Key Words: PET-CT, testicular cancer, seminoma, Epistemonikos, GRADE.
Resumen
INTRODUCCIÓN
En pacientes con cáncer testicular avanzado tipo seminoma que tienen lesiones residuales post quimioterapia de más de 3 cm, el PET-CT podría seleccionar un subgrupo susceptible de ser manejado con seguimiento, evitando una resección quirúrgica innecesaria de tumor no viable.
MÉTODOS
Realizamos una búsqueda en Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante el cribado de múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, analizamos los datos de los estudios primarios, realizamos un metanálisis y preparamos una tabla de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Identificamos tres revisiones sistemáticas que en conjunto incluyeron 11 estudios primarios, de los cuales, ninguno es un ensayo aleatorizado. Concluimos que el uso de PET-CT en la evaluación de masas residuales post quimioterapia en pacientes con cáncer testicular tipo seminoma podría evitar un porcentaje importante de cirugías innecesarias (certeza de la evidencia baja). Además, el uso de PET-CT podría presentar balances riesgo/beneficio y costo/beneficio favorables en el manejo de pacientes con cáncer testicular tipo seminoma. Sin embargo, se requieren revisiones sistemáticas y estudios primarios que evalúen directamente el impacto diagnóstico del test.
Problem
Testicular germ cell cancer is the most common cancer among males aged 15-44. Testicular germ cell tumors can be divided into two categories according to clinical management: seminoma and non-seminoma. About 30% of testicular germ cell cancers are diagnosed with metastatic disease or eventually develop systemic recurrences. For these cases, therapy is still administered with curative intent and consists on platinum-based chemotherapy plus surgery, in order to remove post-treatment residual masses. Survival rates for treated patients are among the highest in metastatic solid tumors, reaching up to 90% of cured patients in low-risk cases. Therefore, current studies seek to minimize treatment-related toxicities [1].
In seminoma germ cell tumors, treatment induces a desmoplastic reaction in the tumor tissue that increases both the complexity and the morbidity of its surgical resection. Furthermore, in several cases, these lesions contain unviable tumor tissue, regardless of their size. Considering these features, the safety of clinical follow up without surgery of post-chemotherapy lesions measuring <3 cm is widely accepted [2].
Nowadays, positron emission tomography-computed tomography (PET-CT) has become a commonly used technique to select testicular seminoma patients that could potentially benefit of surveillance of post-treatment residual masses, avoiding surgical resection, even in lesions> 3cm.
Methods
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
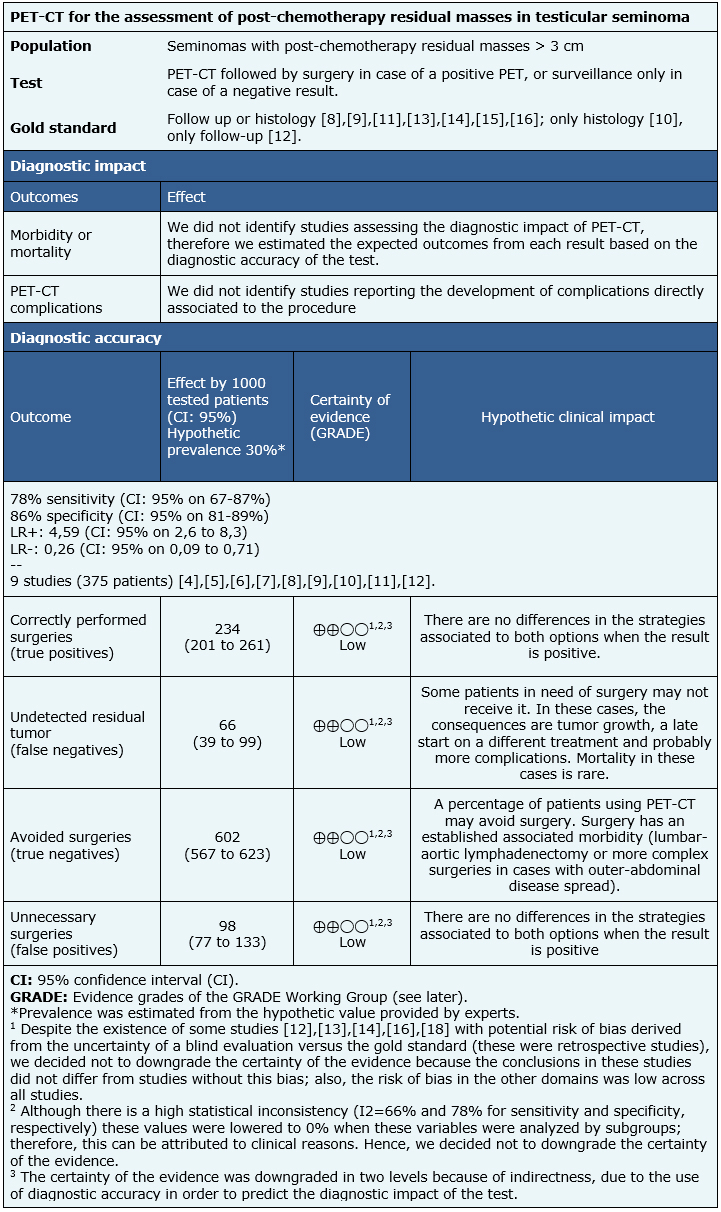
We found three systematic reviews [3], [4], [5] including eleven primary studies across 13 references [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]; however, none of them assessed the diagnostic impact of PET-CT. Instead, they reported the diagnostic accuracy of the test. This summary involves only testicular seminoma patients. Therefore, one of the abovementioned references was excluded due to the use of a separate analysis for seminomas and non-seminomas [4]. Another reference was also excluded because of methodological limitations [5]. Two studies [6], [7] were also excluded because their data could not be entered into the meta-analysis. Hence, this table and the summary in general are based on nine primary studies [8], [9], [10], [11], [12], [13], [14], [15], [16], using the diagnostic accuracy data provided by one of the reviews [3]. |
|
What types of patients were included* |
Average age reported in these studies ranged from 29 to 42.5 years. Six studies exclusively reported testicular seminoma [9], [10], [11], [12], [13], [15], and other three included both seminoma and non-seminoma [8], [14], [16]. The profile of tumor markers could not be obtained from the systematic review included in this analysis [3]. A study included post-chemotherapy patients after the first line of treatment [8]; the rest were post-chemotherapy in the first-line and/or as salvage treatment [9], [10], [11], [12], [13], [14], [15], [16]. One study also included post-radiotherapy patients [13]. |
|
What types of interventions were included* |
All studies used 18F-fluoro-deoxy-glucose (18F-FDG) [8], [9], [10], [11], [12], [13], [14], [15], [16]. Four studies used PET-CT/FDG [6], [13], [14], [17]. Post-chemotherapy time varied between 4-12 weeks. The 18F-FDG dosing and administration followed standardized protocols by each participating center. All studies compared against CT [8], [9], [10], [11], [12], [13], [14], [15], [16]. Gold standard utilized were: follow up in one study [12], histology obtained from surgery [10] and follow up or histology in the other seven studies [8], [9], [11], [13], [14], [15], [16]. |
|
What types of outcomes |
Given the absence of studies on the diagnostic impact, the diagnostic properties of this test were reported including sensitivity, specificity, predictive value (both positive and negative), and positive and negative likelihood ratio (LR+ and LR-, respectively) to identify the presence of viable tumor in post-chemotherapy residual masses. The reported follow up time ranged from 4 to 12 months. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
We could not identify systematic reviews assessing the diagnostic impact of PET-CT; the evidence was limited to diagnostic accuracy. Data and results were obtained from one systematic review [3] that included nine primary studies [8], [9], [10], [11], [12], [13], [14], [15], [16], whose diagnostic accuracy data were entered into a meta-analysis.
The summary of findings is as follows:
- The assessment of postchemotherapy residual masses by PET-CT in testicular seminoma patients may potentially lower the number of unnecessary surgeries (low certainty of evidence).

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
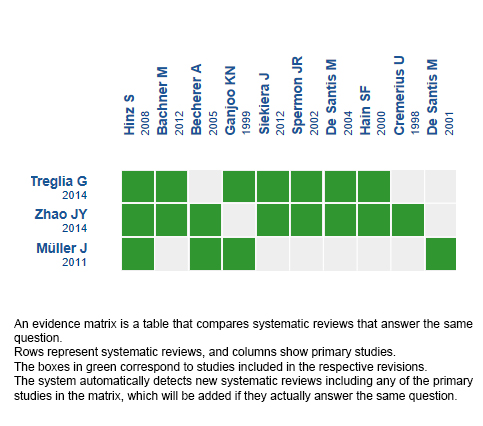
Follow the link to access the interactive version: PET/CT for the management of testicular seminoma with residual mass after chemotherapy.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
En pacientes con cáncer testicular avanzado tipo seminoma que tienen lesiones residuales post quimioterapia de más de 3 cm, el PET-CT podría seleccionar un subgrupo susceptible de ser manejado con seguimiento, evitando una resección quirúrgica innecesaria de tumor no viable.
MÉTODOS
Realizamos una búsqueda en Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante el cribado de múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, analizamos los datos de los estudios primarios, realizamos un metanálisis y preparamos una tabla de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Identificamos tres revisiones sistemáticas que en conjunto incluyeron 11 estudios primarios, de los cuales, ninguno es un ensayo aleatorizado. Concluimos que el uso de PET-CT en la evaluación de masas residuales post quimioterapia en pacientes con cáncer testicular tipo seminoma podría evitar un porcentaje importante de cirugías innecesarias (certeza de la evidencia baja). Además, el uso de PET-CT podría presentar balances riesgo/beneficio y costo/beneficio favorables en el manejo de pacientes con cáncer testicular tipo seminoma. Sin embargo, se requieren revisiones sistemáticas y estudios primarios que evalúen directamente el impacto diagnóstico del test.
 Authors:
Oscar Corsi[1,2], Gabriel Rada[1,2,3], José Peña[2,4,5,6]
Authors:
Oscar Corsi[1,2], Gabriel Rada[1,2,3], José Peña[2,4,5,6]
Affiliation:
[1] Departamento de Medicina Interna, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Centro Evidencia UC, Pontificia Universidad Católica de Chile, Santiago, Chile
[4] Departamento Hemato-Oncología, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[5] Centro de Cáncer Nuestra Señora de la Esperanza, Red de Salud UC-Christus, Santiago, Chile
[6] Centro Oncológico, Complejo Asistencial Dr. Sótero del Río, Santiago, Chile
E-mail: jepena@uc.cl
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Diagonal Paraguay 476 Santiago Chile

Citation: Corsi O, Rada G, Peña J. PET-CT for the management of testicular seminoma patients with post-chemotherapy residual masses. Medwave 2019;19(4):e7625 doi: 10.5867/medwave.2019.04.7625
Submission date: 4/7/2018
Acceptance date: 5/4/2019
Publication date: 6/5/2019
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Haugnes HS, Stephenson AJ, Feldman DR. Beyond stage I germ cell tumors: current status regarding treatment and long-term toxicities. Am Soc Clin Oncol Educ Book. 2014:e180-90. | CrossRef | PubMed |
- Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA. 2008 Feb 13;299(6):672-84. | CrossRef | PubMed |
- Treglia G, Sadeghi R, Annunziata S, Caldarella C, Bertagna F, Giovanella L. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in the postchemotherapy management of patients with seminoma: systematic review and meta-analysis. Biomed Res Int. 2014;2014:852681. | CrossRef | PubMed | PMC |
- Zhao JY, Ma XL, Li YY, Zhang BL, Li MM, Ma XL, Liu L. Diagnostic accuracy of 18F-FDG-PET in patients with testicular cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(8):3525-31. | PubMed |
- Müller J, Schrader AJ, Jentzmik F, Schrader M. [Assessment of residual tumours after systemic treatment of metastatic seminoma: ¹⁸F-2-fluoro-2-deoxy-D-glucose positron emission tomography - meta-analysis of diagnostic value]. Urologe A. 2011 Mar;50(3):322-7. | CrossRef | PubMed |
- Cremerius U, Effert PJ, Adam G, Sabri O, Zimmy M, Wagenknecht G, Jakse G, Buell U. FDG PET for detection and therapy control of metastatic germ cell tumor. J Nucl Med. 1998 May;39(5):815-22. | PubMed |
- Akbulut Z, Canda AE, Atmaca AF, Caglayan A, Asil E, Balbay MD. Is positron emission tomography reliable to predict post-chemotherapy retroperitoneal lymph node involvement in advanced germ cell tumors of the testis? Urol J. 2011 Spring;8(2):120-6. | PubMed |
- Spermon JR, De Geus-Oei LF, Kiemeney LA, Witjes JA, Oyen WJ. The role of (18)fluoro-2-deoxyglucose positron emission tomography in initial staging and re-staging after chemotherapy for testicular germ cell tumours. BJU Int. 2002 Apr;89(6):549-56. | PubMed |
- Lewis DA, Tann M, Kesler K, McCool A, Foster RS, Einhorn LH. Positron emission tomography scans in postchemotherapy seminoma patients with residual masses: a retrospective review from Indiana University Hospital. J Clin Oncol. 2006 Dec 1;24(34):e54-5. | PubMed |
- Hinz S, Schrader M, Kempkensteffen C, Bares R, Brenner W, Krege S, Franzius C, Kliesch S, Heicappel R, Miller K, de Wit M. The role of positron emission tomography in the evaluation of residual masses after chemotherapy for advanced stage seminoma. J Urol. 2008 Mar;179(3):936-40; discussion 940. | CrossRef | PubMed |
- Bachner M, Loriot Y, Gross-Goupil M, Zucali PA, Horwich A, Germa-Lluch JR, Kollmannsberger C, Stoiber F, Fléchon A, Oechsle K, Gillessen S, Oldenburg J, Cohn-Cedermark G, Daugaard G, Morelli F, Sella A, Harland S, Kerst M, Gampe J, Dittrich C, Fizazi K, De Santis M. 2-¹⁸fluoro-deoxy-D-glucose positron emission tomography (FDG-PET) for postchemotherapy seminoma residual lesions: a retrospective validation of the SEMPET trial. Ann Oncol. 2012 Jan;23(1):59-64. | CrossRef | PubMed |
- Ganjoo KN, Chan RJ, Sharma M, Einhorn LH. Positron emission tomography scans in the evaluation of postchemotherapy residual masses in patients with seminoma. J Clin Oncol. 1999 Nov;17(11):3457-60. | PubMed |
- Siekiera J, Małkowski B, Jóźwicki W, Jasiński M, Wronczewski A, Pietrzak T, Chmielowska E, Petrus A, Kamecki K, Mikołajczak W, Kraśnicki K, Chłosta P, Drewa T. Can we rely on PET in the follow-up of advanced seminoma patients? Urol Int. 2012;88(4):405-9. | CrossRef | PubMed |
- Ambrosini V, Zucchini G, Nicolini S, Berselli A, Nanni C, Allegri V, Martoni A, Rubello D, Cricca A, Fanti S. 18F-FDG PET/CT impact on testicular tumours clinical management. Eur J Nucl Med Mol Imaging. 2014 Apr;41(4):668-73. Erratum in: Eur J Nucl Med Mol Imaging. 2014 Mar;41(3):585. | PubMed |
- De Santis M, Becherer A, Bokemeyer C, Stoiber F, Oechsle K, Sellner F, Lang A, Kletter K, Dohmen BM, Dittrich C, Pont J. 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. J Clin Oncol. 2004 Mar 15;22(6):1034-9. | PubMed |
- Hain SF, O'Doherty MJ, Timothy AR, Leslie MD, Harper PG, Huddart RA. Fluorodeoxyglucose positron emission tomography in the evaluation of germ cell tumours at relapse. British journal of cancer. 2000;83(7):863-9.
- Becherer A, De Santis M, Karanikas G, Szabó M, Bokemeyer C, Dohmen BM, Pont J, Dudczak R, Dittrich C, Kletter K. FDG PET is superior to CT in the prediction of viable tumour in post-chemotherapy seminoma residuals. Eur J Radiol. 2005 May;54(2):284-8. | PubMed |
- De Santis M, Bokemeyer C, Becherer A, Stoiber F, Oechsle K, Kletter K, Dohmen BM, Dittrich C, Pont J. Predictive impact of 2-18fluoro-2-deoxy-D-glucose positron emission tomography for residual postchemotherapy masses in patients with bulky seminoma. J Clin Oncol. 2001 Sep 1;19(17):3740-4. Erratum in: J Clin Oncol 2001 Dec 1;19(23):4355. | PubMed |
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Testicular cancer 2.2017 [cited Nov 28, 2017] | Link |
- Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP, Nicolai N, Oldenburg J; European Association of Urology. Guidelines on Testicular Cancer: 2015 Update. Eur Urol. 2015 Dec;68(6):1054-68. | CrossRef | PubMed |
- Loriot Y. Therapeutic Strategy Guided by PET-TDM for Patients With Grade I or Metastatic Seminoma. NCT01887340. | Link |
 Haugnes HS, Stephenson AJ, Feldman DR. Beyond stage I germ cell tumors:
current status regarding treatment and long-term toxicities. Am Soc Clin Oncol
Educ Book. 2014:e180-90. | CrossRef | PubMed |
Haugnes HS, Stephenson AJ, Feldman DR. Beyond stage I germ cell tumors:
current status regarding treatment and long-term toxicities. Am Soc Clin Oncol
Educ Book. 2014:e180-90. | CrossRef | PubMed | Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced
testicular cancer. JAMA. 2008 Feb 13;299(6):672-84. | CrossRef | PubMed |
Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced
testicular cancer. JAMA. 2008 Feb 13;299(6):672-84. | CrossRef | PubMed | Treglia G, Sadeghi R, Annunziata S, Caldarella C, Bertagna F, Giovanella L. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in the postchemotherapy management of patients with seminoma: systematic review and meta-analysis. Biomed Res Int. 2014;2014:852681. | CrossRef | PubMed | PMC |
Treglia G, Sadeghi R, Annunziata S, Caldarella C, Bertagna F, Giovanella L. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in the postchemotherapy management of patients with seminoma: systematic review and meta-analysis. Biomed Res Int. 2014;2014:852681. | CrossRef | PubMed | PMC | Zhao JY, Ma XL, Li YY, Zhang BL, Li MM, Ma XL, Liu L. Diagnostic accuracy of 18F-FDG-PET in patients with testicular cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(8):3525-31. | PubMed |
Zhao JY, Ma XL, Li YY, Zhang BL, Li MM, Ma XL, Liu L. Diagnostic accuracy of 18F-FDG-PET in patients with testicular cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(8):3525-31. | PubMed | Müller J, Schrader AJ, Jentzmik F, Schrader M. [Assessment of residual tumours after systemic treatment of metastatic seminoma: ¹⁸F-2-fluoro-2-deoxy-D-glucose positron emission tomography - meta-analysis of diagnostic value]. Urologe A. 2011 Mar;50(3):322-7. | CrossRef | PubMed |
Müller J, Schrader AJ, Jentzmik F, Schrader M. [Assessment of residual tumours after systemic treatment of metastatic seminoma: ¹⁸F-2-fluoro-2-deoxy-D-glucose positron emission tomography - meta-analysis of diagnostic value]. Urologe A. 2011 Mar;50(3):322-7. | CrossRef | PubMed | Cremerius U, Effert PJ, Adam G, Sabri O, Zimmy M, Wagenknecht G, Jakse G, Buell U. FDG PET for detection and therapy control of metastatic germ cell tumor. J Nucl Med. 1998 May;39(5):815-22. | PubMed |
Cremerius U, Effert PJ, Adam G, Sabri O, Zimmy M, Wagenknecht G, Jakse G, Buell U. FDG PET for detection and therapy control of metastatic germ cell tumor. J Nucl Med. 1998 May;39(5):815-22. | PubMed | Akbulut Z, Canda AE, Atmaca AF, Caglayan A, Asil E, Balbay MD. Is positron emission tomography reliable to predict post-chemotherapy retroperitoneal lymph node involvement in advanced germ cell tumors of the testis? Urol J. 2011 Spring;8(2):120-6. | PubMed |
Akbulut Z, Canda AE, Atmaca AF, Caglayan A, Asil E, Balbay MD. Is positron emission tomography reliable to predict post-chemotherapy retroperitoneal lymph node involvement in advanced germ cell tumors of the testis? Urol J. 2011 Spring;8(2):120-6. | PubMed | Spermon JR, De Geus-Oei LF, Kiemeney LA, Witjes JA, Oyen WJ. The role of (18)fluoro-2-deoxyglucose positron emission tomography in initial staging and re-staging after chemotherapy for testicular germ cell tumours. BJU Int. 2002 Apr;89(6):549-56. | PubMed |
Spermon JR, De Geus-Oei LF, Kiemeney LA, Witjes JA, Oyen WJ. The role of (18)fluoro-2-deoxyglucose positron emission tomography in initial staging and re-staging after chemotherapy for testicular germ cell tumours. BJU Int. 2002 Apr;89(6):549-56. | PubMed | Lewis DA, Tann M, Kesler K, McCool A, Foster RS, Einhorn LH. Positron emission tomography scans in postchemotherapy seminoma patients with residual masses: a retrospective review from Indiana University Hospital. J Clin Oncol. 2006 Dec 1;24(34):e54-5. | PubMed |
Lewis DA, Tann M, Kesler K, McCool A, Foster RS, Einhorn LH. Positron emission tomography scans in postchemotherapy seminoma patients with residual masses: a retrospective review from Indiana University Hospital. J Clin Oncol. 2006 Dec 1;24(34):e54-5. | PubMed | Hinz S, Schrader M, Kempkensteffen C, Bares R, Brenner W, Krege S, Franzius C, Kliesch S, Heicappel R, Miller K, de Wit M. The role of positron emission tomography in the evaluation of residual masses after chemotherapy for advanced stage seminoma. J Urol. 2008 Mar;179(3):936-40; discussion 940. | CrossRef | PubMed |
Hinz S, Schrader M, Kempkensteffen C, Bares R, Brenner W, Krege S, Franzius C, Kliesch S, Heicappel R, Miller K, de Wit M. The role of positron emission tomography in the evaluation of residual masses after chemotherapy for advanced stage seminoma. J Urol. 2008 Mar;179(3):936-40; discussion 940. | CrossRef | PubMed | Bachner M, Loriot Y, Gross-Goupil M, Zucali PA, Horwich A, Germa-Lluch JR, Kollmannsberger C, Stoiber F, Fléchon A, Oechsle K, Gillessen S, Oldenburg J, Cohn-Cedermark G, Daugaard G, Morelli F, Sella A, Harland S, Kerst M, Gampe J, Dittrich C, Fizazi K, De Santis M. 2-¹⁸fluoro-deoxy-D-glucose positron emission tomography (FDG-PET) for postchemotherapy seminoma residual lesions: a retrospective validation of the SEMPET trial. Ann Oncol. 2012 Jan;23(1):59-64. | CrossRef | PubMed |
Bachner M, Loriot Y, Gross-Goupil M, Zucali PA, Horwich A, Germa-Lluch JR, Kollmannsberger C, Stoiber F, Fléchon A, Oechsle K, Gillessen S, Oldenburg J, Cohn-Cedermark G, Daugaard G, Morelli F, Sella A, Harland S, Kerst M, Gampe J, Dittrich C, Fizazi K, De Santis M. 2-¹⁸fluoro-deoxy-D-glucose positron emission tomography (FDG-PET) for postchemotherapy seminoma residual lesions: a retrospective validation of the SEMPET trial. Ann Oncol. 2012 Jan;23(1):59-64. | CrossRef | PubMed | Ganjoo KN, Chan RJ, Sharma M, Einhorn LH. Positron emission tomography scans in the evaluation of postchemotherapy residual masses in patients with seminoma. J Clin Oncol. 1999 Nov;17(11):3457-60. | PubMed |
Ganjoo KN, Chan RJ, Sharma M, Einhorn LH. Positron emission tomography scans in the evaluation of postchemotherapy residual masses in patients with seminoma. J Clin Oncol. 1999 Nov;17(11):3457-60. | PubMed | Siekiera J, Małkowski B, Jóźwicki W, Jasiński M, Wronczewski A, Pietrzak T, Chmielowska E, Petrus A, Kamecki K, Mikołajczak W, Kraśnicki K, Chłosta P, Drewa T. Can we rely on PET in the follow-up of advanced seminoma patients? Urol Int. 2012;88(4):405-9. | CrossRef | PubMed |
Siekiera J, Małkowski B, Jóźwicki W, Jasiński M, Wronczewski A, Pietrzak T, Chmielowska E, Petrus A, Kamecki K, Mikołajczak W, Kraśnicki K, Chłosta P, Drewa T. Can we rely on PET in the follow-up of advanced seminoma patients? Urol Int. 2012;88(4):405-9. | CrossRef | PubMed | Ambrosini V, Zucchini G, Nicolini S, Berselli A, Nanni C, Allegri V, Martoni A, Rubello D, Cricca A, Fanti S. 18F-FDG PET/CT impact on testicular tumours clinical management. Eur J Nucl Med Mol Imaging. 2014 Apr;41(4):668-73. Erratum in: Eur J Nucl Med Mol Imaging. 2014 Mar;41(3):585. | PubMed |
Ambrosini V, Zucchini G, Nicolini S, Berselli A, Nanni C, Allegri V, Martoni A, Rubello D, Cricca A, Fanti S. 18F-FDG PET/CT impact on testicular tumours clinical management. Eur J Nucl Med Mol Imaging. 2014 Apr;41(4):668-73. Erratum in: Eur J Nucl Med Mol Imaging. 2014 Mar;41(3):585. | PubMed | De Santis M, Becherer A, Bokemeyer C, Stoiber F, Oechsle K, Sellner F, Lang A, Kletter K, Dohmen BM, Dittrich C, Pont J. 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. J Clin Oncol. 2004 Mar 15;22(6):1034-9. | PubMed |
De Santis M, Becherer A, Bokemeyer C, Stoiber F, Oechsle K, Sellner F, Lang A, Kletter K, Dohmen BM, Dittrich C, Pont J. 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. J Clin Oncol. 2004 Mar 15;22(6):1034-9. | PubMed | Hain SF, O'Doherty MJ, Timothy AR, Leslie MD, Harper PG, Huddart RA. Fluorodeoxyglucose positron emission tomography in the evaluation of germ cell tumours at relapse. British journal of cancer. 2000;83(7):863-9.
Hain SF, O'Doherty MJ, Timothy AR, Leslie MD, Harper PG, Huddart RA. Fluorodeoxyglucose positron emission tomography in the evaluation of germ cell tumours at relapse. British journal of cancer. 2000;83(7):863-9.  Becherer A, De Santis M, Karanikas G, Szabó M, Bokemeyer C, Dohmen BM, Pont J, Dudczak R, Dittrich C, Kletter K. FDG PET is superior to CT in the prediction of viable tumour in post-chemotherapy seminoma residuals. Eur J Radiol. 2005 May;54(2):284-8. | PubMed |
Becherer A, De Santis M, Karanikas G, Szabó M, Bokemeyer C, Dohmen BM, Pont J, Dudczak R, Dittrich C, Kletter K. FDG PET is superior to CT in the prediction of viable tumour in post-chemotherapy seminoma residuals. Eur J Radiol. 2005 May;54(2):284-8. | PubMed | De Santis M, Bokemeyer C, Becherer A, Stoiber F, Oechsle K, Kletter K, Dohmen BM, Dittrich C, Pont J. Predictive impact of 2-18fluoro-2-deoxy-D-glucose positron emission tomography for residual postchemotherapy masses in patients with bulky seminoma. J Clin Oncol. 2001 Sep 1;19(17):3740-4. Erratum in: J Clin Oncol 2001 Dec 1;19(23):4355. | PubMed |
De Santis M, Bokemeyer C, Becherer A, Stoiber F, Oechsle K, Kletter K, Dohmen BM, Dittrich C, Pont J. Predictive impact of 2-18fluoro-2-deoxy-D-glucose positron emission tomography for residual postchemotherapy masses in patients with bulky seminoma. J Clin Oncol. 2001 Sep 1;19(17):3740-4. Erratum in: J Clin Oncol 2001 Dec 1;19(23):4355. | PubMed | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Testicular cancer 2.2017 [cited Nov 28, 2017] | Link |
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Testicular cancer 2.2017 [cited Nov 28, 2017] | Link | Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP, Nicolai N, Oldenburg J; European Association of Urology. Guidelines on Testicular Cancer: 2015 Update. Eur Urol. 2015 Dec;68(6):1054-68. | CrossRef | PubMed |
Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP, Nicolai N, Oldenburg J; European Association of Urology. Guidelines on Testicular Cancer: 2015 Update. Eur Urol. 2015 Dec;68(6):1054-68. | CrossRef | PubMed | Loriot Y. Therapeutic Strategy Guided by PET-TDM for Patients With Grade I or Metastatic Seminoma. NCT01887340. | Link |
Loriot Y. Therapeutic Strategy Guided by PET-TDM for Patients With Grade I or Metastatic Seminoma. NCT01887340. | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








