Key Words: hypothyroidism, combination therapy, addition of LT3, Epistemonikos, GRADE.
Abstract
INTRODUCTION
The usual supplementation for hypothyroidism is based on monotherapy with levothyroxine. However, some patients persist with symptoms attributable to the deficit of
thyroid hormone. It has been suggested that the a combined treatment with liothyronine and levothyroxine would provide a greater benefit.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified three systematic reviews including twelve primary studies overall, all of which were randomized trials. We concluded that the addition of liothyronine to the treatment of hypothyroidism has minimal or no effect on fatigue and quality of life. It probably does not improve mood, pain or function cognitive, and it would not reduce body weight.
Problem
Hypothyroidism is a common disease, characterized by a deficiency of endogenous thyroid hormone, which affects 0.5 to 4.1% of the world population [1]. The standard treatment of hypothyroidism, regardless its etiology, is monotherapy with levothyroxine (LT4), the synthetic form of thyroxine, adjusted for the concentration of thyroid stimulating hormone (TSH). Despite achieving normal TSH, approximately 5-10% of patients report residual symptoms of hypothyroidism [2].
The active thyroid hormone is triiodothyronine (T3). Eighty percent comes from the peripheral conversion of thyroxine (T4) to T3, mainly in the liver, and the remaining 20% is produced by the thyroid gland together with T4 [3], in a T3: T4 ratio of 1:5 [1]. Considering physiology, a treatment relying exclusively on LT4 would be deficient since optimum levels of T3 are not reached. Supplementation with T3 may be necessary in patients with hypothyroidism treated exclusively with LT4.
The objective of this summary was to evaluate the effects of the combination of LT4 and liothyronine (LT3) as a treatment for hypothyroidism.
Methods
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found three systematic reviews [3], [4], [5] that included 12 primary studies reported in 13 references [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], all corresponding to randomized trials. |
|
What types of patients were included* |
All trials included patients with hypothyroidism in previous treatment with LT4 with stabilized thyroid hormone levels prior to the intervention, most of them for more than two months. One trial [8] did not mention the time with stable thyroid hormone levels. Another trial [13] included patients with an indeterminate long time of previous hormonal stabilization. Regarding the etiology of hypothyroidism, three trials [14], [16], [17] exclusively included patients with hypothyroidism due to chronic thyroiditis, while in the rest of the trials patients with hypothyroidism of different etiologies were included: chronic thyroiditis, post-thyroidectomy (for cancer) and post-radiation [5]. Regarding the gender of the included patients, two trials [8], [10] included only women and one trial [6] conducted separate analyses: one with the total population and another excluding the male population [7]. In the rest of the trials, both women and men were included, the former constituting the vast majority of patients, except in one of the trials [17]. The age of the patients ranged between 18 and 76 years. |
|
What types of interventions were included* |
All trials evaluated the combined treatment with LT4 + LT3 as an intervention. Regarding the dose used in the intervention, seven trials [6], [8], [9], [11], [12], [14], [17] used the usual dose of LT4 minus 50 ug/day; one trial [16] used the usual dose minus 25 ug/day, and another [13] used the daily dose minus a 5%. In the remaining three trials [10], [15], [18] an arbitrary dose of LT4 (75 ug/day, ≥50 ug day and 80 ug/day respectively) was used. The doses of LT3 varied. All trials compared the intervention against the standard treatment (LT4). Nine trials [6], [8], [9], [11], [12], [13], [14], [16], [17] administered the usual dose, in two trials [10], [18] 100 ug/day and in one trial [15] ≥100ug/day. |
|
What types of outcomes |
The trials evaluated multiple outcomes, which were grouped by the systematic reviews as follows:
The average follow-up of the trials was 12 weeks, with a range between 5 and 39 weeks. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
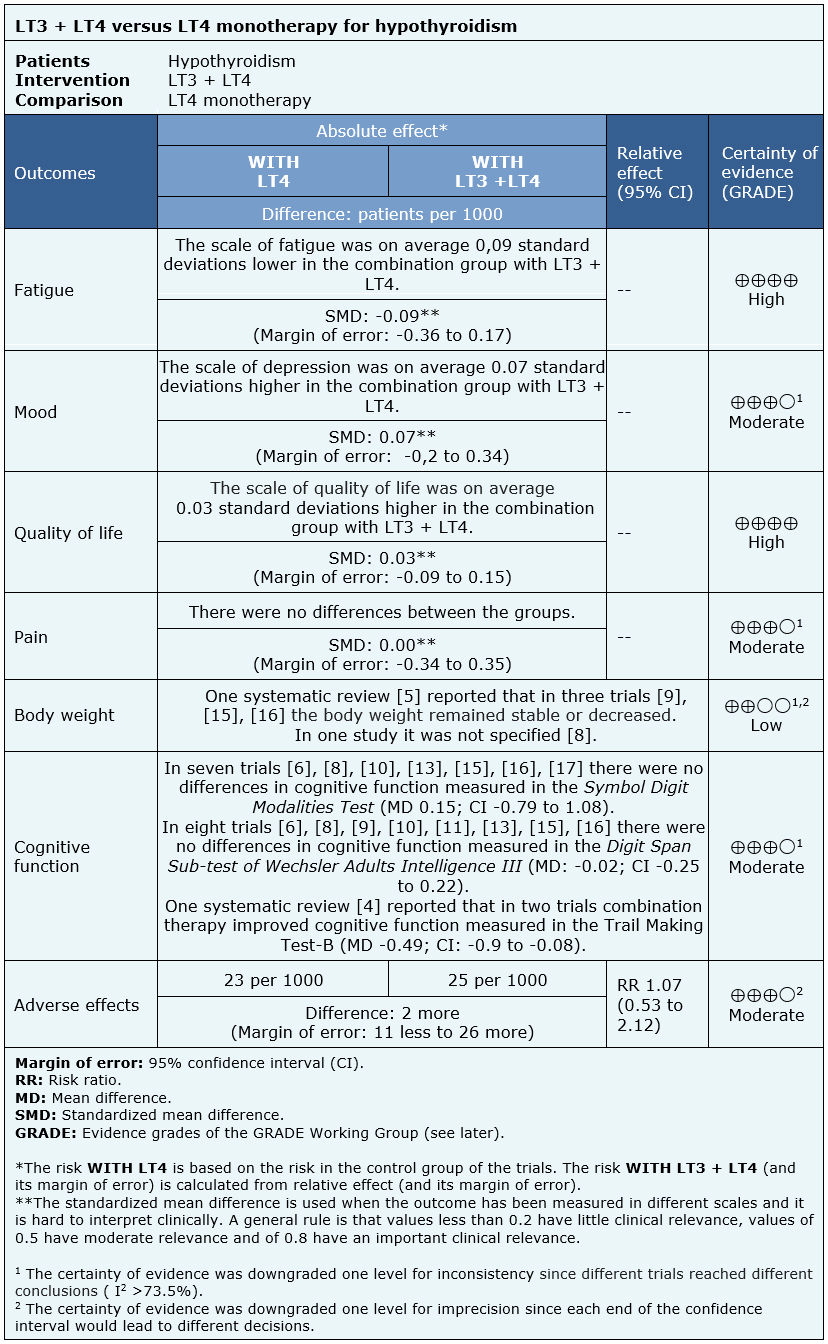
The information on the effects of the combined treatment of hypothyroidism with LT3 + LT4 versus monotherapy with LT4 in hypothyroidism is based on 12 randomized controlled trials.
Four trials assessed pain (936 patients) [12], [14], [15], [17], seven trials assessed quality of life (1068 patients) [9], [10], [12], [13], [14], [15], [17], 11 evaluated mood (1563 patients) [6], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], six assessed fatigue (350 patients) [6], [10], [11], [13], [16], [17] and 11 evaluated adverse effects (1247 patients) [6], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]. None of the systematic reviews presented data that could be re-analyzed and incorporated into a meta-analysis for body weight and cognitive function outcomes, so a narrative summary was prepared based on the results as reported by the reviews.
The summary of findings is as follows:
- The use of LT3 + LT4 compared to monotherapy with LT4 in hypothyroidism results in little or no difference in fatigue. The certainty of the evidence is high.
- The use of LT3 + LT4 compared to monotherapy with LT4 in hypothyroidism probably results in little or no difference in mood. The certainty of the evidence is moderate.
- The use of LT3 + LT4 compared to monotherapy with LT4 in hypothyroidism results in little or no difference in quality of life. The certainty of the evidence is high.
- The use of LT3 + LT4 compared to monotherapy with LT4 in hypothyroidism probably results in little or no difference in pain. The certainty of the evidence is moderate.
- The use of LT3 + LT4 compared to monotherapy with LT4 in hypothyroidism could result in little or no difference in body weight, but the certainty of the evidence is low.
- The use of LT3 + LT4 compared to monotherapy with LT4 in hypothyroidism probably results in little or no difference in cognitive function. The certainty of the evidence is moderate.
- The use of LT3 + LT4 compared to monotherapy with LT4 in hypothyroidism probably results in little or no difference in adverse effects. The certainty of the evidence is moderate.
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
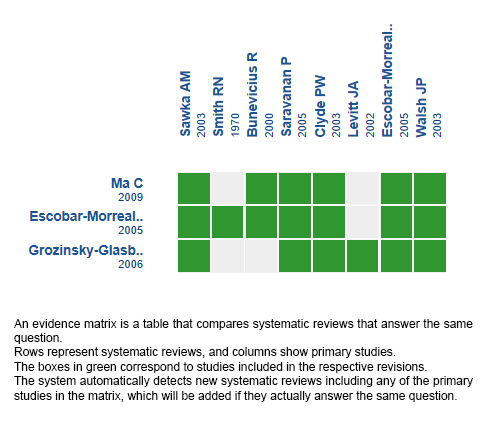
Follow the link to access the interactive version: Combination therapy with thyroxine-triiodothyronine versus thyroxine monotherapy for hypothyroidism
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
La suplementación habitual del hipotiroidismo se basa en la monoterapia con levotiroxina, sin embargo, algunos pacientes persisten con síntomas atribuibles al déficit de hormona tiroidea. Debido a esto se ha planteado que el uso de un tratamiento combinado con liotironina y levotiroxina otorgaría un mayor beneficio.
MÉTODOS
Realizamos una búsqueda en Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante el cribado de múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, analizamos los datos de los estudios primarios, realizamos un metanálisis y preparamos una tabla de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Identificamos tres revisiones sistemáticas que en conjunto incluyeron 12 estudios primarios, todos correspondientes a ensayos aleatorizados. Concluimos que la adición de liotironina al tratamiento del hipotiroidismo tiene un efecto mínimo o nulo sobre fatiga y calidad de vida. Probablemente tampoco mejora estado de ánimo, dolor ni función cognitiva, y no reduciría el peso corporal.
 Authors:
Alexandra Fischman[1,2], José Miguel Domínguez[2,3]
Authors:
Alexandra Fischman[1,2], José Miguel Domínguez[2,3]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Endocrinología, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: jdomingu@uc.cl
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Diagonal Paraguay 476 Santiago Chile

Citation: Fischman A, Domínguez J. Combined therapy with levothyroxine and liothyronine for hypothyroidism. Medwave 2018;18(8):e7375 doi: 10.5867/medwave.2018.08.7375
Submission date: 27/11/2018
Acceptance date: 4/12/2018
Publication date: 14/12/2018
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Kraut E, Farahani P. A Systematic Review of Clinical Practice Guidelines´ Recommendations on Levothyroxine Therapy Alone versus Combination Therapy (LT4 plus LT3) for Hypothyroidism. Clin Invest Med 2015; 38 (6): E305-E313. | CrossRef | PubMed |
- Hennessey J.V, Espaillat R. Current evidence for the treatment of hypothyroidism with levothyroxine/levotriiodothyronine combination therapy versus levothyroxine monotherapy. Int J Clin Pract 2018; 72: e13062
- Escobar-Morreale, Botella-Carretero. REVIEW: Treatment of Hypothyroidism with Combinations of Levothyroxine plus Liothyronine.
- Ma Chao, Xie Jiawei. Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism.
- Grozinsky-Glasberg, Fraser. Thyroxine-Triiodothyronine Combination Therapy Versus Thyroxine Monotherapy for Clinical Hypothyroidism: Meta-Analysis of Randomized Controlled Trials.
- Bunevicius R, Karanavicius G, Zalinkevicius R, Prange AJ Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med 1999; 340:424–429.
- Bunevicius R, Prange AJ. Mental improvement after replacement therapy with thyroxine plus triiodothyronine: relationship to cause of hypothyroidism. Int J Neuropsychopharmacol 2000; 3:167–174.
- Bunevicius R, Jakubonien N, Jurkevicius R, Cernicat J, Lasas L, Prange Jr AJ 2002 Thyroxine vs thyroxine plus triiodothyronine in treatment of hypothyroidism after thyroidectomy for Graves’ disease. Endocrine 18:129–133.
- Clyde PW, Harari AE, Getka EJ, Shakir KM. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA 2003; 290:2952–2958.
- Escobar-Morreale HF, Botella-Carretero JI, Gómez-Bueno M, Galán JM, Barrios V, Sancho J, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med 2005; 142:412–424.
- Rodriguez T, Lavis VR, Meininger JC, Kapadia AS, Stafford LF. Substitution of liothyronine at a 1 : 5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract 2005; 11:223–233.
- Saravanan P, Simmons DJ, Greenwood R, Peters TJ, Dayan CM. Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. J Clin Endocrinol Metab 2005; 90:805–812.
- Siegmund W, Spieker K, Weike AI, Giessmann T, Modess C, et al. Replacement therapy with levothyroxine plus triiodothyronine (bioavailable molar ratio 14 : 1) is not superior to thyroxine alone to improve well-being and cognitive performance in hypothyroidism. Clin Endocrinol (Oxf) 2004; 60:750–757.
- Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. Does a combination regimen of thyroxine (T4) and 3,5,30-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab 2003; 88:4551–4555.
- Walsh JP, Shiels L, Lim EM, Bhagat CI, Ward LC, et al. Combined thyroxine/ liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab 2003; 88:4543–4550.
- Appelhof BC, Fliers E, Wekking EM, Schene AH, Huyser J, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab 2005; 90:2666–2674.
- Levitt JA, Silverberg J, T4 plus T3 for hypothyroidism: a double-blind comparison with usual T4. Proc of the 74th Annual Meeting of the American Thyroid Association, Los Angeles, CA, 2002.
- Smith RN, Taylor SA, Massey JC 1970 Controlled clinical trial of combined triiodothyronine and thyroxine in the treatment of hypothyroidism. Br Med J 4:145–148.
- Wiersinga W.M, Duntas L, Fadeyev V, Nygaard B, Vanderpump M.P.J. 2012 ETA Guidelines: The Use of L-T4 + L-T3 in the Treatment of Hypothyroidism. Eur Thyroid J 2012;(1):55–71.
- Jonklaas J, Bianco A.C, Bauer A.J, Burman K.D, Cappola A.R, Celi F.S et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014 Dec;24(12):1670-751.
- Nygaard B, Jensen EW, Kvetny J, Jarlov A, Faber J. Effect of combination therapy with thyroxine (T4) and 3,5,3`-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. European Journal of Endocrinology 2009; 161: 895-902.
- Yu Kun. Combination L-T4 and L-T3 therapy versus L-T4 therapy in hypothyroidism: a systematic review and meta analysis. | Link |
- Combined Replacement Therapy With Levothyroxine and Liothyronine in Thyroidectomized Patients: Effects on Peripheral Tissues. A Prospective, Randomized, Controlled, Double-blind Study. | Link |
- Substitutive therapy of hypothyroid patients with L-thyroxine (T4) plus T3 sulfate (T3S). A Phase II, open-label, single centre, parallel group study on therapeutic efficacy and tolerability - ND. | Link |
- Combined Therapy With L-Thyroxine and L-Triiodothyronine Compared to L-Thyroxine Alone in the Treatment of Primary Hypothyroidism. | Link |
- Effect of T4-T3 Combination Therapy Versus T4 Monotherapy in Patients With Hypothyroidism,a Double Blind Randomized Cross-Over Study. | Link |
 Kraut E, Farahani P. A Systematic Review of Clinical Practice Guidelines´ Recommendations on Levothyroxine Therapy Alone versus Combination Therapy (LT4 plus LT3) for Hypothyroidism. Clin Invest Med 2015; 38 (6): E305-E313. | CrossRef | PubMed |
Kraut E, Farahani P. A Systematic Review of Clinical Practice Guidelines´ Recommendations on Levothyroxine Therapy Alone versus Combination Therapy (LT4 plus LT3) for Hypothyroidism. Clin Invest Med 2015; 38 (6): E305-E313. | CrossRef | PubMed | Hennessey J.V, Espaillat R. Current evidence for the treatment of hypothyroidism with levothyroxine/levotriiodothyronine combination therapy versus levothyroxine monotherapy. Int J Clin Pract 2018; 72: e13062
Hennessey J.V, Espaillat R. Current evidence for the treatment of hypothyroidism with levothyroxine/levotriiodothyronine combination therapy versus levothyroxine monotherapy. Int J Clin Pract 2018; 72: e13062  Escobar-Morreale, Botella-Carretero. REVIEW: Treatment of Hypothyroidism with Combinations of Levothyroxine plus Liothyronine.
Escobar-Morreale, Botella-Carretero. REVIEW: Treatment of Hypothyroidism with Combinations of Levothyroxine plus Liothyronine.  Ma Chao, Xie Jiawei. Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism.
Ma Chao, Xie Jiawei. Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism.  Grozinsky-Glasberg, Fraser. Thyroxine-Triiodothyronine Combination Therapy Versus Thyroxine Monotherapy for Clinical Hypothyroidism: Meta-Analysis of Randomized Controlled Trials.
Grozinsky-Glasberg, Fraser. Thyroxine-Triiodothyronine Combination Therapy Versus Thyroxine Monotherapy for Clinical Hypothyroidism: Meta-Analysis of Randomized Controlled Trials.  Bunevicius R, Karanavicius G, Zalinkevicius R, Prange AJ Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med 1999; 340:424–429.
Bunevicius R, Karanavicius G, Zalinkevicius R, Prange AJ Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med 1999; 340:424–429.  Bunevicius R, Prange AJ. Mental improvement after replacement therapy with thyroxine plus triiodothyronine: relationship to cause of hypothyroidism. Int J Neuropsychopharmacol 2000; 3:167–174.
Bunevicius R, Prange AJ. Mental improvement after replacement therapy with thyroxine plus triiodothyronine: relationship to cause of hypothyroidism. Int J Neuropsychopharmacol 2000; 3:167–174.  Bunevicius R, Jakubonien N, Jurkevicius R, Cernicat J, Lasas L, Prange Jr AJ 2002 Thyroxine vs thyroxine plus triiodothyronine in treatment of hypothyroidism after thyroidectomy for Graves’ disease. Endocrine 18:129–133.
Bunevicius R, Jakubonien N, Jurkevicius R, Cernicat J, Lasas L, Prange Jr AJ 2002 Thyroxine vs thyroxine plus triiodothyronine in treatment of hypothyroidism after thyroidectomy for Graves’ disease. Endocrine 18:129–133.  Clyde PW, Harari AE, Getka EJ, Shakir KM. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA 2003; 290:2952–2958.
Clyde PW, Harari AE, Getka EJ, Shakir KM. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA 2003; 290:2952–2958.  Escobar-Morreale HF, Botella-Carretero JI, Gómez-Bueno M, Galán JM, Barrios V, Sancho J, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med 2005; 142:412–424.
Escobar-Morreale HF, Botella-Carretero JI, Gómez-Bueno M, Galán JM, Barrios V, Sancho J, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med 2005; 142:412–424.  Rodriguez T, Lavis VR, Meininger JC, Kapadia AS, Stafford LF. Substitution of liothyronine at a 1 : 5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract 2005; 11:223–233.
Rodriguez T, Lavis VR, Meininger JC, Kapadia AS, Stafford LF. Substitution of liothyronine at a 1 : 5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract 2005; 11:223–233.  Saravanan P, Simmons DJ, Greenwood R, Peters TJ, Dayan CM. Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. J Clin Endocrinol Metab 2005; 90:805–812.
Saravanan P, Simmons DJ, Greenwood R, Peters TJ, Dayan CM. Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. J Clin Endocrinol Metab 2005; 90:805–812.  Siegmund W, Spieker K, Weike AI, Giessmann T, Modess C, et al. Replacement therapy with levothyroxine plus triiodothyronine (bioavailable molar ratio 14 : 1) is not superior to thyroxine alone to improve well-being and cognitive performance in hypothyroidism. Clin Endocrinol (Oxf) 2004; 60:750–757.
Siegmund W, Spieker K, Weike AI, Giessmann T, Modess C, et al. Replacement therapy with levothyroxine plus triiodothyronine (bioavailable molar ratio 14 : 1) is not superior to thyroxine alone to improve well-being and cognitive performance in hypothyroidism. Clin Endocrinol (Oxf) 2004; 60:750–757.  Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. Does a combination regimen of thyroxine (T4) and 3,5,30-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab 2003; 88:4551–4555.
Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. Does a combination regimen of thyroxine (T4) and 3,5,30-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab 2003; 88:4551–4555.  Walsh JP, Shiels L, Lim EM, Bhagat CI, Ward LC, et al. Combined thyroxine/ liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab 2003; 88:4543–4550.
Walsh JP, Shiels L, Lim EM, Bhagat CI, Ward LC, et al. Combined thyroxine/ liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab 2003; 88:4543–4550.  Appelhof BC, Fliers E, Wekking EM, Schene AH, Huyser J, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab 2005; 90:2666–2674.
Appelhof BC, Fliers E, Wekking EM, Schene AH, Huyser J, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab 2005; 90:2666–2674.  Levitt JA, Silverberg J, T4 plus T3 for hypothyroidism: a double-blind comparison with usual T4. Proc of the 74th Annual Meeting of the American Thyroid Association, Los Angeles, CA, 2002.
Levitt JA, Silverberg J, T4 plus T3 for hypothyroidism: a double-blind comparison with usual T4. Proc of the 74th Annual Meeting of the American Thyroid Association, Los Angeles, CA, 2002.  Smith RN, Taylor SA, Massey JC 1970 Controlled clinical trial of combined triiodothyronine and thyroxine in the treatment of hypothyroidism. Br Med J 4:145–148.
Smith RN, Taylor SA, Massey JC 1970 Controlled clinical trial of combined triiodothyronine and thyroxine in the treatment of hypothyroidism. Br Med J 4:145–148.  Wiersinga W.M, Duntas L, Fadeyev V, Nygaard B, Vanderpump M.P.J. 2012 ETA Guidelines: The Use of L-T4 + L-T3 in the Treatment of Hypothyroidism. Eur Thyroid J 2012;(1):55–71.
Wiersinga W.M, Duntas L, Fadeyev V, Nygaard B, Vanderpump M.P.J. 2012 ETA Guidelines: The Use of L-T4 + L-T3 in the Treatment of Hypothyroidism. Eur Thyroid J 2012;(1):55–71.  Jonklaas J, Bianco A.C, Bauer A.J, Burman K.D, Cappola A.R, Celi F.S et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014 Dec;24(12):1670-751.
Jonklaas J, Bianco A.C, Bauer A.J, Burman K.D, Cappola A.R, Celi F.S et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014 Dec;24(12):1670-751.  Nygaard B, Jensen EW, Kvetny J, Jarlov A, Faber J. Effect of combination therapy with thyroxine (T4) and 3,5,3`-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. European Journal of Endocrinology 2009; 161: 895-902.
Nygaard B, Jensen EW, Kvetny J, Jarlov A, Faber J. Effect of combination therapy with thyroxine (T4) and 3,5,3`-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. European Journal of Endocrinology 2009; 161: 895-902.  Yu Kun. Combination L-T4 and L-T3 therapy versus L-T4 therapy in hypothyroidism: a systematic review and meta analysis. | Link |
Yu Kun. Combination L-T4 and L-T3 therapy versus L-T4 therapy in hypothyroidism: a systematic review and meta analysis. | Link | Combined Replacement Therapy With Levothyroxine and Liothyronine in Thyroidectomized Patients: Effects on Peripheral Tissues. A Prospective, Randomized, Controlled, Double-blind Study. | Link |
Combined Replacement Therapy With Levothyroxine and Liothyronine in Thyroidectomized Patients: Effects on Peripheral Tissues. A Prospective, Randomized, Controlled, Double-blind Study. | Link | Substitutive therapy of hypothyroid patients with L-thyroxine (T4) plus T3 sulfate (T3S). A Phase II, open-label, single centre, parallel group study on therapeutic efficacy and tolerability - ND. | Link |
Substitutive therapy of hypothyroid patients with L-thyroxine (T4) plus T3 sulfate (T3S). A Phase II, open-label, single centre, parallel group study on therapeutic efficacy and tolerability - ND. | Link | Combined Therapy With L-Thyroxine and L-Triiodothyronine Compared to L-Thyroxine Alone in the Treatment of Primary Hypothyroidism. | Link |
Combined Therapy With L-Thyroxine and L-Triiodothyronine Compared to L-Thyroxine Alone in the Treatment of Primary Hypothyroidism. | Link | Effect of T4-T3 Combination Therapy Versus T4 Monotherapy in Patients With Hypothyroidism,a Double Blind Randomized Cross-Over Study. | Link |
Effect of T4-T3 Combination Therapy Versus T4 Monotherapy in Patients With Hypothyroidism,a Double Blind Randomized Cross-Over Study. | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








