nerve inactivity and the occurrence of syncope followed this response. These authors also observed significant reductions in baroreflex responses in patients who were in decubitus; they therefore postulated that a decreased baroreflex response could explain the inability of these patients to increase efferent sympathetic activity in response to a drop in blood pressure. These results are consistent with those presented by Bechir et al. [41], who also found that patients with vasovagal syncope also presented a diminished baroreflex response during the orthostatic challenge and the microneurography. Interestingly, these patients had an increased sympathetic tone in basal conditions. This increase in basal sympathetic vasomotor tone modulation could deplete their reserve and prevent the increase of efferent sympathetic vasomotor tone to support peripheral resistances during orthostatic stress. However, remarks made by Vaddadi et al. [42], discredit the idea that the final "trigger" responsible for a vasovagal orthostatic reaction is the inhibition of the sympathetic nervous system, demonstrating that the efferent sympathetic activity (measured by microneurography) remained normal in nine of ten patients. In these circumstances, an alternative hypothesis could be that a reduction in cardiac output is the predominant physiological event that determines the hypotension. In a recent study conducted in 56 patients with suspected vasovagal syncope, undergoing a tilt test, a reduction of 50% in the cardiac output was observed, while the systemic vascular resistances remained stable until the time of the presyncope [43].
Functional disorders of the proteins of sympathetic nerves
Vaddadi et al. [44] compared two groups of patients with vasovagal syncope: those with normal baseline supine blood pressure (systolic > 100 mmHg) with those with supine low blood pressure (systolic ≤ 100 mmHg). Both types of patients had important cushioning of the concentrations of plasma norepinephrine ("overflow" or "spill" [spillover] norepinephrine) in response to the tilt test, indicating a failure of the sympathetic nervous system to respond to the postural challenge. To explain these results, they analyzed the proteins involved in the sympathetic nerve transmission (synthesis, storage, release and reuptake of norepinephrine). Patients with vasovagal syncope of Group 1, which were "normotensive" at decubitus, showed a reduced expression of tyrosine hydroxylase. This enzyme is responsible for norepinephrine production, so a decreased level of it could explain the decrease in the concentration of this neurotransmitter. In contrast, patients of Group 2 (hypotensive in decubitus) showed elevated concentrations of the norepinephrine transporter. The norepinephrine transporter is responsible for the clearance of norepinephrine in the synaptic cleft to terminate the neural signal. An increased expression of the norepinephrine transporter could depurate norepinephrine faster, reducing the compensatory vasoconstriction and predisposing to postural hypotension. Later on, we will discuss the potential therapeutic applications of this finding.
Myocardial adrenergic innervation
Kochiadakis et al. [25] showed that patients with vasovagal syncope had a high degree of disturbance of myocardial adrenergic innervation and multiple adrenergic innervation defects. The semi-quantitative analysis of adrenergic innervations showed a significantly lower H/M (heart/mediastinum) ratio in the syncopal patients compared with the control group. On the contrary, the washout rate of the 123I-MIBG was significantly greater in patients with vasovagal syncope. They suggest a possible predominance of cardiac adrenergic activity in those with abnormal cardiac MIBG scintigraphy. The decreased MIBG uptake could be an indirect consequence of either chronically increased circulating norepinephrine concentrations or an increase in myocardial norepinephrine release. The increased washout rate could also reflect increased cardiac sympathetic nerve activity.
Genetics of the sympathetic nervous system in vasovagal syncope
It has been well demonstrated that patients with vasovagal syncope frequently have a first-degree relative who is also affected [45]. Although genetic participation in the vasovagal syncope has been debated, some evidence suggests that there is one major genetic component: the adrenergic receptors. Studies have shown an association between positive tilt test in patients with syncope and the presence of polymorphisms in two adrenergic receptors, the Arg389Gly of beta-1 [46] and the Arg347Cys of the alpha-1a (α1a) [47]. Unfortunately, these findings have not been replicated.
Beta-blockers in vasovagal syncope
Based on the information previously presented, it is not a surprise that beta-blockers were employed in the treatment of vasovagal syncope from the very beginning [48]. Although initial results in the use of beta-blockers in uncontrolled clinical studies or when compared with standard treatment seemed encouraging, there are several controlled studies, compared to placebo, in which a real effect of these drugs has not been demonstrated (Table 2). However, it is important to describe some of the limitations of those studies. First, it must be considered that all the published studies have a small sample size; some of them included less than 10 patients. It is also important to mention that the follow up was short (Table 2) in most of them. The only randomized study with an adequate sample size was POST (Prevention of Syncope Trial) [49] and it could not demonstrate any benefit with the use of beta-blockers. However, it should be noted that a fixed dose of metoprolol was used for all the patients, without individualizing the dosage, which probably justifies the absence of positive results. A fixed dose of metoprolol may affect the outcomes since not all patients tolerate it. In our clinical practice, we have learned that, depending on the subject, sometimes it is necessary to start with very low doses of metoprolol (25 - 50 mg/day) and increase it progressively every 2-4 weeks. In the mentioned study (POST), the researchers increased the dose from 50 mg to 100 mg bid in 3-5 days. This may explain the high incidence of abandonment of the study in the group taking metoprolol (22%). However, a sub analysis of the study showed that metoprolol was effective in patients over 42 years [50]. Supporting this finding, Benditt et al. [51] reported that in these patients the differences related to age were related to the amount of circulating catecholamines. They found that although basal epinephrine and norepinephrine concentrations were similar in young subjects and those over 40 years, the ratio of epinephrine and norepinephrine greatly increased in younger patients. Based on these findings, there is actually underway a placebo-controlled study on the use of metoprolol for subjects with vasovagal syncope over 40-years-old [52]. In a review, Raj and Coffin [53], propose that beta-blockers may be a reasonable therapeutic option for patients with vasovagal syncope and systemic arterial hypertension.
Table 2. Summary of studies of beta-adrenergic blockers in vasovagal syncope*
Vyas et al. [54] published a meta-analysis of all the studies on the different treatment options for vasovagal syncope. Thirteen studies were included in the analysis of patients treated with beta-blockers. In this meta-analysis there were significant differences in favor of beta-blockers when the 13 studies were pooled. A systematic review of the literature showed that the treatment with beta-blockers was significantly better in comparison with standard therapy. However, no differences in all studies were found when compared to placebo, other drugs, or pacemakers [55].
Pharmacological properties of beta-blockers and their effect on vasovagal syncope
Some aspects that cannot be overcome by the meta-analyses and systematic reviews are the different pharmacological properties of beta-blockers. The most relevant issue in subjects with vasovagal syncope is the intrinsic sympathetic activity, which limits the bradycardia effect of some beta-blockers, such as pindolol. Although this effect might be useful to avoid more bradycardia in subjects with low heart rate tendency, it may also be associated with palpitations, which could limit its use. In this regard, dose titration is a very important issue not yet addressed by previous studies, which is also important to the different specificity of the target organs of beta-blockers. The differences between non-selective antagonists (with or without alpha antagonist activity) and the selective beta-antagonist-1 have neither been addressed adequately. The use of agents with arteriolar vasodilator activity (e.g. labetalol, carvedilol, nebivolol) might be more useful in the subgroup of patients who also suffer from reactive hypertension, but could harm patients prone to very low blood pressure. Finally, the affinity of some beta-blockers, such as pindolol, for the subtype 1A serotonin receptors (5-HT1A) for depression could also be the basis of a part of the effect in vasovagal syncope [56].
Norepinephrine reuptake inhibitors in vasovagal syncope
As mentioned, the reuptake of norepinephrine in the synaptic cleft, made possible by the norepinephrine transporter, is the main mechanism of inactivation of norepinephrine. Therefore, the action of this transporter is a key factor in the regulation of norepinephrine. Increased concentrations of the norepinephrine transporter or an increase in its function would result in a greater inactivation of norepinephrine and vice versa, (a decreased concentration or a lower activity of the norepinephrine transporter would imply greater action on adrenergic receptors). In a case with postural orthostatic tachycardia syndrome, Goldstein et al. [57] found a mutation that decreased the norepinephrine transporter function by 98%, demonstrating for the first time a relation between a diminished function of this transporter with clinical sympathetic hyperactivity, manifested by postural tachycardia.
On the other hand, an increased activity of the norepinephrine transporter, clearing faster the neurotransmitter, would reduce the compensatory vasoconstriction predisposing to postural hypotension. This theoretical possibility is supported by several studies. Schroeder et al. [58] demonstrated a better tolerance to tilt test under norepinephrine transporter inhibitor treatment (reboxetine) compared to placebo in 18 healthy subjects. Reboxetine is a drug primarily used as an antidepressant. Under placebo, a vasovagal reaction occurred in 50% of individuals (9 of 18), while only one individual fainted (5%) under reboxetine. They expanded their observation with 51 healthy subjects with no history of syncope [59]. In this group, they compared two inhibitors of norepinephrine transporter: sibutramine (a drug used for weight loss) or reboxetine. They showed again a greater tolerance for passive orthostatism during the tilt test; the duration of the test before patient presented a vasovagal reaction increased from 29 ± 2 to 35 ± 1 min (P = 0.001) when the subject was under the effect of any of these two inhibitors. An excellent review of these findings and their implications was posted by the main author of these works in 2012 [60]. Sheldon et al. [61] reported that five of seven very symptomatic patients with multiple recurrences of vasovagal syncope (32 events on average per month) treated with sibutramine showed a reduction in the frequency of fainting by more than 50%. Sibutramine was withdrawn from the market in several countries, including Mexico, when a study showed increased rate of cardiovascular events (myocardial infarction and cerebrovascular disease) in people with pre-existing heart disease. Ramirez et al. [62] reported that atomoxetine, an inhibitor of the norepinephrine transporter approved for the treatment of attention deficit disorder, could increase the blood pressure in individuals with autonomic failure. Atomoxetine was even better than midodrine in improving blood pressure when adopting orthostatic position in a group of 65 patients with severe autonomic failure (caused by Parkinson's disease, multiple systemic atrophy or pure autonomic failure). Therefore, atomoxetine could be a therapeutic option for vasovagal syncope due to its availability in many countries and the possibility of using pediatric dosing.
Conclusions
At present, beta-blockers are considered a placebo because there is no strong evidence to support their use. However, further studies on the use of beta-blockers in vasovagal syncope are urgently needed based on the information presented in this document and the fact that they are still used in clinical practice due to the excellent response informed by clinical experts. A multicenter clinical trial (POST-5) is ongoing in another attempt to prove this hypothesis. Recent studies on the inhibition of reuptake of norepinephrine with specific agents have shown promising results on this entity and merits further investigation.
Notes
From the editor
The authors originally submitted this article in Spanish and English. The Journal has not copyedited the English version.
Conflicts of interests
The authors completed the ICMJE conflicts of interest declaration form, translated to Spanish by Medwave, and declare not having received funding for the preparation of this report, not having any financial relationships with organizations that could have interests in the published article in the last three years, and not having other relations or activities that might influence the article´s content. Forms can be requested to the responsible author or the editorial direction of the Journal.
Funding
The authors declare that there was no funding coming from external sources.

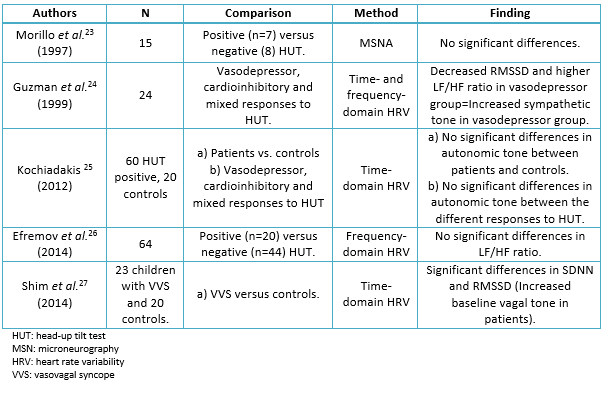 Table 1. Studies on baseline autonomic tone in vasovagal syncope
Table 1. Studies on baseline autonomic tone in vasovagal syncope

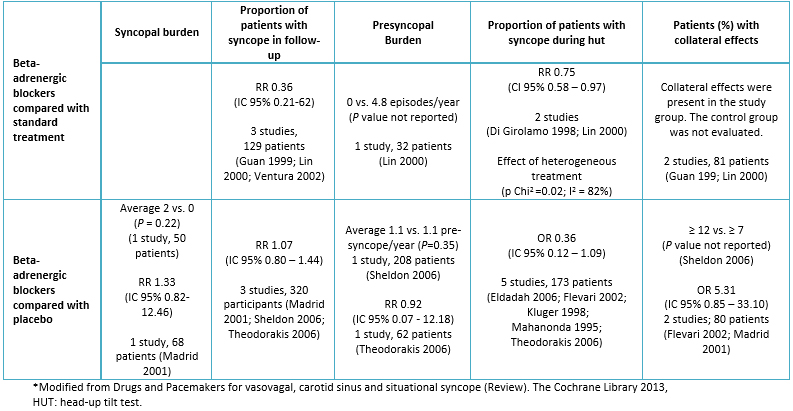 Table 2. Summary of studies of beta-adrenergic blockers in vasovagal syncope*
Table 2. Summary of studies of beta-adrenergic blockers in vasovagal syncope*
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

El síncope vasovagal o neurocardiogénico es una situación clínica común, y así como en otras entidades asociadas con la intolerancia ortostática, la condición de base es una disfunción del sistema nervioso autónomo. En este artículo se revisan diversos aspectos sobre el síncope vasovagal, incluyendo su relación con la intolerancia ortostática y el papel que juega el sistema nervioso autónomo. Se da una breve reseña histórica del problema, así como una descripción de la forma en que han evolucionado los términos y conceptos asociados al mismo. Se hace un análisis sobre la respuesta del sistema nervioso simpático al estrés ortostático, la fisiología del sistema barorreflejo y los cambios neurohumorales que ocurren. Se muestra evidencia sobre el papel del sistema nervioso autónomo, incluyendo estudios sobre variabilidad de la frecuencia cardiaca, microneurografía, inervación cardiaca y estudios genéticos moleculares. Finalmente, se describen diferentes estudios sobre el uso de betabloqueadores e inhibidores del transportador de noradrenalina (sibutramina, reboxetina) y la justificación de su uso en la prevención de este tipo de síncope.
 Authors:
Manlio F. Márquez [1], Jorge Rafael Gómez-Flores [1], Jesús A. González-Hermosillo [2], Teresita de Jesús Ruíz-Siller [1], Manuel Cárdenas [1]
Authors:
Manlio F. Márquez [1], Jorge Rafael Gómez-Flores [1], Jesús A. González-Hermosillo [2], Teresita de Jesús Ruíz-Siller [1], Manuel Cárdenas [1]
Affiliation:
[1] Manuel Cárdenas Departamento de Electrofisiología, Instituto Nacional de Cardiología Ignacio Chávez, Ciudad de México, México
[2] Departamento de Proyectos de Innovación y Desarrollo, Instituto Nacional de Cardiología Ignacio Chávez, Ciudad de México, México
E-mail: manlio.marquez@gmail.com
Author address:
[1] Juan Badiano 1
Colonia Sección XVI
Delegación Tlalpan
Distrito Federal
México

Citation: Márquez MF, Gómez-Flores JR, González-Hermosillo JA, Ruíz-Siller TJ, Cárdenas M. Role of the sympathetic nervous system in vasovagal syncope and rationale for beta-blockers and norepinephrine transporter inhibitors. Medwave 2016;16(Suppl 4):e6824 doi: 10.5867/medwave.2016.6824
Publication date: 29/12/2016
Origin: This article is part of the Special Supplement 4 of Cardiology whose guest editor is Dr. Alberto Morales Salinas, Cardiocentro "Ernesto Che Guevara", Villa Clara; National Cardiology Group, Ministry of Public Health, Cuba

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Hermosillo AG, Márquez MF, Jáuregui-Renaud K, Cárdenas M. Orthostatic hypotension, 2001. Cardiol Rev. 2001 Nov-Dec;9(6):339-47. | PubMed |
- Medow MS, Stewart JM, Sanyal S, Mumtaz A, Sica D, Frishman WH. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol Rev. 2008 Jan-Feb;16(1):4-20. | PubMed |
- Mathias CJ, Deguchi K, Schatz I. Observations on recurrent syncope and presyncope in 641 patients. Lancet. 2001 Feb 3;357(9253):348-53. | PubMed |
- Celaya Cota M de J, Márquez MF. Evaluación de síncope en el Servicio de Urgencias. In: Manual de Urgencias Cardiovasculares. Cuarta. México: Mc Graw Hill;2012.
- Allende R, Cázares Campos I, Márquez MF. Neuropatía. En: Atención Integral Del Paciente Diabético. Cuarta. México: Mc Graw Hill; 2011.
- Cárdenas M, Vallejo M, Martínez-Palomino G, Paredes-Balderas G, Sandoval-Rubio LA, et al. Prevalencia de síncope en una muestra de mujeres mexicanas residentes en la ciudad de México. Arch Cardiol México. 79(3):197-200. | Link |
- Martínez-Martínez LA, Mora T, Vargas A, Fuentes-Iniestra M, Martínez-Lavín M. Sympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: a review of case-control studies. J Clin Rheumatol. 2014 Apr;20(3):146-50. | CrossRef | PubMed |
- Lerma C, Martinez-Martinez LA, Ruiz N, Vargas A, Infante O, Martinez-Lavin M. Fibromyalgia beyond reductionism. Heart rhythm fractal analysis to assess autonomic nervous system resilience. Scand J Rheumatol. 2016;45(2):151-7. | CrossRef | PubMed |
- Lobban TC. Syncope: a patient and family perspective. Cardiol Clin. 2013 Feb;31(1):1-8. | CrossRef | PubMed |
- Eppinger H, Hess L. Die Vagotonie. Berlin: Eine Klinische Studie; 1910.
- Delgado G, Estañol-Vidal B. Vagotonía. La tesis recepcional de Salvador Zubirán. Rev Invest Clin. 2012;64(4):387-398. | Link |
- Lewis T. A Lecture on vasovagal syncope and the carotid sinus mechanism. Br Med J. 1932 May 14;1(3723):873-6. | PubMed |
- Wooley CF. Jacob Mendez DaCosta: medical teacher, clinician, and clinical investigator. Am J Cardiol. 1982 Nov;50(5):1145-8. | PubMed |
- Jones E. Historical approaches to post-combat disorders. Philos Trans R Soc Lond B Biol Sci. 2006 Apr 29;361(1468):533-42. | PubMed |
- Levine SA. The origin of the term neurocirculatory asthenia. N Engl J Med. 1965 Sep 9;273:604-5. | PubMed |
- Chávez I. Cinco Lecciones de Clínica Cardiológica. Méndez Oteo; 1933.
- Wood P. Da Costa's Syndrome: Aetiology. Lecture III. Br Med J. 1941 Jun 7;1(4196):845-51. | PubMed |
- Ríos-Martínez BP, Huitrón-Cervantes G, Márquez MF, González-Hermosillo JA, Rangel-Rodríguez GA, Pedraza-Moctezuma LG. Estudio descriptivo de la personalidad y psicopatología en pacientes con síncope vasovagal. Arch Cardiol México. 79(3):207-211. | Link |
- Lerma A, Lerma C, Márquez MF, Cárdenas M, Hermosillo AG. Correlation of syncopal burden with anxiety symptoms score in recurrent vasovagal syncope. Int J Cardiol. 2013 Jun 5;166(1):266-7. | CrossRef | PubMed |
- Davis SD, Kator SF, Wonnett JA, Pappas BL, Sall JL. Neurally mediated hypotension in fatigued Gulf War veterans: a preliminary report. Am J Med Sci. 2000 Feb;319(2):89-95. | PubMed |
- Haley RW, Charuvastra E, Shell WE, Buhner DM, Marshall WW, Biggs MM, et al. Cholinergic autonomic dysfunction in veterans with Gulf War illness: confirmation in a population-based sample. JAMA Neurol. 2013 Feb;70(2):191-200. | CrossRef | PubMed |
- Li M, Xu C, Yao W, Mahan CM, Kang HK, Sandbrink F, et al.Self-reported post-exertional fatigue in Gulf War veterans: roles of autonomic testing. Front Neurosci. 2014 Jan 7;7:269. | CrossRef | PubMed |
- Morillo CA, Eckberg DL, Ellenbogen KA, Beightol LA, Hoag JB, Tahvanainen KU, et al. Vagal and sympathetic mechanisms in patients with orthostatic vasovagal syncope. Circulation. 1997 Oct 21;96(8):2509-13. | PubMed |
- Guzmán CE, Sánchez GM, Márquez MF, Hermosillo AG, Cárdenas M. Differences in heart rate variability between cardioinhibitory and vasodepressor responses to head-up tilt table testing. Arch Med Res. 1999 May-Jun;30(3):203-11. | PubMed |
- Kochiadakis G, Marketou M, Koukouraki S, Parthenakis F, Chlouverakis G, Karkavitsas N, et al. Cardiac autonomic disturbances in patients with vasovagal syndrome: comparison between iodine-123-metaiodobenzylguanidine myocardial scintigraphy and heart rate variability. Europace. 2012 Sep;14 (9):1352-8. | CrossRef | PubMed |
- Efremov K, Brisinda D, Venuti A, Iantorno E, Cataldi C, Fioravanti F, Fenici R. Heart rate variability analysis during head-up tilt test predicts nitroglycerine-induced syncope. Open Heart. 2014 Jun 14;1(1):e000063. | CrossRef | PubMed |
- Shim SH, Park SY, Moon SN, Oh JH, Lee JY, Kim HH, Han JW, et al. Baseline heart rate variability in children and adolescents with vasovagal syncope. Korean J Pediatr. 2014 Apr;57(4):193-8. | CrossRef | PubMed |
- Estañol B, Porras-Betancourt M, Padilla-Leyva MÁ, Sentíes-Madrid H. Breve historia del reflejo barorreceptor: de Claude Bernard a Arthur C. Guyton. Ilustrada con algunos experimentos clásicos. Arch Cardiol México. 81(4):330-336. | Link |
- Chapleau MW, Cunningham JT, Sullivan MJ, Wachtel RE, Abboud FM. Structural versus functional modulation of the arterial baroreflex. Hypertension. 1995 Aug;26(2):341-7. | PubMed |
- Hinghofer-Szalkay H, König EM, Sauseng-Fellegger G, Zambo-Polz C. Biphasic blood volume changes with lower body suction in humans. Am J Physiol. 1992 Oct;263(4 Pt 2):H1270-5. | PubMed |
- Hinghofer-Szalkay H, Lackner HK, Rössler A, Narath B, Jantscher A, Goswami N. Hormonal and plasma volume changes after presyncope. Eur J Clin Invest. 2011 Nov;41(11):1180-5. | CrossRef | PubMed |
- Charkoudian N, Rabbitts JA. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc. 2009 Sep;84(9):822-30. | CrossRef | PubMed |
- Mosqueda-Garcia R, Furlan R, Fernandez-Violante R, Desai T, Snell M, Jarai Z, et al. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest. 1997 Jun 1;99(11):2736-44. | PubMed |
- Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM, Robertson D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. J Appl Physiol (1985). 1998 Mar;84(3):914-21. | PubMed |
- Meredith IT, Eisenhofer G, Lambert GW, Jennings GL, Thompson J, Esler MD. Plasma norepinephrine responses to head-up tilt are misleading in autonomic failure. Hypertension. 1992 Jun;19(6 Pt 2):628-33. | PubMed |
- Thorén P. Role of cardiac vagal C-fibers in cardiovascular control. Rev Physiol Biochem Pharmacol. 1979;86:1-94. | PubMed |
- Kaufmann H. Neurally mediated syncope: pathogenesis, diagnosis, and treatment. Neurology. 1995 Apr;45(4 Suppl 5):S12-8. | PubMed |
- Novak V, Honos G, Schondorf R. Is the heart "empty' at syncope? J Auton Nerv Syst. 1996 Aug 27;60(1-2):83-92. | PubMed |
- Liu JE, Hahn RT, Stein KM, Markowitz SM, Okin PM, Devereux RB, et al. Left ventricular geometry and function preceding neurally mediated syncope. Circulation. 2000 Feb 22;101(7):777-83. | PubMed |
- Jardine DL, Ikram H, Frampton CM, Frethey R, Bennett SI, Crozier IG. Autonomic control of vasovagal syncope. Am J Physiol. 1998 Jun;274(6 Pt2):H2110-5. | PubMed |
- Béchir M, Binggeli C, Corti R, Chenevard R, Spieker L, Ruschitzka F, et al. Dysfunctional baroreflex regulation of sympathetic nerve activity in patients with vasovagal syncope. Circulation. 2003 Apr 1;107(12):1620-5. | CrossRef | PubMed |
- Vaddadi G, Esler MD, Dawood T, Lambert E. Persistence of muscle sympathetic nerve activity during vasovagal syncope. Eur Heart J. 2010 Aug;31 (16):2027-33. | CrossRef | PubMed |
- Verheyden B, Liu J, van Dijk N, Westerhof BE, Reybrouck T, Aubert AE, et al. Steep fall in cardiac output is main determinant of hypotension during drug-free and nitroglycerine-induced orthostatic vasovagal syncope. Heart Rhythm. 2008 Dec;5(12):1695-701. | CrossRef | PubMed |
- Vaddadi G, Guo L, Esler M, Socratous F, Schlaich M, Chopra R, et al. Recurrent postural vasovagal syncope: sympathetic nervous system phenotypes. Circ Arrhythm Electrophysiol. 2011 Oct;4(5):711-8. | CrossRef | PubMed |
- Márquez MF, Urias KI, Hermosillo AG, Jardón JL, Iturralde P, Colín L, et al. Familial vasovagal syncope. Europace. 2005 Sep;7(5):472-4. | PubMed |
- Márquez MF, Hernández-Pacheco G, Hermosillo AG, Gómez JR, Cárdenas M, Vargas-Alarcón G. The Arg389Gly beta1-adrenergic receptor gene polymorphism and susceptibility to faint during head-up tilt test. Europace. 2007 Aug;9(8):585-8. | PubMed |
- Hernández-Pacheco G, González-Hermosillo A, Murata C, Yescas P, Espínola-Zavaleta N, Martínez M, et al. Arg347Cys polymorphism of α1a-adrenergic receptor in vasovagal syncope. Case-control study in a Mexican population. Auton Neurosci. 2014 Jul;183:66-71. | CrossRef | PubMed |
- Márquez MF, Urias-Medina K, Gómez-Flores J, Sobrino A, Sotomayor-González A, González-Hermosillo A, et al. [Comparison of metoprolol vs clonazepam as a first treatment choice among patients with neurocardiogenic syncope]. Gac Med Mex. 2008 Nov-Dec;144(6):503-7. | PubMed |
- Sheldon R, Connolly S, Rose S, Klingenheben T, Krahn A, Morillo C, et al. Prevention of Syncope Trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation. 2006 Mar 7;113(9):1164-70. | PubMed |
- Sheldon RS, Morillo CA, Klingenheben T, Krahn AD, Sheldon A, Rose MS. Age-dependent effect of β-blockers in preventing vasovagal syncope. Circ Arrhythm Electrophysiol. 2012 Oct;5(5):920-6. | CrossRef | PubMed |
- Benditt DG, Detloff BL, Adkisson WO, Lu F, Sakaguchi S, Schussler S, et al. Age-dependence of relative change in circulating epinephrine and norepinephrine concentrations during tilt-induced vasovagal syncope. Heart Rhythm. 2012 Nov;9(11):1847-52. | CrossRef | PubMed |
- Raj SR, Faris PD, Semeniuk L, Manns B, Krahn AD, Morillo CA, et al. Rationale for the Assessment of Metoprolol in the Prevention of Vasovagal Syncope in Aging Subjects Trial (POST5). Am Heart J. 2016 Apr;174:89-94. | CrossRef | PubMed |
- Raj SR, Coffin ST. Medical therapy and physical maneuvers in the treatment of the vasovagal syncope and orthostatic hypotension. Prog Cardiovasc Dis. 2013 Jan-Feb;55(4):425-33. | CrossRef | PubMed |
- Vyas A, Swaminathan PD, Zimmerman MB, Olshansky B. Are treatments for vasovagal syncope effective? A meta-analysis. Int J Cardiol. 2013 Sep 1;167(5):1906-11. | CrossRef | PubMed |
- Romme JJ, Reitsma JB, Black CN, Colman N, Scholten RJ, Wieling W, et al. Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst Rev. 2011 Oct 5;(10):CD004194. | CrossRef | PubMed |
- Pérez V, Puiigdemont D, Gilaberte I, Alvarez E, Artigas F; Grup de Recerca en Trastorns Afectius. Augmentation of fluoxetine's antidepressant action by pindolol: analysis of clinical, pharmacokinetic, and methodologic factors. J Clin Psychopharmacol. 2001 Feb;21(1):36-45. | PubMed |
- Goldstein DS, Holmes C, Frank SM, Dendi R, Cannon RO 3rd, Sharabi Y, et al. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation. 2002 Oct 29;106(18):2358-65. | PubMed |
- Schroeder C, Tank J, Boschmann M, et al. Selective norepinephrine reuptake inhibition as a human model of orthostatic intolerance. Circulation. 2002 Jan 22;105(3):347-53. | CrossRef | PubMed |
- Schroeder C, Birkenfeld AL, Mayer AF, Tank J, Diedrich A, Luft FC, et al. Norepinephrine transporter inhibition prevents tilt-induced pre-syncope. J Am Coll Cardiol. 2006 Aug 1;48(3):516-22. | PubMed |
- Schroeder C, Jordan J. Norepinephrine transporter function and human cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012 Dec 1;30(11):H1273-82. | CrossRef | PubMed |
- Sheldon RS, Ritchie D, McRae M, Raj S. Norepinephrine transport inhibition for treatment of vasovagal syncope. J Cardiovasc Electrophysiol. 2013 Jul;24(7):799-803. | CrossRef | PubMed |
- Ramirez CE, Okamoto LE, Arnold AC, Gamboa A, Diedrich A, Choi L, et al. Efficacy of atomoxetine versus midodrine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2014 Dec;64(6):1235-40. | CrossRef | PubMed |
 Hermosillo AG, Márquez MF, Jáuregui-Renaud K, Cárdenas M. Orthostatic hypotension, 2001. Cardiol Rev. 2001 Nov-Dec;9(6):339-47. | PubMed |
Hermosillo AG, Márquez MF, Jáuregui-Renaud K, Cárdenas M. Orthostatic hypotension, 2001. Cardiol Rev. 2001 Nov-Dec;9(6):339-47. | PubMed | Medow MS, Stewart JM, Sanyal S, Mumtaz A, Sica D, Frishman WH. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol Rev. 2008 Jan-Feb;16(1):4-20. | PubMed |
Medow MS, Stewart JM, Sanyal S, Mumtaz A, Sica D, Frishman WH. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol Rev. 2008 Jan-Feb;16(1):4-20. | PubMed | Mathias CJ, Deguchi K, Schatz I. Observations on recurrent syncope and presyncope in 641 patients. Lancet. 2001 Feb 3;357(9253):348-53. | PubMed |
Mathias CJ, Deguchi K, Schatz I. Observations on recurrent syncope and presyncope in 641 patients. Lancet. 2001 Feb 3;357(9253):348-53. | PubMed | Celaya Cota M de J, Márquez MF. Evaluación de síncope en el Servicio de Urgencias. In: Manual de Urgencias Cardiovasculares. Cuarta. México: Mc Graw Hill;2012.
Celaya Cota M de J, Márquez MF. Evaluación de síncope en el Servicio de Urgencias. In: Manual de Urgencias Cardiovasculares. Cuarta. México: Mc Graw Hill;2012.  Allende R, Cázares Campos I, Márquez MF. Neuropatía. En: Atención Integral Del Paciente Diabético. Cuarta. México: Mc Graw Hill; 2011.
Allende R, Cázares Campos I, Márquez MF. Neuropatía. En: Atención Integral Del Paciente Diabético. Cuarta. México: Mc Graw Hill; 2011.  Cárdenas M, Vallejo M, Martínez-Palomino G, Paredes-Balderas G, Sandoval-Rubio LA, et al. Prevalencia de síncope en una muestra de mujeres mexicanas residentes en la ciudad de México. Arch Cardiol México. 79(3):197-200. | Link |
Cárdenas M, Vallejo M, Martínez-Palomino G, Paredes-Balderas G, Sandoval-Rubio LA, et al. Prevalencia de síncope en una muestra de mujeres mexicanas residentes en la ciudad de México. Arch Cardiol México. 79(3):197-200. | Link | Martínez-Martínez LA, Mora T, Vargas A, Fuentes-Iniestra M, Martínez-Lavín M. Sympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: a review of case-control studies. J Clin Rheumatol. 2014 Apr;20(3):146-50. | CrossRef | PubMed |
Martínez-Martínez LA, Mora T, Vargas A, Fuentes-Iniestra M, Martínez-Lavín M. Sympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: a review of case-control studies. J Clin Rheumatol. 2014 Apr;20(3):146-50. | CrossRef | PubMed | Lerma C, Martinez-Martinez LA, Ruiz N, Vargas A, Infante O, Martinez-Lavin M. Fibromyalgia beyond reductionism. Heart rhythm fractal analysis to assess autonomic nervous system resilience. Scand J Rheumatol. 2016;45(2):151-7. | CrossRef | PubMed |
Lerma C, Martinez-Martinez LA, Ruiz N, Vargas A, Infante O, Martinez-Lavin M. Fibromyalgia beyond reductionism. Heart rhythm fractal analysis to assess autonomic nervous system resilience. Scand J Rheumatol. 2016;45(2):151-7. | CrossRef | PubMed | Lobban TC. Syncope: a patient and family perspective. Cardiol Clin. 2013 Feb;31(1):1-8. | CrossRef | PubMed |
Lobban TC. Syncope: a patient and family perspective. Cardiol Clin. 2013 Feb;31(1):1-8. | CrossRef | PubMed | Eppinger H, Hess L. Die Vagotonie. Berlin: Eine Klinische Studie; 1910.
Eppinger H, Hess L. Die Vagotonie. Berlin: Eine Klinische Studie; 1910.  Delgado G, Estañol-Vidal B. Vagotonía. La tesis recepcional de Salvador Zubirán. Rev Invest Clin. 2012;64(4):387-398. | Link |
Delgado G, Estañol-Vidal B. Vagotonía. La tesis recepcional de Salvador Zubirán. Rev Invest Clin. 2012;64(4):387-398. | Link | Lewis T. A Lecture on vasovagal syncope and the carotid sinus mechanism. Br Med J. 1932 May 14;1(3723):873-6. | PubMed |
Lewis T. A Lecture on vasovagal syncope and the carotid sinus mechanism. Br Med J. 1932 May 14;1(3723):873-6. | PubMed | Wooley CF. Jacob Mendez DaCosta: medical teacher, clinician, and clinical investigator. Am J Cardiol. 1982 Nov;50(5):1145-8. | PubMed |
Wooley CF. Jacob Mendez DaCosta: medical teacher, clinician, and clinical investigator. Am J Cardiol. 1982 Nov;50(5):1145-8. | PubMed | Jones E. Historical approaches to post-combat disorders. Philos Trans R Soc Lond B Biol Sci. 2006 Apr 29;361(1468):533-42. | PubMed |
Jones E. Historical approaches to post-combat disorders. Philos Trans R Soc Lond B Biol Sci. 2006 Apr 29;361(1468):533-42. | PubMed | Levine SA. The origin of the term neurocirculatory asthenia. N Engl J Med. 1965 Sep 9;273:604-5. | PubMed |
Levine SA. The origin of the term neurocirculatory asthenia. N Engl J Med. 1965 Sep 9;273:604-5. | PubMed | Chávez I. Cinco Lecciones de Clínica Cardiológica. Méndez Oteo; 1933.
Chávez I. Cinco Lecciones de Clínica Cardiológica. Méndez Oteo; 1933.  Wood P. Da Costa's Syndrome: Aetiology. Lecture III. Br Med J. 1941 Jun 7;1(4196):845-51. | PubMed |
Wood P. Da Costa's Syndrome: Aetiology. Lecture III. Br Med J. 1941 Jun 7;1(4196):845-51. | PubMed | Ríos-Martínez BP, Huitrón-Cervantes G, Márquez MF, González-Hermosillo JA, Rangel-Rodríguez GA, Pedraza-Moctezuma LG. Estudio descriptivo de la personalidad y psicopatología en pacientes con síncope vasovagal. Arch Cardiol México. 79(3):207-211. | Link |
Ríos-Martínez BP, Huitrón-Cervantes G, Márquez MF, González-Hermosillo JA, Rangel-Rodríguez GA, Pedraza-Moctezuma LG. Estudio descriptivo de la personalidad y psicopatología en pacientes con síncope vasovagal. Arch Cardiol México. 79(3):207-211. | Link | Lerma A, Lerma C, Márquez MF, Cárdenas M, Hermosillo AG. Correlation of syncopal burden with anxiety symptoms score in recurrent vasovagal syncope. Int J Cardiol. 2013 Jun 5;166(1):266-7. | CrossRef | PubMed |
Lerma A, Lerma C, Márquez MF, Cárdenas M, Hermosillo AG. Correlation of syncopal burden with anxiety symptoms score in recurrent vasovagal syncope. Int J Cardiol. 2013 Jun 5;166(1):266-7. | CrossRef | PubMed | Davis SD, Kator SF, Wonnett JA, Pappas BL, Sall JL. Neurally mediated hypotension in fatigued Gulf War veterans: a preliminary report. Am J Med Sci. 2000 Feb;319(2):89-95. | PubMed |
Davis SD, Kator SF, Wonnett JA, Pappas BL, Sall JL. Neurally mediated hypotension in fatigued Gulf War veterans: a preliminary report. Am J Med Sci. 2000 Feb;319(2):89-95. | PubMed | Haley RW, Charuvastra E, Shell WE, Buhner DM, Marshall WW, Biggs MM, et al. Cholinergic autonomic dysfunction in veterans with Gulf War illness: confirmation in a population-based sample. JAMA Neurol. 2013 Feb;70(2):191-200. | CrossRef | PubMed |
Haley RW, Charuvastra E, Shell WE, Buhner DM, Marshall WW, Biggs MM, et al. Cholinergic autonomic dysfunction in veterans with Gulf War illness: confirmation in a population-based sample. JAMA Neurol. 2013 Feb;70(2):191-200. | CrossRef | PubMed | Li M, Xu C, Yao W, Mahan CM, Kang HK, Sandbrink F, et al.Self-reported post-exertional fatigue in Gulf War veterans: roles of autonomic testing. Front Neurosci. 2014 Jan 7;7:269. | CrossRef | PubMed |
Li M, Xu C, Yao W, Mahan CM, Kang HK, Sandbrink F, et al.Self-reported post-exertional fatigue in Gulf War veterans: roles of autonomic testing. Front Neurosci. 2014 Jan 7;7:269. | CrossRef | PubMed | Morillo CA, Eckberg DL, Ellenbogen KA, Beightol LA, Hoag JB, Tahvanainen KU, et al. Vagal and sympathetic mechanisms in patients with orthostatic vasovagal syncope. Circulation. 1997 Oct 21;96(8):2509-13. | PubMed |
Morillo CA, Eckberg DL, Ellenbogen KA, Beightol LA, Hoag JB, Tahvanainen KU, et al. Vagal and sympathetic mechanisms in patients with orthostatic vasovagal syncope. Circulation. 1997 Oct 21;96(8):2509-13. | PubMed | Guzmán CE, Sánchez GM, Márquez MF, Hermosillo AG, Cárdenas M. Differences in heart rate variability between cardioinhibitory and vasodepressor responses to head-up tilt table testing. Arch Med Res. 1999 May-Jun;30(3):203-11. | PubMed |
Guzmán CE, Sánchez GM, Márquez MF, Hermosillo AG, Cárdenas M. Differences in heart rate variability between cardioinhibitory and vasodepressor responses to head-up tilt table testing. Arch Med Res. 1999 May-Jun;30(3):203-11. | PubMed | Kochiadakis G, Marketou M, Koukouraki S, Parthenakis F, Chlouverakis G, Karkavitsas N, et al. Cardiac autonomic disturbances in patients with vasovagal syndrome: comparison between iodine-123-metaiodobenzylguanidine myocardial scintigraphy and heart rate variability. Europace. 2012 Sep;14 (9):1352-8. | CrossRef | PubMed |
Kochiadakis G, Marketou M, Koukouraki S, Parthenakis F, Chlouverakis G, Karkavitsas N, et al. Cardiac autonomic disturbances in patients with vasovagal syndrome: comparison between iodine-123-metaiodobenzylguanidine myocardial scintigraphy and heart rate variability. Europace. 2012 Sep;14 (9):1352-8. | CrossRef | PubMed | Efremov K, Brisinda D, Venuti A, Iantorno E, Cataldi C, Fioravanti F, Fenici R. Heart rate variability analysis during head-up tilt test predicts nitroglycerine-induced syncope. Open Heart. 2014 Jun 14;1(1):e000063. | CrossRef | PubMed |
Efremov K, Brisinda D, Venuti A, Iantorno E, Cataldi C, Fioravanti F, Fenici R. Heart rate variability analysis during head-up tilt test predicts nitroglycerine-induced syncope. Open Heart. 2014 Jun 14;1(1):e000063. | CrossRef | PubMed | Shim SH, Park SY, Moon SN, Oh JH, Lee JY, Kim HH, Han JW, et al. Baseline heart rate variability in children and adolescents with vasovagal syncope. Korean J Pediatr. 2014 Apr;57(4):193-8. | CrossRef | PubMed |
Shim SH, Park SY, Moon SN, Oh JH, Lee JY, Kim HH, Han JW, et al. Baseline heart rate variability in children and adolescents with vasovagal syncope. Korean J Pediatr. 2014 Apr;57(4):193-8. | CrossRef | PubMed | Estañol B, Porras-Betancourt M, Padilla-Leyva MÁ, Sentíes-Madrid H. Breve historia del reflejo barorreceptor: de Claude Bernard a Arthur C. Guyton. Ilustrada con algunos experimentos clásicos. Arch Cardiol México. 81(4):330-336. | Link |
Estañol B, Porras-Betancourt M, Padilla-Leyva MÁ, Sentíes-Madrid H. Breve historia del reflejo barorreceptor: de Claude Bernard a Arthur C. Guyton. Ilustrada con algunos experimentos clásicos. Arch Cardiol México. 81(4):330-336. | Link | Chapleau MW, Cunningham JT, Sullivan MJ, Wachtel RE, Abboud FM. Structural versus functional modulation of the arterial baroreflex. Hypertension. 1995 Aug;26(2):341-7. | PubMed |
Chapleau MW, Cunningham JT, Sullivan MJ, Wachtel RE, Abboud FM. Structural versus functional modulation of the arterial baroreflex. Hypertension. 1995 Aug;26(2):341-7. | PubMed | Hinghofer-Szalkay H, König EM, Sauseng-Fellegger G, Zambo-Polz C. Biphasic blood volume changes with lower body suction in humans. Am J Physiol. 1992 Oct;263(4 Pt 2):H1270-5. | PubMed |
Hinghofer-Szalkay H, König EM, Sauseng-Fellegger G, Zambo-Polz C. Biphasic blood volume changes with lower body suction in humans. Am J Physiol. 1992 Oct;263(4 Pt 2):H1270-5. | PubMed | Hinghofer-Szalkay H, Lackner HK, Rössler A, Narath B, Jantscher A, Goswami N. Hormonal and plasma volume changes after presyncope. Eur J Clin Invest. 2011 Nov;41(11):1180-5. | CrossRef | PubMed |
Hinghofer-Szalkay H, Lackner HK, Rössler A, Narath B, Jantscher A, Goswami N. Hormonal and plasma volume changes after presyncope. Eur J Clin Invest. 2011 Nov;41(11):1180-5. | CrossRef | PubMed | Charkoudian N, Rabbitts JA. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc. 2009 Sep;84(9):822-30. | CrossRef | PubMed |
Charkoudian N, Rabbitts JA. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc. 2009 Sep;84(9):822-30. | CrossRef | PubMed | Mosqueda-Garcia R, Furlan R, Fernandez-Violante R, Desai T, Snell M, Jarai Z, et al. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest. 1997 Jun 1;99(11):2736-44. | PubMed |
Mosqueda-Garcia R, Furlan R, Fernandez-Violante R, Desai T, Snell M, Jarai Z, et al. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest. 1997 Jun 1;99(11):2736-44. | PubMed | Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM, Robertson D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. J Appl Physiol (1985). 1998 Mar;84(3):914-21. | PubMed |
Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM, Robertson D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. J Appl Physiol (1985). 1998 Mar;84(3):914-21. | PubMed | Meredith IT, Eisenhofer G, Lambert GW, Jennings GL, Thompson J, Esler MD. Plasma norepinephrine responses to head-up tilt are misleading in autonomic failure. Hypertension. 1992 Jun;19(6 Pt 2):628-33. | PubMed |
Meredith IT, Eisenhofer G, Lambert GW, Jennings GL, Thompson J, Esler MD. Plasma norepinephrine responses to head-up tilt are misleading in autonomic failure. Hypertension. 1992 Jun;19(6 Pt 2):628-33. | PubMed | Thorén P. Role of cardiac vagal C-fibers in cardiovascular control. Rev Physiol Biochem Pharmacol. 1979;86:1-94. | PubMed |
Thorén P. Role of cardiac vagal C-fibers in cardiovascular control. Rev Physiol Biochem Pharmacol. 1979;86:1-94. | PubMed | Kaufmann H. Neurally mediated syncope: pathogenesis, diagnosis, and treatment. Neurology. 1995 Apr;45(4 Suppl 5):S12-8. | PubMed |
Kaufmann H. Neurally mediated syncope: pathogenesis, diagnosis, and treatment. Neurology. 1995 Apr;45(4 Suppl 5):S12-8. | PubMed | Novak V, Honos G, Schondorf R. Is the heart "empty' at syncope? J Auton Nerv Syst. 1996 Aug 27;60(1-2):83-92. | PubMed |
Novak V, Honos G, Schondorf R. Is the heart "empty' at syncope? J Auton Nerv Syst. 1996 Aug 27;60(1-2):83-92. | PubMed | Liu JE, Hahn RT, Stein KM, Markowitz SM, Okin PM, Devereux RB, et al. Left ventricular geometry and function preceding neurally mediated syncope. Circulation. 2000 Feb 22;101(7):777-83. | PubMed |
Liu JE, Hahn RT, Stein KM, Markowitz SM, Okin PM, Devereux RB, et al. Left ventricular geometry and function preceding neurally mediated syncope. Circulation. 2000 Feb 22;101(7):777-83. | PubMed | Jardine DL, Ikram H, Frampton CM, Frethey R, Bennett SI, Crozier IG. Autonomic control of vasovagal syncope. Am J Physiol. 1998 Jun;274(6 Pt2):H2110-5. | PubMed |
Jardine DL, Ikram H, Frampton CM, Frethey R, Bennett SI, Crozier IG. Autonomic control of vasovagal syncope. Am J Physiol. 1998 Jun;274(6 Pt2):H2110-5. | PubMed | Béchir M, Binggeli C, Corti R, Chenevard R, Spieker L, Ruschitzka F, et al. Dysfunctional baroreflex regulation of sympathetic nerve activity in patients with vasovagal syncope. Circulation. 2003 Apr 1;107(12):1620-5. | CrossRef | PubMed |
Béchir M, Binggeli C, Corti R, Chenevard R, Spieker L, Ruschitzka F, et al. Dysfunctional baroreflex regulation of sympathetic nerve activity in patients with vasovagal syncope. Circulation. 2003 Apr 1;107(12):1620-5. | CrossRef | PubMed | Vaddadi G, Esler MD, Dawood T, Lambert E. Persistence of muscle sympathetic nerve activity during vasovagal syncope. Eur Heart J. 2010 Aug;31 (16):2027-33. | CrossRef | PubMed |
Vaddadi G, Esler MD, Dawood T, Lambert E. Persistence of muscle sympathetic nerve activity during vasovagal syncope. Eur Heart J. 2010 Aug;31 (16):2027-33. | CrossRef | PubMed | Verheyden B, Liu J, van Dijk N, Westerhof BE, Reybrouck T, Aubert AE, et al. Steep fall in cardiac output is main determinant of hypotension during drug-free and nitroglycerine-induced orthostatic vasovagal syncope. Heart Rhythm. 2008 Dec;5(12):1695-701. | CrossRef | PubMed |
Verheyden B, Liu J, van Dijk N, Westerhof BE, Reybrouck T, Aubert AE, et al. Steep fall in cardiac output is main determinant of hypotension during drug-free and nitroglycerine-induced orthostatic vasovagal syncope. Heart Rhythm. 2008 Dec;5(12):1695-701. | CrossRef | PubMed | Vaddadi G, Guo L, Esler M, Socratous F, Schlaich M, Chopra R, et al. Recurrent postural vasovagal syncope: sympathetic nervous system phenotypes. Circ Arrhythm Electrophysiol. 2011 Oct;4(5):711-8. | CrossRef | PubMed |
Vaddadi G, Guo L, Esler M, Socratous F, Schlaich M, Chopra R, et al. Recurrent postural vasovagal syncope: sympathetic nervous system phenotypes. Circ Arrhythm Electrophysiol. 2011 Oct;4(5):711-8. | CrossRef | PubMed | Márquez MF, Urias KI, Hermosillo AG, Jardón JL, Iturralde P, Colín L, et al. Familial vasovagal syncope. Europace. 2005 Sep;7(5):472-4. | PubMed |
Márquez MF, Urias KI, Hermosillo AG, Jardón JL, Iturralde P, Colín L, et al. Familial vasovagal syncope. Europace. 2005 Sep;7(5):472-4. | PubMed | Márquez MF, Hernández-Pacheco G, Hermosillo AG, Gómez JR, Cárdenas M, Vargas-Alarcón G. The Arg389Gly beta1-adrenergic receptor gene polymorphism and susceptibility to faint during head-up tilt test. Europace. 2007 Aug;9(8):585-8. | PubMed |
Márquez MF, Hernández-Pacheco G, Hermosillo AG, Gómez JR, Cárdenas M, Vargas-Alarcón G. The Arg389Gly beta1-adrenergic receptor gene polymorphism and susceptibility to faint during head-up tilt test. Europace. 2007 Aug;9(8):585-8. | PubMed | Hernández-Pacheco G, González-Hermosillo A, Murata C, Yescas P, Espínola-Zavaleta N, Martínez M, et al. Arg347Cys polymorphism of α1a-adrenergic receptor in vasovagal syncope. Case-control study in a Mexican population. Auton Neurosci. 2014 Jul;183:66-71. | CrossRef | PubMed |
Hernández-Pacheco G, González-Hermosillo A, Murata C, Yescas P, Espínola-Zavaleta N, Martínez M, et al. Arg347Cys polymorphism of α1a-adrenergic receptor in vasovagal syncope. Case-control study in a Mexican population. Auton Neurosci. 2014 Jul;183:66-71. | CrossRef | PubMed | Márquez MF, Urias-Medina K, Gómez-Flores J, Sobrino A, Sotomayor-González A, González-Hermosillo A, et al. [Comparison of metoprolol vs clonazepam as a first treatment choice among patients with neurocardiogenic syncope]. Gac Med Mex. 2008 Nov-Dec;144(6):503-7. | PubMed |
Márquez MF, Urias-Medina K, Gómez-Flores J, Sobrino A, Sotomayor-González A, González-Hermosillo A, et al. [Comparison of metoprolol vs clonazepam as a first treatment choice among patients with neurocardiogenic syncope]. Gac Med Mex. 2008 Nov-Dec;144(6):503-7. | PubMed | Sheldon R, Connolly S, Rose S, Klingenheben T, Krahn A, Morillo C, et al. Prevention of Syncope Trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation. 2006 Mar 7;113(9):1164-70. | PubMed |
Sheldon R, Connolly S, Rose S, Klingenheben T, Krahn A, Morillo C, et al. Prevention of Syncope Trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation. 2006 Mar 7;113(9):1164-70. | PubMed | Sheldon RS, Morillo CA, Klingenheben T, Krahn AD, Sheldon A, Rose MS. Age-dependent effect of β-blockers in preventing vasovagal syncope. Circ Arrhythm Electrophysiol. 2012 Oct;5(5):920-6. | CrossRef | PubMed |
Sheldon RS, Morillo CA, Klingenheben T, Krahn AD, Sheldon A, Rose MS. Age-dependent effect of β-blockers in preventing vasovagal syncope. Circ Arrhythm Electrophysiol. 2012 Oct;5(5):920-6. | CrossRef | PubMed | Benditt DG, Detloff BL, Adkisson WO, Lu F, Sakaguchi S, Schussler S, et al. Age-dependence of relative change in circulating epinephrine and norepinephrine concentrations during tilt-induced vasovagal syncope. Heart Rhythm. 2012 Nov;9(11):1847-52. | CrossRef | PubMed |
Benditt DG, Detloff BL, Adkisson WO, Lu F, Sakaguchi S, Schussler S, et al. Age-dependence of relative change in circulating epinephrine and norepinephrine concentrations during tilt-induced vasovagal syncope. Heart Rhythm. 2012 Nov;9(11):1847-52. | CrossRef | PubMed | Raj SR, Faris PD, Semeniuk L, Manns B, Krahn AD, Morillo CA, et al. Rationale for the Assessment of Metoprolol in the Prevention of Vasovagal Syncope in Aging Subjects Trial (POST5). Am Heart J. 2016 Apr;174:89-94. | CrossRef | PubMed |
Raj SR, Faris PD, Semeniuk L, Manns B, Krahn AD, Morillo CA, et al. Rationale for the Assessment of Metoprolol in the Prevention of Vasovagal Syncope in Aging Subjects Trial (POST5). Am Heart J. 2016 Apr;174:89-94. | CrossRef | PubMed | Raj SR, Coffin ST. Medical therapy and physical maneuvers in the treatment of the vasovagal syncope and orthostatic hypotension. Prog Cardiovasc Dis. 2013 Jan-Feb;55(4):425-33. | CrossRef | PubMed |
Raj SR, Coffin ST. Medical therapy and physical maneuvers in the treatment of the vasovagal syncope and orthostatic hypotension. Prog Cardiovasc Dis. 2013 Jan-Feb;55(4):425-33. | CrossRef | PubMed | Vyas A, Swaminathan PD, Zimmerman MB, Olshansky B. Are treatments for vasovagal syncope effective? A meta-analysis. Int J Cardiol. 2013 Sep 1;167(5):1906-11. | CrossRef | PubMed |
Vyas A, Swaminathan PD, Zimmerman MB, Olshansky B. Are treatments for vasovagal syncope effective? A meta-analysis. Int J Cardiol. 2013 Sep 1;167(5):1906-11. | CrossRef | PubMed | Romme JJ, Reitsma JB, Black CN, Colman N, Scholten RJ, Wieling W, et al. Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst Rev. 2011 Oct 5;(10):CD004194. | CrossRef | PubMed |
Romme JJ, Reitsma JB, Black CN, Colman N, Scholten RJ, Wieling W, et al. Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst Rev. 2011 Oct 5;(10):CD004194. | CrossRef | PubMed | Pérez V, Puiigdemont D, Gilaberte I, Alvarez E, Artigas F; Grup de Recerca en Trastorns Afectius. Augmentation of fluoxetine's antidepressant action by pindolol: analysis of clinical, pharmacokinetic, and methodologic factors. J Clin Psychopharmacol. 2001 Feb;21(1):36-45. | PubMed |
Pérez V, Puiigdemont D, Gilaberte I, Alvarez E, Artigas F; Grup de Recerca en Trastorns Afectius. Augmentation of fluoxetine's antidepressant action by pindolol: analysis of clinical, pharmacokinetic, and methodologic factors. J Clin Psychopharmacol. 2001 Feb;21(1):36-45. | PubMed | Goldstein DS, Holmes C, Frank SM, Dendi R, Cannon RO 3rd, Sharabi Y, et al. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation. 2002 Oct 29;106(18):2358-65. | PubMed |
Goldstein DS, Holmes C, Frank SM, Dendi R, Cannon RO 3rd, Sharabi Y, et al. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation. 2002 Oct 29;106(18):2358-65. | PubMed | Schroeder C, Tank J, Boschmann M, et al. Selective norepinephrine reuptake inhibition as a human model of orthostatic intolerance. Circulation. 2002 Jan 22;105(3):347-53. | CrossRef | PubMed |
Schroeder C, Tank J, Boschmann M, et al. Selective norepinephrine reuptake inhibition as a human model of orthostatic intolerance. Circulation. 2002 Jan 22;105(3):347-53. | CrossRef | PubMed | Schroeder C, Birkenfeld AL, Mayer AF, Tank J, Diedrich A, Luft FC, et al. Norepinephrine transporter inhibition prevents tilt-induced pre-syncope. J Am Coll Cardiol. 2006 Aug 1;48(3):516-22. | PubMed |
Schroeder C, Birkenfeld AL, Mayer AF, Tank J, Diedrich A, Luft FC, et al. Norepinephrine transporter inhibition prevents tilt-induced pre-syncope. J Am Coll Cardiol. 2006 Aug 1;48(3):516-22. | PubMed | Schroeder C, Jordan J. Norepinephrine transporter function and human cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012 Dec 1;30(11):H1273-82. | CrossRef | PubMed |
Schroeder C, Jordan J. Norepinephrine transporter function and human cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012 Dec 1;30(11):H1273-82. | CrossRef | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








