Key Words: Diabetic macular edema, aflibercept, dexamethasone, Epistemonikos, GRADE.
Abstract
INTRODUCTION
Diabetic macular edema is a frequent pathology that causes gradual deterioration of visual acuity, which does not have a standardized treatment. The anti-vascular endothelial growth factor (anti-VEGF) drugs and corticosteroids are widely used, especially aflibercept and dexamethasone, respectively, but it is unclear which one is best.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
No evidence that compared the interventions directly in the population of interest was found, so systematic reviews that provide an estimate of the effect indirectly using network meta-analysis were selected. We identified two systematic reviews that together included four primary studies, all randomized trials. We concluded that we are uncertain whether aflibercept compared to dexamethasone improves visual acuity or is safer, as the certainty of the evidence has been assessed as very low.
Problem
Diabetic macular edema is a manifestation of diabetic retinopathy, one of the most frequent eye diseases worldwide. The usual presentation of this condition is gradual deterioration of visual acuity, which without treatment is usually associated with a poor prognosis. For many years, laser therapy was used to reduce symptoms, but its results and multiple complications increased the urge to find alternative therapies. Nowadays, the most commonly used treatment by specialists is periodic intravitreal injection of anti-vascular endothelial growth factor drugs (anti-VEGF), among which aflibercept stands out, mainly due to its longer half-life, and corticosteroids, more specifically dexamethasone. Despite the above, there is no clear superiority between them, so it is important to summarize the evidence that allows us to compare both treatments..
Methods
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
No evidence that compares the interventions directly in the population of interest was found, so systematic reviews that provide an estimate of the effect indirectly using network meta-analysis were selected. We found two systematic reviews [1], [2], which included four primary studies [3], [4], [5], [6], all randomized trials. This summary is based on the latter, since they evaluate the intervention in the population of interest, but against a different comparison. |
|
What types of patients were included* |
All trials included patients with the diagnosis of diabetic macular edema. None of the reviews reported if all the included patients had central involvement. |
|
What types of interventions were included* |
Two trials evaluated aflibercept [5], [6]: one compared it against other medications [4] and the other trial compared it against placebo [6]. Two trials evaluated dexamethasone [3], [5]: one evaluated the addition of dexamethasone to laser treatment [3] and the other compared it against another drug [5]. |
|
What types of outcomes |
The trials reported multiple outcomes, which were grouped by systematic reviews as follows:
|
*Information about primary studies is not extracted directly from primary studies but from identified systematic reviews, unless otherwise stated.
Summary of findings
No direct evidence that compares aflibercept with dexamethasone in diabetic macular edema was found. Information of the effects of aflibercept compared to dexamethasone in diabetic macular edema is based on two systematic reviews that performed an indirect comparison using network meta-analyses <a data-dropdown="drop1" href="#">[1]</a>,<a data-dropdown="drop2" href="#">[2]</a>.
Since no studies were identified evaluating the question directly, the results in the summary of findings table are based on punctual estimators from each of the systematic reviews. All reviews <a data-dropdown="drop1" href="#">[1]</a>, <a data-dropdown="drop2" href="#">[2]</a> assessed visual acuity outcomes and adverse events.
The summary of findings is as follows:
- We are uncertain whether aflibercept compared to dexamethasone improves visual acuity as the certainty of the evidence has been assessed as very low.
- We are uncertain whether aflibercept compared to dexamethasone reduces adverse events as the certainty of the evidence has been assessed as very low.
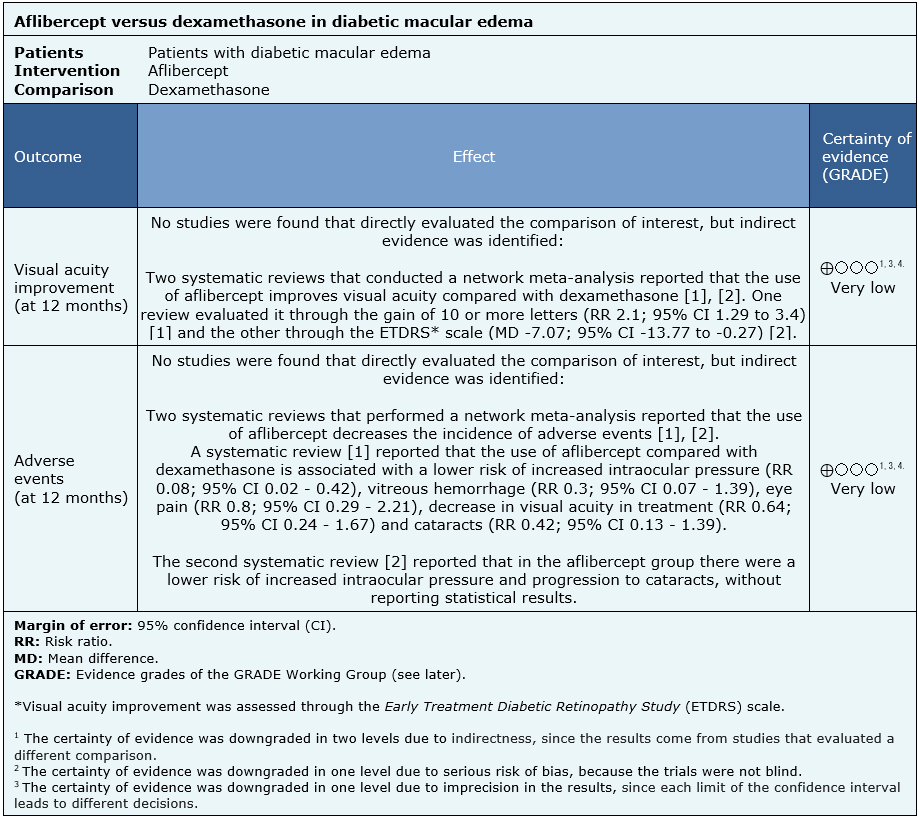
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
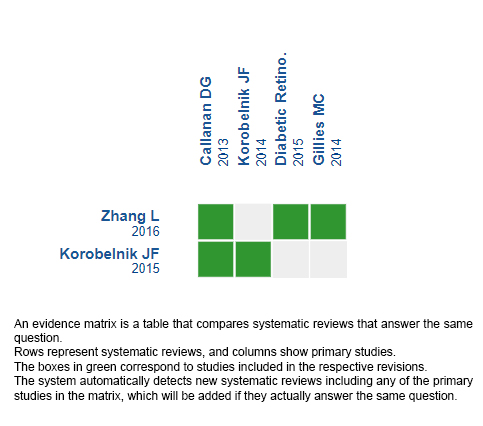
Follow the link to access the interactive version: Aflibercept versus dexamethasone in diabetic macular edema.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
El edema macular diabético es una patología frecuente, causante de deterioro gradual de la agudeza visual, que no tiene un tratamiento estandarizado. Los fármacos anti factor del crecimiento vascular endotelial (anti-VEGF) y los corticoides se encuentran entre los tratamientos más ampliamente utilizados, destacando aflibercept y dexametasona, respectivamente, sin haber una clara superioridad entre ambas terapias.
MÉTODOS
Realizamos una búsqueda en Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante el cribado de múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, analizamos los datos de los estudios primarios, realizamos un meta análisis y preparamos una tabla de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
No se encontró evidencia que compare las intervenciones directamente en la población de interés, por lo que se seleccionaron revisiones sistemáticas que entregan una estimación del efecto de manera indirecta, mediante la técnica de metanálisis de comparaciones múltiples (metanálisis en red). Identificamos dos revisiones sistemáticas que en conjunto incluyeron cuatro estudios primarios, todos ensayos aleatorizados. Concluimos que no es posible establecer con claridad si usar aflibercept comparado con dexametasona aumenta la agudeza visual o es más seguro, debido a que la certeza de la evidencia existente ha sido evaluada como muy baja.
 Authors:
Rodolfo Garretón[1,2], Raúl González[1,2]
Authors:
Rodolfo Garretón[1,2], Raúl González[1,2]
Affiliation:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Diagonal Paraguay 476 Santiago Chile
E-mail: raulgonzalezc@gmail.com

Citation: Garretón R, González R. Aflibercept compared with dexamethasone in diabetic macular edema. Medwave 2021;21(04):e8166 doi: 10.5867/medwave.2021.04.8166
Submission date: 13/11/2019
Acceptance date: 26/12/2019
Publication date: 4/5/2021
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Centro Evidencia UC in collaboration with the Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Korobelnik J, Kleijnen J, Lang S, Birnie R, Leadley R, Misso K et al. Systematic review and mixed treatment comparison of intravitreal aflibercept with other therapies for diabetic macular edema (DME). BMC Ophthalmology. 2015;15(1).
- Zhang L, Wang W, Gao Y, Lan J, Xie L. The Efficacy and Safety of Current Treatments in Diabetic Macular Edema: A Systematic Review and Network Meta-Analysis. PLOS ONE. 2016;11(7):e0159553.
- Callanan D, Gupta S, Boyer D, Ciulla T, Singer M, Kuppermann B et al. Dexamethasone Intravitreal Implant in Combination with Laser Photocoagulation for the Treatment of Diffuse Diabetic Macular Edema. Ophthalmology. 2013;120(9):1843-1851.
- Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. New England Journal of Medicine. 2015;372(13):1193-1203.
- Gillies M, Lim L, Campain A, Quin G, Salem W, Li J et al. A Randomized Clinical Trial of Intravitreal Bevacizumab versus Intravitreal Dexamethasone for Diabetic Macular Edema. Ophthalmology. 2014;121(12):2473-2481.
- Korobelnik J, Do D, Schmidt-Erfurth U, Boyer D, Holz F, Heier J et al. Intravitreal Aflibercept for Diabetic Macular Edema. Ophthalmology. 2014;121(11):2247-2254.
- American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern® Guidelines. Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2017. | Link |
- Manejo de las complicaciones oculares de la diabetes. Retinopatía Diabética y Edema Macular. “Guías de Práctica Clínica de la SERV” | Link |
- Haoyu Li, Wenhua Xu, Hao Chen, Zhongxu Tian. Comparison between ozurdex and intravitreal anti vascular endothelial growth factor (anti-VEGF) treatment for diabetic macular edema: a meta-analysis. PROSPERO 2018 CRD42018109883. | Link |
 Korobelnik J, Kleijnen J, Lang S, Birnie R, Leadley R, Misso K et al. Systematic review and mixed treatment comparison of intravitreal aflibercept with other therapies for diabetic macular edema (DME). BMC Ophthalmology. 2015;15(1).
Korobelnik J, Kleijnen J, Lang S, Birnie R, Leadley R, Misso K et al. Systematic review and mixed treatment comparison of intravitreal aflibercept with other therapies for diabetic macular edema (DME). BMC Ophthalmology. 2015;15(1).  Zhang L, Wang W, Gao Y, Lan J, Xie L. The Efficacy and Safety of Current Treatments in Diabetic Macular Edema: A Systematic Review and Network Meta-Analysis. PLOS ONE. 2016;11(7):e0159553.
Zhang L, Wang W, Gao Y, Lan J, Xie L. The Efficacy and Safety of Current Treatments in Diabetic Macular Edema: A Systematic Review and Network Meta-Analysis. PLOS ONE. 2016;11(7):e0159553.  Callanan D, Gupta S, Boyer D, Ciulla T, Singer M, Kuppermann B et al. Dexamethasone Intravitreal Implant in Combination with Laser Photocoagulation for the Treatment of Diffuse Diabetic Macular Edema. Ophthalmology. 2013;120(9):1843-1851.
Callanan D, Gupta S, Boyer D, Ciulla T, Singer M, Kuppermann B et al. Dexamethasone Intravitreal Implant in Combination with Laser Photocoagulation for the Treatment of Diffuse Diabetic Macular Edema. Ophthalmology. 2013;120(9):1843-1851.  Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. New England Journal of Medicine. 2015;372(13):1193-1203.
Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. New England Journal of Medicine. 2015;372(13):1193-1203.  Gillies M, Lim L, Campain A, Quin G, Salem W, Li J et al. A Randomized Clinical Trial of Intravitreal Bevacizumab versus Intravitreal Dexamethasone for Diabetic Macular Edema. Ophthalmology. 2014;121(12):2473-2481.
Gillies M, Lim L, Campain A, Quin G, Salem W, Li J et al. A Randomized Clinical Trial of Intravitreal Bevacizumab versus Intravitreal Dexamethasone for Diabetic Macular Edema. Ophthalmology. 2014;121(12):2473-2481.  Korobelnik J, Do D, Schmidt-Erfurth U, Boyer D, Holz F, Heier J et al. Intravitreal Aflibercept for Diabetic Macular Edema. Ophthalmology. 2014;121(11):2247-2254.
Korobelnik J, Do D, Schmidt-Erfurth U, Boyer D, Holz F, Heier J et al. Intravitreal Aflibercept for Diabetic Macular Edema. Ophthalmology. 2014;121(11):2247-2254.  American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern® Guidelines. Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2017. | Link |
American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern® Guidelines. Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2017. | Link | Manejo de las complicaciones oculares de la diabetes. Retinopatía Diabética y Edema Macular. “Guías de Práctica Clínica de la SERV” | Link |
Manejo de las complicaciones oculares de la diabetes. Retinopatía Diabética y Edema Macular. “Guías de Práctica Clínica de la SERV” | Link | Haoyu Li, Wenhua Xu, Hao Chen, Zhongxu Tian. Comparison between ozurdex and intravitreal anti vascular endothelial growth factor (anti-VEGF) treatment for diabetic macular edema: a meta-analysis. PROSPERO 2018 CRD42018109883. | Link |
Haoyu Li, Wenhua Xu, Hao Chen, Zhongxu Tian. Comparison between ozurdex and intravitreal anti vascular endothelial growth factor (anti-VEGF) treatment for diabetic macular edema: a meta-analysis. PROSPERO 2018 CRD42018109883. | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








