Key Words: Plaque psoriasis, biological treatments, secukinumab, Epistemonikos, GRADE.
Abstract
INTRODUCTION
Biological treatments have appeared as the main alternative for the management of patients with plaque psoriasis that do not respond to conventional treatment. So, evaluating its actual efficacy and safety is needed.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified 21 systematic reviews including ten studies overall, of which all were randomized trials. We concluded secukinumab achieves clinical improvement in patients with plaque psoriasis, although it is probably associated with serious adverse effects.
Problem
Approximately 20% of patients with plaque psoriasis have moderate to severe disease [1], requiring conventional systemic treatment, such as methotrexate, cyclosporine or acitretin, or phototherapy. However, for a large number of patients, these treatments are not sufficient, due to a limited therapeutic effect or to the presence of adverse effects.
In the search for more effective and safe treatments, biological therapies have emerged in the last years as an alternative. Secukinumab is a human monoclonal antibody that selectively neutralizes interleukin-17A, which has a major role in the pathogenesis of psoriasis. We aimed to evaluate the efficacy and safety of secukinumab in patients with plaque psoriasis.
Methods
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found 21 systematic reviews [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22] that included ten primary studies, reported in 23 references [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], all corresponding to randomized controlled trials. However, four of these trials were excluded from our analysis: two because the intervention was not administered in usual doses [27], [39] and two because no comparison was made against placebo [32], [33], [34], [44], [45]. This table and the summary in general are based on the six randomized trials that answer the question of interest [23], [28], [31], [35], [37], [40]. |
|
What types of patients were included* |
All trials included adult patients older than 18 years with plaque psoriasis on the trunk, upper and lower limbs, not including scalp, with moderate to severe disease determined by PASI ≥ 12, IGA 3-4, BSA ≥ 10%, without response to topical treatment, phototherapy or conventional systemic treatment. Two trials [23], [37] excluded patients who had received biological treatment with anti-interleukin-17. |
|
What types of interventions were included* |
Four trials [23], [28], [31], [37] included subcutaneous secukinumab 150 mg or 300 mg weekly for four weeks and then in monthly doses. One trial [35] included an intervention group with subcutaneous secukinumab 150 mg at weeks 0,4 and 8. Another trial [40] included three intervention groups: subcutaneous secukinumab 150 mg in single dose; subcutaneous secukinumab 150 mg at weeks 0,4 and 8; and subcutaneous secukinumab 150 mg at weeks 0, 1, 2 and 4. |
|
What types of outcomes |
The trials evaluated multiple outcomes, which were grouped in the systematic reviews as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
The information about the effects of secukinumab is based on six randomized trials including 2531 patients [23], [28], [31], [35], [37], [40].
All trials reported PASI 75 and PGA/IGA (2531 patients) [23], [28], [31], [35], [37], [40]. Five trials reported PASI 90 and serious adverse events (2482 patients) [23], [28], [31], [37], [40]. Two trials reported DLQI (1708 patients) [28], [31].
The summary of findings is as follows:
- Secukinumab leads to an improvement in PASI 75 and PASI 90 score in patients with moderate to severe plaque psoriasis. The certainty of the evidence is high.
- Secukinumab leads to an improvement in PGA/IGA score in patients with moderate to severe plaque psoriasis. The certainty of the evidence is high.
- Secukinumab probably leads to an improvement in DLQI score in patients with moderate to severe plaque psoriasis. The certainty of the evidence is moderate.
- Secukinumab probably leads to an increase in serious adverse events in patients with moderate to severe plaque psoriasis. The certainty of the evidence is moderate.
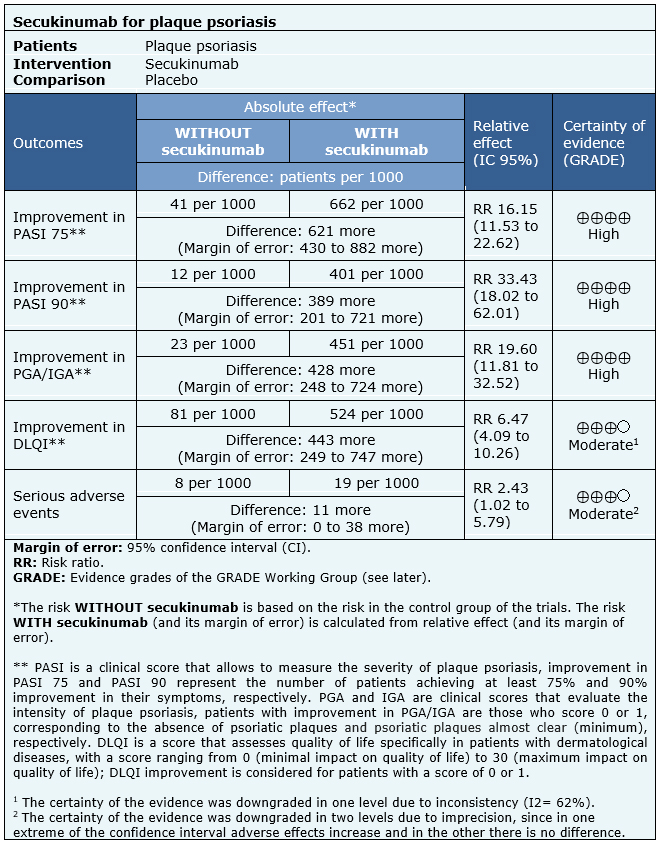
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
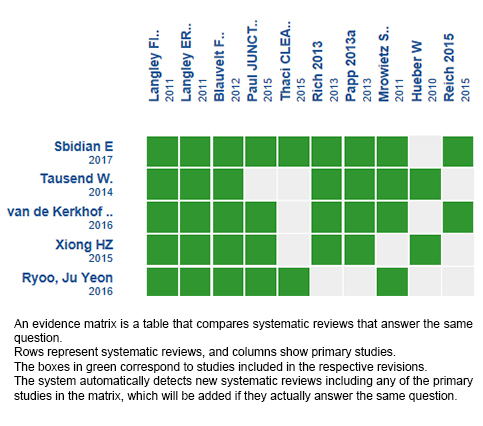
Follow the link to access the interactive version: Secukinumab for plaque psoriasis.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
Los tratamientos biológicos han aparecido como principal alternativa para el manejo de los pacientes con psoriasis en placa que no responden a tratamiento convencional, resultando necesario evaluar su real efectividad y seguridad.
MÉTODOS
Realizamos una búsqueda en Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante el cribado de múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, analizamos los datos de los estudios primarios, realizamos un metanálisis y preparamos una tabla de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Identificamos 21 revisiones sistemáticas que en conjunto incluyeron diez estudios primarios, todos correspondientes a ensayos aleatorizados. Concluimos que secukinumab logra mejoría clínica en pacientes con psoriasis en placa, aunque probablemente se asocia a efectos adversos graves.
 Authors:
Gonzalo Ordenes-Cavieres[1,2], Romina Andino-Navarrete[2,3]
Authors:
Gonzalo Ordenes-Cavieres[1,2], Romina Andino-Navarrete[2,3]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Dermatología, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: rominaandino@gmail.com
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Diagonal Paraguay 476 Santiago Chile

Citation: Ordenes-Cavieres G, Andino-Navarrete R. Secukinumab for plaque psoriasis. Medwave 2018;18(7):e7363 doi: 10.5867/medwave.2018.07.7363
Submission date: 6/11/2018
Acceptance date: 27/11/2018
Publication date: 30/11/2018
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA, Korman NJ, Beutner KR, Bhushan R. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008 May;58(5):826-50. | CrossRef | PubMed |
- Ryoo JY, Yang HJ, Ji E, Yoo BK. Meta-analysis of the Efficacy and Safety of Secukinumab for the Treatment of Plaque Psoriasis. Ann Pharmacother. 2016 May;50(5):341-51. | CrossRef | PubMed |
- Nast A, Jacobs A, Rosumeck S, Werner RN. Efficacy and Safety of Systemic Long-Term Treatments for Moderate-to-Severe Psoriasis: A Systematic Review and Meta-Analysis. J Invest Dermatol. 2015 Nov;135(11):2641-2648. | CrossRef | PubMed |
- Yiu ZZ, Exton LS, Jabbar-Lopez Z, Mohd Mustapa MF, Samarasekera EJ, Burden AD, Murphy R, Owen CM, Parslew R, Venning V, Ashcroft DM, Griffiths CE, Smith CH, Warren RB. Risk of Serious Infections in Patients with Psoriasis on Biologic Therapies: A Systematic Review and Meta-Analysis. J Invest Dermatol. 2016 Aug;136(8):1584-91. | CrossRef | PubMed | PMC |
- Rungapiromnan W, Yiu ZZN, Warren RB, Griffiths CEM, Ashcroft DM. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2017 Apr;176(4):890-901. | CrossRef | PubMed | PMC |
- de Carvalho AV, Duquia RP, Horta BL, Bonamigo RR. Efficacy of Immunobiologic and Small Molecule Inhibitor Drugs for Psoriasis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Drugs R D. 2017 Mar;17(1):29-51. | CrossRef | PubMed | PMC |
- Tausend W, Downing C, Tyring S. Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab. J Cutan Med Surg. 2014 May-Jun;18(3):156-69. | PubMed |
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014 Jul;15(3):165-80. | CrossRef | PubMed |
- Xiong HZ, Gu JY, He ZG, Chen WJ, Zhang X, Wang JY, Shi YL. Efficacy and safety of secukinumab in the treatment of moderate to severe plaque psoriasis: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2015 Mar 15;8(3):3156-72. | PubMed | PMC |
- Chen Y, Qian T, Zhang D, Yan H, Hao F. Clinical efficacy and safety of anti-IL-17 agents for the treatment of patients with psoriasis. Immunotherapy. 2015;7(9):1023-37. | CrossRef | PubMed |
- Wu D, Hou SY, Zhao S, Hou LX, Jiao T, Xu NN, Zhang N. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017 Jun;31(6):992-1003. | CrossRef | PubMed |
- Jabbar-Lopez ZK, Yiu ZZN, Ward V, Exton LS, Mohd Mustapa MF, Samarasekera E, Burden AD, Murphy R, Owen CM, Parslew R, Venning V, Warren RB, Smith CH. Quantitative Evaluation of Biologic Therapy Options for Psoriasis: A Systematic Review and Network Meta-Analysis. J Invest Dermatol. 2017 Aug;137(8):1646-1654. | CrossRef | PubMed | PMC |
- Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017 Jul;177(1):47-62. | CrossRef | PubMed |
- Sbidian E, Chaimani A, Garcia-Doval I, Do G, Hua C, Mazaud C, Droitcourt C, Hughes C, Ingram JR, Naldi L, Chosidow O, Le Cleach L. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017 Dec 22;12:CD011535. | CrossRef | PubMed |
- Gómez-García F, Epstein D, Isla-Tejera B, Lorente A, Vélez García-Nieto A, Ruano J. Short-term efficacy and safety of new biological agents targeting the interleukin-23-T helper 17 pathway for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis. Br J Dermatol. 2017 Mar;176(3):594-603. | CrossRef | PubMed |
- Théréné C, Brenaut E, Barnetche T, Misery L. Efficacy of Systemic Treatments of Psoriasis on Pruritus: A Systemic Literature Review and Meta-Analysis. J Invest Dermatol. 2018 Jan;138(1):38-4 | CrossRef | PubMed |
- o DJ, Amin M, Bhutani T, Wu JJ. A systematic review of active comparator controlled clinical trials in patients with moderate-to-severe psoriasis. J Dermatolog Treat. 2017 Nov 22:1-8. | CrossRef | PubMed |
- Loos AM, Liu S, Segel C, Ollendorf DA, Pearson SD, Linder JA. Comparative Effectiveness of Targeted Immunomodulators for the Treatment of Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis. J Am Acad Dermatol. 2018 Feb 10. pii: S0190-9622(18)30213-5. | CrossRef | PubMed |
- van de Kerkhof PC, Griffiths CE, Reich K, Leonardi CL, Blauvelt A, Tsai TF, Gong Y, Huang J, Papavassilis C, Fox T. Secukinumab long-term safety experience: A pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016 Jul;75(1):83-98.e4. | CrossRef | PubMed |
- Warren RB, Brnabic A, Saure D, Langley RG, See K, Wu JJ, Schacht A, Mallbris L, Nast A. Matching-adjusted indirect comparison of efficacy in patients with moderate-to-severe plaque psoriasis treated with ixekizumab vs. secukinumab. Br J Dermatol. 2017 Nov 24. | CrossRef | PubMed |
- Fan XD, Xia X, Zhang CY, Kong WQ, Zhou CY, DU B. [A systematic review of anti-interleukin-17 antibody in the treatment of plaque psoriasis]. Nan Fang Yi Ke Da Xue Xue Bao. 2017 Sep 20;37(9):1274-1279. Chinese. | PubMed |
- Armstrong AW, Betts KA, Signorovitch JE, Sundaram M, Li J, Ganguli AX, Wu EQ. Number needed to treat and costs per responder among biologic treatments for moderate-to-severe psoriasis: a network meta-analysis. Curr Med Res Opin. 2018 Apr 23:1-9. | CrossRef | PubMed |
- Blauvelt A, Prinz JC, Gottlieb AB, Kingo K, Sofen H, Ruer-Mulard M, Singh V, Pathan R, Papavassilis C, Cooper S; FEATURE Study Group. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015 Feb;172(2):484-93. | CrossRef | PubMed |
- Novartis Pharmaceuticals. Extension Study of Secukinumab Prefilled Syringes in Subjects With Moderate to Severe Chronic Plaque-type Psoriasis Completing Preceding Secukinumab Phase III Studies. clinicaltrials.gov. 2012. | CrossRef | Link |
- Novartis Pharmaceuticals. First Study of Secukinumab in Pre-filled Syringes in Subjects With Chronic Plaque-type Psoriasis: Response at 12 Weeks (FEATURE). clinicaltrials.gov. 2012. | Link |
- Novartis Pharmaceuticals. Extension Study of Secukinumab Prefilled Syringes in Subjects With Moderate to Severe Chronic Plaque-type Psoriasis Completing Preceding Psoriasis Phase III Studies With Secukinumab. clinicaltrials.gov. 2012. | Link |
- Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH; Psoriasis Study Group, Durez P, Tak PP, Gomez-Reino JJ; Rheumatoid Arthritis Study Group, Foster CS, Kim RY, Samson CM, Falk NS, Chu DS, Callanan D, Nguyen QD; Uveitis Study Group, Rose K, Haider A, Di Padova F. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010 Oct 6;2(52):52ra72. | CrossRef | PubMed |
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C; ERASURE Study Group; FIXTURE Study Group. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014 Jul 24;371(4):326-38. | CrossRef | PubMed |
- Ohtsuki M, Morita A, Abe M, Takahashi H, Seko N, Karpov A, Shima T, Papavassilis C, Nakagawa H; ERASURE Study Japanese subgroup. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014 Dec;41(12):1039-46. | CrossRef | PubMed |
- Novartis Pharmaceuticals. Efficacy and Safety of Subcutaneous Secukinumab for Moderate to Severe Chronic Plaque-type Psoriasis for up to 1 Year (Erasure). clinicaltrials.gov. 2011. | Link |
- Gottlieb AB, Langley RG, Philipp S, Sigurgeirsson B, Blauvelt A, Martin R, Papavassilis C, Mpofu S. Secukinumab Improves Physical Function in Subjects With Plaque Psoriasis and Psoriatic Arthritis: Results from Two Randomized, Phase 3 Trials. J Drugs Dermatol. 2015 Aug;14(8):821-33. | PubMed |
- Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, Szepietowski JC, Regnault P, Thurston H, Papavassilis C; SCULPTURE Study Group. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: A randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015 Jul;73(1):27-36.e1. | CrossRef | PubMed |
- Thaçi D, Humeniuk J, Frambach Y, Bissonnette R, Goodman JJ, Shevade S, Gong Y, Papavassilis C; STATURE study group. Secukinumab in psoriasis: randomized, controlled phase 3 trial results assessing the potential to improve treatment response in partial responders (STATURE). Br J Dermatol. 2015 Sep;173(3):777-87. | CrossRef | PubMed |
- Novartis Pharmaceuticals. Efficacy and Safety of Subcutaneous Secukinumab (AIN457) for Moderate to Severe Chronic Plaque-type Psoriasis Assessing Different Doses and Dose Regimens (SCULPTURE). clinicaltrials.gov. 2011 | Link |
- Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, Haemmerle S, Thurston HJ, Papavassilis C, Richards HB. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013 Feb;168(2):412-21. | CrossRef | PubMed |
- Sigurgeirsson B, Kircik L, Nemoto O, Mikazans I, Haemmerle S, Thurston HJ, Papavassilis C, Richards HB. Secukinumab improves the signs and symptoms of moderate-to-severe plaque psoriasis in subjects with involvement of hands and/or feet: subanalysis of a randomized, double-blind, placebo-controlled, phase 2 dose-ranging study. J Eur Acad Dermatol Venereol. 2014 Aug;28(8):1127-9. | CrossRef | PubMed |
- Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, Guindon C, You R, Papavassilis C; JUNCTURE study group. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015 Jun;29(6):1082-90. | CrossRef | PubMed |
- Lacour JP, Paul C, Jazayeri S, Papanastasiou P, Xu C, Nyirady J, Fox T, Papavassilis C. Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: results of the randomized controlled JUNCTURE trial. J Eur Acad Dermatol Venereol. 2017 May;31(5):847-856. | CrossRef | PubMed |
- Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M, Gratton D, Kunynetz RA, Poulin Y, Rosoph LA, Stingl G, Bauer WM, Salter JM, Falk TM, Blödorn-Schlicht NA, Hueber W, Sommer U, Schumacher MM, Peters T, Kriehuber E, Lee DM, Wieczorek GA, Kolbinger F, Bleul CC. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol. 2015 Jul;24(7):529-35. | CrossRef | PubMed | PMC |
- Rich P, Sigurgeirsson B, Thaci D, Ortonne JP, Paul C, Schopf RE, Morita A, Roseau K, Harfst E, Guettner A, Machacek M, Papavassilis C. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013 Feb;168(2):402-11. | CrossRef | PubMed |
- Paul C, Reich K, Gottlieb AB, Mrowietz U, Philipp S, Nakayama J, Harfst E, Guettner A, Papavassilis C; CAIN457A2211 study group. Secukinumab improves hand, foot and nail lesions in moderate-to-severe plaque psoriasis: subanalysis of a randomized, double-blind, placebo-controlled, regimen-finding phase 2 trial. J Eur Acad Dermatol Venereol. 2014 Dec;28(12):1670-5. | CrossRef | PubMed |
- Augustin M, Abeysinghe S, Mallya U, Qureshi A, Roskell N, McBride D, Papavassillis C, Gelfand J. Secukinumab treatment of plaque psoriasis shows early improvement in DLQI response - results of a phase II regimen-finding trial. J Eur Acad Dermatol Venereol. 2016 Apr;30(4):645-9. | CrossRef | PubMed | PMC |
- Novartis Pharmaceuticals. AIN457 regimen finding extension study in patients with moderate to severe psoriasis. clinicaltrials.gov. 2010. | Link |
- Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, Ziv M, Pinter A, Hugot S, You R, Milutinovic M. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015 Sep;73(3):400-9. | CrossRef | PubMed |
- Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, Ziv M, Pinter A, Vender R, Hugot S, You R, Milutinovic M, Thaçi D. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: Results from the CLEAR study. J Am Acad Dermatol. 2017 Jan;76(1):60-69.e9. | CrossRef | PubMed |
- Nast A, Gisondi P, Ormerod AD, Saiag P, Smith C, Spuls PI, Arenberger P, Bachelez H, Barker J, Dauden E, de Jong EM, Feist E, Jacobs A, Jobling R, Kemény L, Maccarone M, Mrowietz U, Papp KA, Paul C, Reich K, Rosumeck S, Talme T, Thio HB, van de Kerkhof P, Werner RN, Yawalkar N. European S3-Guidelines on the systemic treatment of psoriasis vulgaris--Update 2015--Short version--EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015 Dec;29(12):2277-94. | CrossRef | PubMed |
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, Bale T, Burden AD, Coates LC, Cruickshank M, Hadoke T, MacMahon E, Murphy R, Nelson-Piercy C, Owen CM, Parslew R, Peleva E, Pottinger E, Samarasekera EJ, Stoddart J, Strudwicke C, Venning VA, Warren RB, Exton LS, Mohd Mustapa MF. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017 Sep;177(3):628-636. | CrossRef | PubMed |
- Corinna Dressler, Miriam Zidane, Alexander Nast. A systematic review and "time-effectiveness" analysis of psoriasis treatments and different treatment sequences. PROSPERO 2017 CRD42017074218. | Link |
- Christine Ballegaard, Tanja Schjødt Jørgensen, Marie Skougaard, Vibeke Strand, Philip J. Mease, Lars E. Kristensen, Lene Dreyer, Alice Gottlieb, Maarten de Wit, Robin Christensen, Simon Tarp. Assessing the importance of trial characteristics as contextual factors when evaluating targeted therapies in patients with psoriatic disease: protocol for an exploratory systematic review and meta-research project. PROSPERO 2016 CRD42016050049. | CrossRef | Link |
- Daniel Ollendorf, Reiner Banken, Foluso Agboola, Katherine Fazioli, Celia Segel. Comparative clinical effectiveness of treatment options for moderate-to-severe plaque psoriasis. PROSPERO 2018 CRD42018088801. | Link |
- Simon Tarp, Lars Erik Bryld, Lars Iversen, Lone Skov, Sigurd Broesby-Olsen, Birgitte Brock, Camilla Mikkelsen, Lis Andersen, Robin Christensen. Comparative effectiveness associated with the use of biologics and small-molecules for psoriasis: protocol for a systematic review and meta-analysis. PROSPERO 2015 CRD42015029122. | Link |
- Catherine Smith, Zarif Jabbar-Lopez, Zenas Yiu, Ellie Samarasekera, M. Firouz Mohd Mustapa, Leslie Exton. In people with psoriasis (all types), what are the clinical effectiveness/efficacy, safety and tolerability of systemic biologics (adalimumab, etanercept, infliximab, secukinumab or ustekinumab) compared with each other, with methotrexate or with placebo?. PROSPERO 2015 CRD42015017538. | Link |
- Juan Ruano, Francisco Gómez-García, Beatriz Isla-Tejera, Jesús Gay-Mimbrera, Macarena Aquilar-Luqe, Marcelino González-Padilla, Juan Luis Sanz-Cabanillas, Antonio Vélez García-Nieto. Pharmacogenetics of agents blocking TNF-a, IL-12/23, and IL17/IL17RA for the treatment of moderate-severe psoriasis: a systematic review and meta-analysis. PROSPERO 2016 CRD42016038769. | Link |
- Francisco Jose Gomez Garcia, Juan Ruano, Beatriz Isla-Tejera, Marcelino Gonzalez Padilla, Juan Luis Sanz Cabanillas, Antonio Velez Garcia Nieto, Macarena Aguilar Luque, Jesus Gay Mimbrera, Pedro Carmona. Short-, medium- and long-term efficacy and safety of biological drugs for moderate to severe plaque psoriasis: systematic review and network meta-analysis. PROSPERO 2017 CRD42017057642. | Link |
- Yayoi Tada, Rei Watanabe, Hisashi Noma. Short-term and long-term effectiveness of biologic agents targeting TNF-a, IL-23, and IL-17 for the treatment of plaque psoriasis: a systematic review and network meta-analysis. PROSPERO 2017 CRD42017063906. | Link |
- Sarah Dewilde, Alison Griffiths, Leticia Barcena, Julie Winstone. Systematic review and network meta-analysis of biological agents for the treatment of moderate to severe and very severe psoriasis. PROSPERO 2016 CRD42016052312. | Link |
- Rhea Jakubzyk, Dr. Alexander Nast, Corinna Dressler. Systematic review of the efficacy, effectiveness and safety of topical and systemic treatments for psoriasis in patients with hepatitis, diabetes mellitus, renal failure, HIV infection, pregnancy or cancer. PROSPERO 2018 CRD42018087908. | Link |
- James G. Krueger, MD, PhD. Safety and Efficacy of Secukinumab in Mild Psoriasis. clinicaltrials.gov. 2017. | Link |
- Novartis Pharmaceuticals. Study to Explore the Effect of Secukinumab, Compared to Placebo, on Fat Tissue and Skin in Plaque Psoriasis Patients ObePso-S. clinicaltrials.gov. 2017. | Link |
- Novartis Pharmaceuticals. Pediatric Study in Children and Adolescents With Severe Plaque Psoriasis. clinicaltrials.gov. 2015. | Link |
 Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA, Korman NJ, Beutner KR, Bhushan R. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008 May;58(5):826-50. | CrossRef | PubMed |
Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA, Korman NJ, Beutner KR, Bhushan R. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008 May;58(5):826-50. | CrossRef | PubMed | Ryoo JY, Yang HJ, Ji E, Yoo BK. Meta-analysis of the Efficacy and Safety of Secukinumab for the Treatment of Plaque Psoriasis. Ann Pharmacother. 2016 May;50(5):341-51. | CrossRef | PubMed |
Ryoo JY, Yang HJ, Ji E, Yoo BK. Meta-analysis of the Efficacy and Safety of Secukinumab for the Treatment of Plaque Psoriasis. Ann Pharmacother. 2016 May;50(5):341-51. | CrossRef | PubMed | Nast A, Jacobs A, Rosumeck S, Werner RN. Efficacy and Safety of Systemic Long-Term Treatments for Moderate-to-Severe Psoriasis: A Systematic Review and Meta-Analysis. J Invest Dermatol. 2015 Nov;135(11):2641-2648. | CrossRef | PubMed |
Nast A, Jacobs A, Rosumeck S, Werner RN. Efficacy and Safety of Systemic Long-Term Treatments for Moderate-to-Severe Psoriasis: A Systematic Review and Meta-Analysis. J Invest Dermatol. 2015 Nov;135(11):2641-2648. | CrossRef | PubMed | Yiu ZZ, Exton LS, Jabbar-Lopez Z, Mohd Mustapa MF, Samarasekera EJ, Burden AD, Murphy R, Owen CM, Parslew R, Venning V, Ashcroft DM, Griffiths CE, Smith CH, Warren RB. Risk of Serious Infections in Patients with Psoriasis on Biologic Therapies: A Systematic Review and Meta-Analysis. J Invest Dermatol. 2016 Aug;136(8):1584-91. | CrossRef | PubMed | PMC |
Yiu ZZ, Exton LS, Jabbar-Lopez Z, Mohd Mustapa MF, Samarasekera EJ, Burden AD, Murphy R, Owen CM, Parslew R, Venning V, Ashcroft DM, Griffiths CE, Smith CH, Warren RB. Risk of Serious Infections in Patients with Psoriasis on Biologic Therapies: A Systematic Review and Meta-Analysis. J Invest Dermatol. 2016 Aug;136(8):1584-91. | CrossRef | PubMed | PMC | Rungapiromnan W, Yiu ZZN, Warren RB, Griffiths CEM, Ashcroft DM. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2017 Apr;176(4):890-901. | CrossRef | PubMed | PMC |
Rungapiromnan W, Yiu ZZN, Warren RB, Griffiths CEM, Ashcroft DM. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2017 Apr;176(4):890-901. | CrossRef | PubMed | PMC | de Carvalho AV, Duquia RP, Horta BL, Bonamigo RR. Efficacy of Immunobiologic and Small Molecule Inhibitor Drugs for Psoriasis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Drugs R D. 2017 Mar;17(1):29-51. | CrossRef | PubMed | PMC |
de Carvalho AV, Duquia RP, Horta BL, Bonamigo RR. Efficacy of Immunobiologic and Small Molecule Inhibitor Drugs for Psoriasis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Drugs R D. 2017 Mar;17(1):29-51. | CrossRef | PubMed | PMC | Tausend W, Downing C, Tyring S. Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab. J Cutan Med Surg. 2014 May-Jun;18(3):156-69. | PubMed |
Tausend W, Downing C, Tyring S. Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab. J Cutan Med Surg. 2014 May-Jun;18(3):156-69. | PubMed | Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014 Jul;15(3):165-80. | CrossRef | PubMed |
Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014 Jul;15(3):165-80. | CrossRef | PubMed | Xiong HZ, Gu JY, He ZG, Chen WJ, Zhang X, Wang JY, Shi YL. Efficacy and safety of secukinumab in the treatment of moderate to severe plaque psoriasis: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2015 Mar 15;8(3):3156-72. | PubMed | PMC |
Xiong HZ, Gu JY, He ZG, Chen WJ, Zhang X, Wang JY, Shi YL. Efficacy and safety of secukinumab in the treatment of moderate to severe plaque psoriasis: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2015 Mar 15;8(3):3156-72. | PubMed | PMC | Chen Y, Qian T, Zhang D, Yan H, Hao F. Clinical efficacy and safety of anti-IL-17 agents for the treatment of patients with psoriasis. Immunotherapy. 2015;7(9):1023-37. | CrossRef | PubMed |
Chen Y, Qian T, Zhang D, Yan H, Hao F. Clinical efficacy and safety of anti-IL-17 agents for the treatment of patients with psoriasis. Immunotherapy. 2015;7(9):1023-37. | CrossRef | PubMed | Wu D, Hou SY, Zhao S, Hou LX, Jiao T, Xu NN, Zhang N. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017 Jun;31(6):992-1003. | CrossRef | PubMed |
Wu D, Hou SY, Zhao S, Hou LX, Jiao T, Xu NN, Zhang N. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017 Jun;31(6):992-1003. | CrossRef | PubMed | Jabbar-Lopez ZK, Yiu ZZN, Ward V, Exton LS, Mohd Mustapa MF, Samarasekera E, Burden AD, Murphy R, Owen CM, Parslew R, Venning V, Warren RB, Smith CH. Quantitative Evaluation of Biologic Therapy Options for Psoriasis: A Systematic Review and Network Meta-Analysis. J Invest Dermatol. 2017 Aug;137(8):1646-1654. | CrossRef | PubMed | PMC |
Jabbar-Lopez ZK, Yiu ZZN, Ward V, Exton LS, Mohd Mustapa MF, Samarasekera E, Burden AD, Murphy R, Owen CM, Parslew R, Venning V, Warren RB, Smith CH. Quantitative Evaluation of Biologic Therapy Options for Psoriasis: A Systematic Review and Network Meta-Analysis. J Invest Dermatol. 2017 Aug;137(8):1646-1654. | CrossRef | PubMed | PMC | Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017 Jul;177(1):47-62. | CrossRef | PubMed |
Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017 Jul;177(1):47-62. | CrossRef | PubMed | Sbidian E, Chaimani A, Garcia-Doval I, Do G, Hua C, Mazaud C, Droitcourt C, Hughes C, Ingram JR, Naldi L, Chosidow O, Le Cleach L. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017 Dec 22;12:CD011535. | CrossRef | PubMed |
Sbidian E, Chaimani A, Garcia-Doval I, Do G, Hua C, Mazaud C, Droitcourt C, Hughes C, Ingram JR, Naldi L, Chosidow O, Le Cleach L. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017 Dec 22;12:CD011535. | CrossRef | PubMed | Gómez-García F, Epstein D, Isla-Tejera B, Lorente A, Vélez García-Nieto A, Ruano J. Short-term efficacy and safety of new biological agents targeting the interleukin-23-T helper 17 pathway for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis. Br J Dermatol. 2017 Mar;176(3):594-603. | CrossRef | PubMed |
Gómez-García F, Epstein D, Isla-Tejera B, Lorente A, Vélez García-Nieto A, Ruano J. Short-term efficacy and safety of new biological agents targeting the interleukin-23-T helper 17 pathway for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis. Br J Dermatol. 2017 Mar;176(3):594-603. | CrossRef | PubMed | Théréné C, Brenaut E, Barnetche T, Misery L. Efficacy of Systemic Treatments of Psoriasis on Pruritus: A Systemic Literature Review and Meta-Analysis. J Invest Dermatol. 2018 Jan;138(1):38-4 | CrossRef | PubMed |
Théréné C, Brenaut E, Barnetche T, Misery L. Efficacy of Systemic Treatments of Psoriasis on Pruritus: A Systemic Literature Review and Meta-Analysis. J Invest Dermatol. 2018 Jan;138(1):38-4 | CrossRef | PubMed | o DJ, Amin M, Bhutani T, Wu JJ. A systematic review of active comparator controlled clinical trials in patients with moderate-to-severe psoriasis. J Dermatolog Treat. 2017 Nov 22:1-8. | CrossRef | PubMed |
o DJ, Amin M, Bhutani T, Wu JJ. A systematic review of active comparator controlled clinical trials in patients with moderate-to-severe psoriasis. J Dermatolog Treat. 2017 Nov 22:1-8. | CrossRef | PubMed | Loos AM, Liu S, Segel C, Ollendorf DA, Pearson SD, Linder JA. Comparative Effectiveness of Targeted Immunomodulators for the Treatment of Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis. J Am Acad Dermatol. 2018 Feb 10. pii: S0190-9622(18)30213-5. | CrossRef | PubMed |
Loos AM, Liu S, Segel C, Ollendorf DA, Pearson SD, Linder JA. Comparative Effectiveness of Targeted Immunomodulators for the Treatment of Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis. J Am Acad Dermatol. 2018 Feb 10. pii: S0190-9622(18)30213-5. | CrossRef | PubMed | van de Kerkhof PC, Griffiths CE, Reich K, Leonardi CL, Blauvelt A, Tsai TF, Gong Y, Huang J, Papavassilis C, Fox T. Secukinumab long-term safety experience: A pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016 Jul;75(1):83-98.e4. | CrossRef | PubMed |
van de Kerkhof PC, Griffiths CE, Reich K, Leonardi CL, Blauvelt A, Tsai TF, Gong Y, Huang J, Papavassilis C, Fox T. Secukinumab long-term safety experience: A pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016 Jul;75(1):83-98.e4. | CrossRef | PubMed | Warren RB, Brnabic A, Saure D, Langley RG, See K, Wu JJ, Schacht A, Mallbris L, Nast A. Matching-adjusted indirect comparison of efficacy in patients with moderate-to-severe plaque psoriasis treated with ixekizumab vs. secukinumab. Br J Dermatol. 2017 Nov 24. | CrossRef | PubMed |
Warren RB, Brnabic A, Saure D, Langley RG, See K, Wu JJ, Schacht A, Mallbris L, Nast A. Matching-adjusted indirect comparison of efficacy in patients with moderate-to-severe plaque psoriasis treated with ixekizumab vs. secukinumab. Br J Dermatol. 2017 Nov 24. | CrossRef | PubMed | Fan XD, Xia X, Zhang CY, Kong WQ, Zhou CY, DU B. [A systematic review of anti-interleukin-17 antibody in the treatment of plaque psoriasis]. Nan Fang Yi Ke Da Xue Xue Bao. 2017 Sep 20;37(9):1274-1279. Chinese. | PubMed |
Fan XD, Xia X, Zhang CY, Kong WQ, Zhou CY, DU B. [A systematic review of anti-interleukin-17 antibody in the treatment of plaque psoriasis]. Nan Fang Yi Ke Da Xue Xue Bao. 2017 Sep 20;37(9):1274-1279. Chinese. | PubMed | Armstrong AW, Betts KA, Signorovitch JE, Sundaram M, Li J, Ganguli AX, Wu EQ. Number needed to treat and costs per responder among biologic treatments for moderate-to-severe psoriasis: a network meta-analysis. Curr Med Res Opin. 2018 Apr 23:1-9. | CrossRef | PubMed |
Armstrong AW, Betts KA, Signorovitch JE, Sundaram M, Li J, Ganguli AX, Wu EQ. Number needed to treat and costs per responder among biologic treatments for moderate-to-severe psoriasis: a network meta-analysis. Curr Med Res Opin. 2018 Apr 23:1-9. | CrossRef | PubMed | Blauvelt A, Prinz JC, Gottlieb AB, Kingo K, Sofen H, Ruer-Mulard M, Singh V, Pathan R, Papavassilis C, Cooper S; FEATURE Study Group. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015 Feb;172(2):484-93. | CrossRef | PubMed |
Blauvelt A, Prinz JC, Gottlieb AB, Kingo K, Sofen H, Ruer-Mulard M, Singh V, Pathan R, Papavassilis C, Cooper S; FEATURE Study Group. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015 Feb;172(2):484-93. | CrossRef | PubMed | Novartis Pharmaceuticals. Extension Study of Secukinumab Prefilled Syringes in Subjects With Moderate to Severe Chronic Plaque-type Psoriasis Completing Preceding Secukinumab Phase III Studies. clinicaltrials.gov. 2012. | CrossRef | Link |
Novartis Pharmaceuticals. Extension Study of Secukinumab Prefilled Syringes in Subjects With Moderate to Severe Chronic Plaque-type Psoriasis Completing Preceding Secukinumab Phase III Studies. clinicaltrials.gov. 2012. | CrossRef | Link | Novartis Pharmaceuticals. First Study of Secukinumab in Pre-filled Syringes in Subjects With Chronic Plaque-type Psoriasis: Response at 12 Weeks (FEATURE). clinicaltrials.gov. 2012. | Link |
Novartis Pharmaceuticals. First Study of Secukinumab in Pre-filled Syringes in Subjects With Chronic Plaque-type Psoriasis: Response at 12 Weeks (FEATURE). clinicaltrials.gov. 2012. | Link | Novartis Pharmaceuticals. Extension Study of Secukinumab Prefilled Syringes in Subjects With Moderate to Severe Chronic Plaque-type Psoriasis Completing Preceding Psoriasis Phase III Studies With Secukinumab. clinicaltrials.gov. 2012. | Link |
Novartis Pharmaceuticals. Extension Study of Secukinumab Prefilled Syringes in Subjects With Moderate to Severe Chronic Plaque-type Psoriasis Completing Preceding Psoriasis Phase III Studies With Secukinumab. clinicaltrials.gov. 2012. | Link | Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH; Psoriasis Study Group, Durez P, Tak PP, Gomez-Reino JJ; Rheumatoid Arthritis Study Group, Foster CS, Kim RY, Samson CM, Falk NS, Chu DS, Callanan D, Nguyen QD; Uveitis Study Group, Rose K, Haider A, Di Padova F. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010 Oct 6;2(52):52ra72. | CrossRef | PubMed |
Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH; Psoriasis Study Group, Durez P, Tak PP, Gomez-Reino JJ; Rheumatoid Arthritis Study Group, Foster CS, Kim RY, Samson CM, Falk NS, Chu DS, Callanan D, Nguyen QD; Uveitis Study Group, Rose K, Haider A, Di Padova F. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010 Oct 6;2(52):52ra72. | CrossRef | PubMed | Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C; ERASURE Study Group; FIXTURE Study Group. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014 Jul 24;371(4):326-38. | CrossRef | PubMed |
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C; ERASURE Study Group; FIXTURE Study Group. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014 Jul 24;371(4):326-38. | CrossRef | PubMed | Ohtsuki M, Morita A, Abe M, Takahashi H, Seko N, Karpov A, Shima T, Papavassilis C, Nakagawa H; ERASURE Study Japanese subgroup. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014 Dec;41(12):1039-46. | CrossRef | PubMed |
Ohtsuki M, Morita A, Abe M, Takahashi H, Seko N, Karpov A, Shima T, Papavassilis C, Nakagawa H; ERASURE Study Japanese subgroup. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014 Dec;41(12):1039-46. | CrossRef | PubMed | Novartis Pharmaceuticals. Efficacy and Safety of Subcutaneous Secukinumab for Moderate to Severe Chronic Plaque-type Psoriasis for up to 1 Year (Erasure). clinicaltrials.gov. 2011. | Link |
Novartis Pharmaceuticals. Efficacy and Safety of Subcutaneous Secukinumab for Moderate to Severe Chronic Plaque-type Psoriasis for up to 1 Year (Erasure). clinicaltrials.gov. 2011. | Link | Gottlieb AB, Langley RG, Philipp S, Sigurgeirsson B, Blauvelt A, Martin R, Papavassilis C, Mpofu S. Secukinumab Improves Physical Function in Subjects With Plaque Psoriasis and Psoriatic Arthritis: Results from Two Randomized, Phase 3 Trials. J Drugs Dermatol. 2015 Aug;14(8):821-33. | PubMed |
Gottlieb AB, Langley RG, Philipp S, Sigurgeirsson B, Blauvelt A, Martin R, Papavassilis C, Mpofu S. Secukinumab Improves Physical Function in Subjects With Plaque Psoriasis and Psoriatic Arthritis: Results from Two Randomized, Phase 3 Trials. J Drugs Dermatol. 2015 Aug;14(8):821-33. | PubMed | Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, Szepietowski JC, Regnault P, Thurston H, Papavassilis C; SCULPTURE Study Group. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: A randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015 Jul;73(1):27-36.e1. | CrossRef | PubMed |
Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, Szepietowski JC, Regnault P, Thurston H, Papavassilis C; SCULPTURE Study Group. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: A randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015 Jul;73(1):27-36.e1. | CrossRef | PubMed | Thaçi D, Humeniuk J, Frambach Y, Bissonnette R, Goodman JJ, Shevade S, Gong Y, Papavassilis C; STATURE study group. Secukinumab in psoriasis: randomized, controlled phase 3 trial results assessing the potential to improve treatment response in partial responders (STATURE). Br J Dermatol. 2015 Sep;173(3):777-87. | CrossRef | PubMed |
Thaçi D, Humeniuk J, Frambach Y, Bissonnette R, Goodman JJ, Shevade S, Gong Y, Papavassilis C; STATURE study group. Secukinumab in psoriasis: randomized, controlled phase 3 trial results assessing the potential to improve treatment response in partial responders (STATURE). Br J Dermatol. 2015 Sep;173(3):777-87. | CrossRef | PubMed | Novartis Pharmaceuticals. Efficacy and Safety of Subcutaneous Secukinumab (AIN457) for Moderate to Severe Chronic Plaque-type Psoriasis Assessing Different Doses and Dose Regimens (SCULPTURE). clinicaltrials.gov. 2011 | Link |
Novartis Pharmaceuticals. Efficacy and Safety of Subcutaneous Secukinumab (AIN457) for Moderate to Severe Chronic Plaque-type Psoriasis Assessing Different Doses and Dose Regimens (SCULPTURE). clinicaltrials.gov. 2011 | Link | Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, Haemmerle S, Thurston HJ, Papavassilis C, Richards HB. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013 Feb;168(2):412-21. | CrossRef | PubMed |
Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, Haemmerle S, Thurston HJ, Papavassilis C, Richards HB. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013 Feb;168(2):412-21. | CrossRef | PubMed | Sigurgeirsson B, Kircik L, Nemoto O, Mikazans I, Haemmerle S, Thurston HJ, Papavassilis C, Richards HB. Secukinumab improves the signs and symptoms of moderate-to-severe plaque psoriasis in subjects with involvement of hands and/or feet: subanalysis of a randomized, double-blind, placebo-controlled, phase 2 dose-ranging study. J Eur Acad Dermatol Venereol. 2014 Aug;28(8):1127-9. | CrossRef | PubMed |
Sigurgeirsson B, Kircik L, Nemoto O, Mikazans I, Haemmerle S, Thurston HJ, Papavassilis C, Richards HB. Secukinumab improves the signs and symptoms of moderate-to-severe plaque psoriasis in subjects with involvement of hands and/or feet: subanalysis of a randomized, double-blind, placebo-controlled, phase 2 dose-ranging study. J Eur Acad Dermatol Venereol. 2014 Aug;28(8):1127-9. | CrossRef | PubMed | Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, Guindon C, You R, Papavassilis C; JUNCTURE study group. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015 Jun;29(6):1082-90. | CrossRef | PubMed |
Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, Guindon C, You R, Papavassilis C; JUNCTURE study group. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015 Jun;29(6):1082-90. | CrossRef | PubMed | Lacour JP, Paul C, Jazayeri S, Papanastasiou P, Xu C, Nyirady J, Fox T, Papavassilis C. Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: results of the randomized controlled JUNCTURE trial. J Eur Acad Dermatol Venereol. 2017 May;31(5):847-856. | CrossRef | PubMed |
Lacour JP, Paul C, Jazayeri S, Papanastasiou P, Xu C, Nyirady J, Fox T, Papavassilis C. Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: results of the randomized controlled JUNCTURE trial. J Eur Acad Dermatol Venereol. 2017 May;31(5):847-856. | CrossRef | PubMed | Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M, Gratton D, Kunynetz RA, Poulin Y, Rosoph LA, Stingl G, Bauer WM, Salter JM, Falk TM, Blödorn-Schlicht NA, Hueber W, Sommer U, Schumacher MM, Peters T, Kriehuber E, Lee DM, Wieczorek GA, Kolbinger F, Bleul CC. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol. 2015 Jul;24(7):529-35. | CrossRef | PubMed | PMC |
Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M, Gratton D, Kunynetz RA, Poulin Y, Rosoph LA, Stingl G, Bauer WM, Salter JM, Falk TM, Blödorn-Schlicht NA, Hueber W, Sommer U, Schumacher MM, Peters T, Kriehuber E, Lee DM, Wieczorek GA, Kolbinger F, Bleul CC. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol. 2015 Jul;24(7):529-35. | CrossRef | PubMed | PMC | Rich P, Sigurgeirsson B, Thaci D, Ortonne JP, Paul C, Schopf RE, Morita A, Roseau K, Harfst E, Guettner A, Machacek M, Papavassilis C. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013 Feb;168(2):402-11. | CrossRef | PubMed |
Rich P, Sigurgeirsson B, Thaci D, Ortonne JP, Paul C, Schopf RE, Morita A, Roseau K, Harfst E, Guettner A, Machacek M, Papavassilis C. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013 Feb;168(2):402-11. | CrossRef | PubMed | Paul C, Reich K, Gottlieb AB, Mrowietz U, Philipp S, Nakayama J, Harfst E, Guettner A, Papavassilis C; CAIN457A2211 study group. Secukinumab improves hand, foot and nail lesions in moderate-to-severe plaque psoriasis: subanalysis of a randomized, double-blind, placebo-controlled, regimen-finding phase 2 trial. J Eur Acad Dermatol Venereol. 2014 Dec;28(12):1670-5. | CrossRef | PubMed |
Paul C, Reich K, Gottlieb AB, Mrowietz U, Philipp S, Nakayama J, Harfst E, Guettner A, Papavassilis C; CAIN457A2211 study group. Secukinumab improves hand, foot and nail lesions in moderate-to-severe plaque psoriasis: subanalysis of a randomized, double-blind, placebo-controlled, regimen-finding phase 2 trial. J Eur Acad Dermatol Venereol. 2014 Dec;28(12):1670-5. | CrossRef | PubMed | Augustin M, Abeysinghe S, Mallya U, Qureshi A, Roskell N, McBride D, Papavassillis C, Gelfand J. Secukinumab treatment of plaque psoriasis shows early improvement in DLQI response - results of a phase II regimen-finding trial. J Eur Acad Dermatol Venereol. 2016 Apr;30(4):645-9. | CrossRef | PubMed | PMC |
Augustin M, Abeysinghe S, Mallya U, Qureshi A, Roskell N, McBride D, Papavassillis C, Gelfand J. Secukinumab treatment of plaque psoriasis shows early improvement in DLQI response - results of a phase II regimen-finding trial. J Eur Acad Dermatol Venereol. 2016 Apr;30(4):645-9. | CrossRef | PubMed | PMC | Novartis Pharmaceuticals. AIN457 regimen finding extension study in patients with moderate to severe psoriasis. clinicaltrials.gov. 2010. | Link |
Novartis Pharmaceuticals. AIN457 regimen finding extension study in patients with moderate to severe psoriasis. clinicaltrials.gov. 2010. | Link | Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, Ziv M, Pinter A, Hugot S, You R, Milutinovic M. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015 Sep;73(3):400-9. | CrossRef | PubMed |
Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, Ziv M, Pinter A, Hugot S, You R, Milutinovic M. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015 Sep;73(3):400-9. | CrossRef | PubMed | Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, Ziv M, Pinter A, Vender R, Hugot S, You R, Milutinovic M, Thaçi D. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: Results from the CLEAR study. J Am Acad Dermatol. 2017 Jan;76(1):60-69.e9. | CrossRef | PubMed |
Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, Ziv M, Pinter A, Vender R, Hugot S, You R, Milutinovic M, Thaçi D. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: Results from the CLEAR study. J Am Acad Dermatol. 2017 Jan;76(1):60-69.e9. | CrossRef | PubMed | Nast A, Gisondi P, Ormerod AD, Saiag P, Smith C, Spuls PI, Arenberger P, Bachelez H, Barker J, Dauden E, de Jong EM, Feist E, Jacobs A, Jobling R, Kemény L, Maccarone M, Mrowietz U, Papp KA, Paul C, Reich K, Rosumeck S, Talme T, Thio HB, van de Kerkhof P, Werner RN, Yawalkar N. European S3-Guidelines on the systemic treatment of psoriasis vulgaris--Update 2015--Short version--EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015 Dec;29(12):2277-94. | CrossRef | PubMed |
Nast A, Gisondi P, Ormerod AD, Saiag P, Smith C, Spuls PI, Arenberger P, Bachelez H, Barker J, Dauden E, de Jong EM, Feist E, Jacobs A, Jobling R, Kemény L, Maccarone M, Mrowietz U, Papp KA, Paul C, Reich K, Rosumeck S, Talme T, Thio HB, van de Kerkhof P, Werner RN, Yawalkar N. European S3-Guidelines on the systemic treatment of psoriasis vulgaris--Update 2015--Short version--EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015 Dec;29(12):2277-94. | CrossRef | PubMed | Smith CH, Jabbar-Lopez ZK, Yiu ZZ, Bale T, Burden AD, Coates LC, Cruickshank M, Hadoke T, MacMahon E, Murphy R, Nelson-Piercy C, Owen CM, Parslew R, Peleva E, Pottinger E, Samarasekera EJ, Stoddart J, Strudwicke C, Venning VA, Warren RB, Exton LS, Mohd Mustapa MF. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017 Sep;177(3):628-636. | CrossRef | PubMed |
Smith CH, Jabbar-Lopez ZK, Yiu ZZ, Bale T, Burden AD, Coates LC, Cruickshank M, Hadoke T, MacMahon E, Murphy R, Nelson-Piercy C, Owen CM, Parslew R, Peleva E, Pottinger E, Samarasekera EJ, Stoddart J, Strudwicke C, Venning VA, Warren RB, Exton LS, Mohd Mustapa MF. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017 Sep;177(3):628-636. | CrossRef | PubMed | Corinna Dressler, Miriam Zidane, Alexander Nast. A systematic review and "time-effectiveness" analysis of psoriasis treatments and different treatment sequences. PROSPERO 2017 CRD42017074218. | Link |
Corinna Dressler, Miriam Zidane, Alexander Nast. A systematic review and "time-effectiveness" analysis of psoriasis treatments and different treatment sequences. PROSPERO 2017 CRD42017074218. | Link | Christine Ballegaard, Tanja Schjødt Jørgensen, Marie Skougaard, Vibeke Strand, Philip J. Mease, Lars E. Kristensen, Lene Dreyer, Alice Gottlieb, Maarten de Wit, Robin Christensen, Simon Tarp. Assessing the importance of trial characteristics as contextual factors when evaluating targeted therapies in patients with psoriatic disease: protocol for an exploratory systematic review and meta-research project. PROSPERO 2016 CRD42016050049. | CrossRef | Link |
Christine Ballegaard, Tanja Schjødt Jørgensen, Marie Skougaard, Vibeke Strand, Philip J. Mease, Lars E. Kristensen, Lene Dreyer, Alice Gottlieb, Maarten de Wit, Robin Christensen, Simon Tarp. Assessing the importance of trial characteristics as contextual factors when evaluating targeted therapies in patients with psoriatic disease: protocol for an exploratory systematic review and meta-research project. PROSPERO 2016 CRD42016050049. | CrossRef | Link | Daniel Ollendorf, Reiner Banken, Foluso Agboola, Katherine Fazioli, Celia Segel. Comparative clinical effectiveness of treatment options for moderate-to-severe plaque psoriasis. PROSPERO 2018 CRD42018088801. | Link |
Daniel Ollendorf, Reiner Banken, Foluso Agboola, Katherine Fazioli, Celia Segel. Comparative clinical effectiveness of treatment options for moderate-to-severe plaque psoriasis. PROSPERO 2018 CRD42018088801. | Link | Simon Tarp, Lars Erik Bryld, Lars Iversen, Lone Skov, Sigurd Broesby-Olsen, Birgitte Brock, Camilla Mikkelsen, Lis Andersen, Robin Christensen. Comparative effectiveness associated with the use of biologics and small-molecules for psoriasis: protocol for a systematic review and meta-analysis. PROSPERO 2015 CRD42015029122. | Link |
Simon Tarp, Lars Erik Bryld, Lars Iversen, Lone Skov, Sigurd Broesby-Olsen, Birgitte Brock, Camilla Mikkelsen, Lis Andersen, Robin Christensen. Comparative effectiveness associated with the use of biologics and small-molecules for psoriasis: protocol for a systematic review and meta-analysis. PROSPERO 2015 CRD42015029122. | Link | Catherine Smith, Zarif Jabbar-Lopez, Zenas Yiu, Ellie Samarasekera, M. Firouz Mohd Mustapa, Leslie Exton. In people with psoriasis (all types), what are the clinical effectiveness/efficacy, safety and tolerability of systemic biologics (adalimumab, etanercept, infliximab, secukinumab or ustekinumab) compared with each other, with methotrexate or with placebo?. PROSPERO 2015 CRD42015017538. | Link |
Catherine Smith, Zarif Jabbar-Lopez, Zenas Yiu, Ellie Samarasekera, M. Firouz Mohd Mustapa, Leslie Exton. In people with psoriasis (all types), what are the clinical effectiveness/efficacy, safety and tolerability of systemic biologics (adalimumab, etanercept, infliximab, secukinumab or ustekinumab) compared with each other, with methotrexate or with placebo?. PROSPERO 2015 CRD42015017538. | Link | Juan Ruano, Francisco Gómez-García, Beatriz Isla-Tejera, Jesús Gay-Mimbrera, Macarena Aquilar-Luqe, Marcelino González-Padilla, Juan Luis Sanz-Cabanillas, Antonio Vélez García-Nieto. Pharmacogenetics of agents blocking TNF-a, IL-12/23, and IL17/IL17RA for the treatment of moderate-severe psoriasis: a systematic review and meta-analysis. PROSPERO 2016 CRD42016038769. | Link |
Juan Ruano, Francisco Gómez-García, Beatriz Isla-Tejera, Jesús Gay-Mimbrera, Macarena Aquilar-Luqe, Marcelino González-Padilla, Juan Luis Sanz-Cabanillas, Antonio Vélez García-Nieto. Pharmacogenetics of agents blocking TNF-a, IL-12/23, and IL17/IL17RA for the treatment of moderate-severe psoriasis: a systematic review and meta-analysis. PROSPERO 2016 CRD42016038769. | Link | Francisco Jose Gomez Garcia, Juan Ruano, Beatriz Isla-Tejera, Marcelino Gonzalez Padilla, Juan Luis Sanz Cabanillas, Antonio Velez Garcia Nieto, Macarena Aguilar Luque, Jesus Gay Mimbrera, Pedro Carmona. Short-, medium- and long-term efficacy and safety of biological drugs for moderate to severe plaque psoriasis: systematic review and network meta-analysis. PROSPERO 2017 CRD42017057642. | Link |
Francisco Jose Gomez Garcia, Juan Ruano, Beatriz Isla-Tejera, Marcelino Gonzalez Padilla, Juan Luis Sanz Cabanillas, Antonio Velez Garcia Nieto, Macarena Aguilar Luque, Jesus Gay Mimbrera, Pedro Carmona. Short-, medium- and long-term efficacy and safety of biological drugs for moderate to severe plaque psoriasis: systematic review and network meta-analysis. PROSPERO 2017 CRD42017057642. | Link | Yayoi Tada, Rei Watanabe, Hisashi Noma. Short-term and long-term effectiveness of biologic agents targeting TNF-a, IL-23, and IL-17 for the treatment of plaque psoriasis: a systematic review and network meta-analysis. PROSPERO 2017 CRD42017063906. | Link |
Yayoi Tada, Rei Watanabe, Hisashi Noma. Short-term and long-term effectiveness of biologic agents targeting TNF-a, IL-23, and IL-17 for the treatment of plaque psoriasis: a systematic review and network meta-analysis. PROSPERO 2017 CRD42017063906. | Link | Sarah Dewilde, Alison Griffiths, Leticia Barcena, Julie Winstone. Systematic review and network meta-analysis of biological agents for the treatment of moderate to severe and very severe psoriasis. PROSPERO 2016 CRD42016052312. | Link |
Sarah Dewilde, Alison Griffiths, Leticia Barcena, Julie Winstone. Systematic review and network meta-analysis of biological agents for the treatment of moderate to severe and very severe psoriasis. PROSPERO 2016 CRD42016052312. | Link | Rhea Jakubzyk, Dr. Alexander Nast, Corinna Dressler. Systematic review of the efficacy, effectiveness and safety of topical and systemic treatments for psoriasis in patients with hepatitis, diabetes mellitus, renal failure, HIV infection, pregnancy or cancer. PROSPERO 2018 CRD42018087908. | Link |
Rhea Jakubzyk, Dr. Alexander Nast, Corinna Dressler. Systematic review of the efficacy, effectiveness and safety of topical and systemic treatments for psoriasis in patients with hepatitis, diabetes mellitus, renal failure, HIV infection, pregnancy or cancer. PROSPERO 2018 CRD42018087908. | Link | James G. Krueger, MD, PhD. Safety and Efficacy of Secukinumab in Mild Psoriasis. clinicaltrials.gov. 2017. | Link |
James G. Krueger, MD, PhD. Safety and Efficacy of Secukinumab in Mild Psoriasis. clinicaltrials.gov. 2017. | Link | Novartis Pharmaceuticals. Study to Explore the Effect of Secukinumab, Compared to Placebo, on Fat Tissue and Skin in Plaque Psoriasis Patients ObePso-S. clinicaltrials.gov. 2017. | Link |
Novartis Pharmaceuticals. Study to Explore the Effect of Secukinumab, Compared to Placebo, on Fat Tissue and Skin in Plaque Psoriasis Patients ObePso-S. clinicaltrials.gov. 2017. | Link | Novartis Pharmaceuticals. Pediatric Study in Children and Adolescents With Severe Plaque Psoriasis. clinicaltrials.gov. 2015. | Link |
Novartis Pharmaceuticals. Pediatric Study in Children and Adolescents With Severe Plaque Psoriasis. clinicaltrials.gov. 2015. | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








