Abreviaturas: Interleukin 5, chronic rhinosinusitis, nasal polyps, Epistemonikos, GRADE.
Abstract
INTRODUCTION
Chronic rhinosinusitis is the inflammation of sinonasal mucosa lasting longer than 12 weeks. Two clinical forms are distinguished: chronic rhinosinusitis with polyps and without polyps. Patients with chronic rhinosinusitis with polyps exhibit high levels of interleukin 5, which promotes differentiation and survival of eosinophils. So, minimizing their circulation has been proposed as a new treatment strategy. However, there is no clarity regarding its real effectiveness.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified three systematic reviews included three primary studies overall, all corresponding to randomized trials. We concluded inhibitors of interleukin 5 might decrease nasal polyps score. Although they might be associated with adverse effects, these would be infrequent and of low severity. However, the certainty of the evidence is low.
Problem
Chronic rhinosinusitis is a chronic inflammatory disease of the sinonasal mucosa lasting longer than 12 weeks. It is estimated that 11.9% and 10.9% of the general population of the United States and Europe, respectively, have chronic rhinosinusitis [1],[2] whose symptoms significantly reduce physical and psychological well-being, affecting quality of life.
Medical treatment traditionally includes nasal washes and topical corticosteroids as maintenance therapy; systemic corticosteroids and antibiotics for exacerbations, and functional endoscopic surgery of paranasal cavities when medical and pharmacological treatment is not effective. However, there are many patients who do not respond or respond partially to treatment. One potential explanation is it focus on the relief of symptoms and reduction of inflammation rather than the cause of the disease.Interleukin 5 is a key mediator of chemotaxis, differentiation, activation and survival of the eosinophils [3]. Inhibiting this pathway would stop the release of toxic products that lead to more and lasting inflammation and formation of polyps in patients with chronic rhinosinusitis [4]. However, it is unclear what are the clinical effects of biological drugs that inhibit circulating interleukin 5, such as mepolizumab or reslizumab.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a section of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found three systematic reviews [5],[6],[7] that included three primary studies [8],[9],[10], all of which were randomized trials. |
|
What types of patients were included* |
All trials [8],[9],[10] included patients older than 18 years. Two trials [9],[10] included patients with a history of chronic rhinosinusitis and nasal polyps (grade 3 or 4) with failure to standard medical therapy or patients with recurrent nasal polyps after surgery (grade 1-4). One trial included patients with severe recurrent bilateral nasal polyposis who required surgery after failure of standard corticosteroid therapy [8]. |
|
What types of interventions were included* |
All trials used interleukin 5 inhibitors: one trial [9] used reslizumab and two trials [8],[10] mepolizumab.Reslizumab was used in doses of 3 mg/kg and 1 mg/kg in a single dose. Mepolizumab was administered in two doses of 750 mg IV separated by 28 days in one trial [10] and in six doses of 750 mg IV separated by 4 weeks in the other [8]. |
|
What types of outcomes |
The trials evaluated multiple outcomes, which were grouped by the systematic reviews as follows:
The average follow-up was 36 weeks, with a range between 25 and 48 weeks. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
The information on the effects of interleukin 5 inhibitors is based on three randomized trials[8],[9],[10] that included 159 patients.
Two trials [9],[10] measured the Nasal Polyp Score and symptoms through questions aboutspecific symptoms, such as nasal obstruction, loss of sense of smell and presence ofrhinorrhea (54 patients). Two trials [8],[10] reported Peak nasal inspiratory flow and adverse effects (135 patients). Only one trial [8] evaluated quality of life using the SNOT-22 specific indicator (105 patients).
The summary of findings is as follows:
- Inhibitors of interleukin 5 might decrease the score of nasal polyps, but the certainty of the evidence is low.
- Inhibitors of interleukin 5 might not have an impact on quality of life measured in SNOT, but the certainty of the evidence is low.
- It is not clear whether interleukin 5 inhibitors reduce symptoms (nasal obstruction, loss of sense of smell and presence of rhinorrhea) because the certainty of the evidence is very low.
- Inhibitors of interleukin 5 might improve PNIF, but the certainty of the evidence is low.
- Inhibitors of interleukin 5 might be associated to adverse effects of low frequency and severity, but the certainty of the evidence is low.
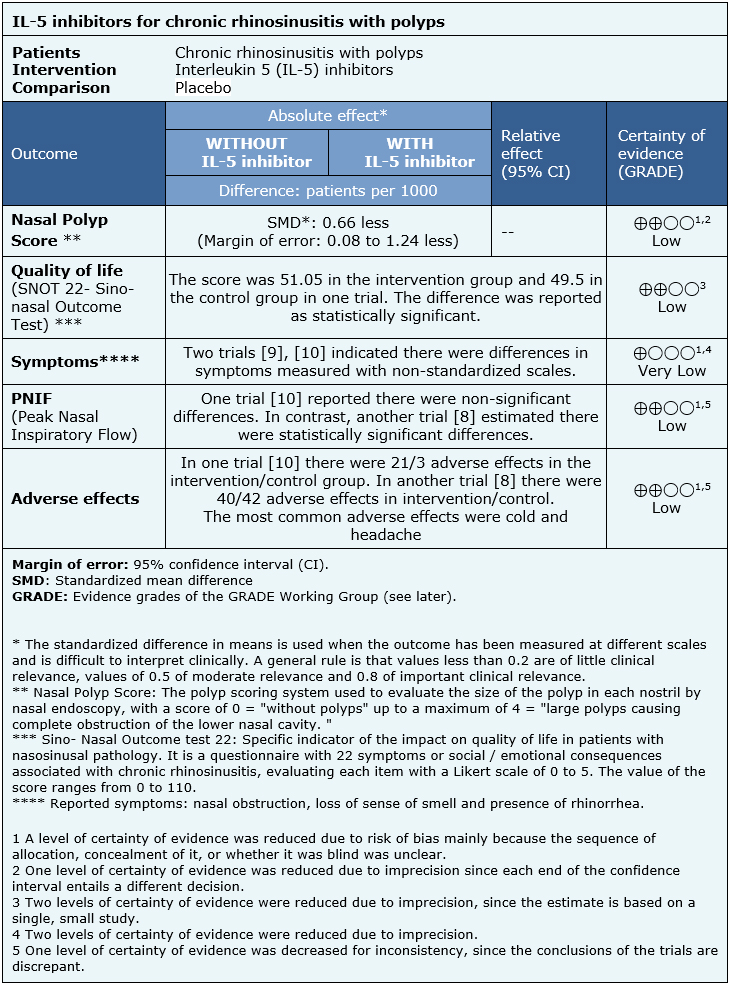
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
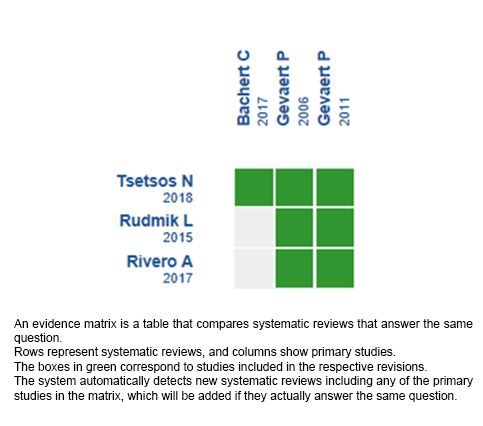
Follow the link to access the interactive version: Anti IL5 for chronic rhinosinusitis with polyps.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
La rinosinusitis crónica es la inflamación de la mucosa nasosinusal de duración superior a 12 semanas. Se distinguen dos formas clínicas: rinosinusitis crónica con pólipos y sin pólipos. Los pacientes con rinosinusitis crónica con pólipos presentan niveles elevados de interleukina 5, la cual promueve la diferenciación y supervivencia de eosinófilos, por lo que se ha propuesto minimizar su circulación como una nueva estrategia de tratamiento. Sin embargo, no hay claridad respecto a su real efectividad.
MÉTODOS
Para responder esta pregunta utilizamos Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante búsquedas en múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, reanalizamos los datos de los estudios primarios, realizamos un metanálisis y preparamos una tabla de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Identificamos tres revisiones sistemáticas que en conjunto incluyeron tres estudios primarios, todos correspondientes a ensayos aleatorizados. Concluimos que los inhibidores de interleukina 5 podrían disminuir el puntaje de pólipos nasales. Si bien podrían asociarse a efectos adversos, estos serían poco frecuentes y de baja severidad. Sin embargo, la certeza de la evidencia es baja.
 Authors:
María José Poblete[1,2], Andrés Rosenbaum[2,3], Matías Winter[2,3]
Authors:
María José Poblete[1,2], Andrés Rosenbaum[2,3], Matías Winter[2,3]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Otorrinolaringología, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: mwinterd@gmail.com
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Diagonal Paraguay 476 Santiago Chile

Citation: Poblete M, Rosenbaum A, Winter M. Anti-interleukin 5 therapy for chronic rhinosinusitis with polyps. Medwave 2018;18(6):e7300 doi: 10.5867/medwave.2018.06.7300
Submission date: 21/9/2018
Acceptance date: 10/10/2018
Publication date: 24/10/2018
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Hirsch AG, Stewart WF, Sundaresan AS, Young AJ, Kennedy TL, Scott Greene J, Feng W, Tan BK, Schleimer RP, Kern RC, Lidder A, Schwartz BS. Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy. 2017 Feb;72(2):274-281 | CrossRef | PubMed | PMC |
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, Bousquet PJ, Brozek G, Bruno A, Dahlén SE, Forsberg B, Gunnbjörnsdóttir M, Kasper L, Krämer U, Kowalski ML, Lange B, Lundbäck B, Salagean E, Todo-Bom A, Tomassen P, Toskala E, van Drunen CM, Bousquet J, Zuberbier T, Jarvis D, Burney P. Chronic rhinosinusitis in European underestimated disease. A GA²LEN study. Allergy. 2011 Sep;66(9):1216-23. | CrossRef | PubMed |
- Gevaert P, Bachert C, Holtappels G, Novo CP, Van der Heyden J, Fransen L, Depraetere S, Walter H, van Cauwenberge P, Tavernier J. Enhanced soluble interleukin-5 receptor alpha expression in nasal polyposis. Allergy. 2003. May;58(5):371-9. | PubMed |
- Bachert C, Gevaert P, Holtappels G, Cuvelier C, van Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinol. 2000 Sep-Oct;14(5):279-90. | PubMed |
- Tsetsos N, Goudakos JK, Daskalakis D, Konstantinidis I, Markou K. Monoclonal antibodies for the treatment of chronic rhinosinusitis with nasal polyposis: a systematic review. Rhinology. 2018 Mar 1;56(1):11-21. | CrossRef | PubMed |
- Rudmik L, Soler ZM. Medical Therapies for Adult Chronic Sinusitis: A Systematic Review. JAMA. 2015 Sep 1;314(9):926-39. | PubMed |
- Rivero A, Liang J. Anti-IgE and Anti-IL5 Biologic Therapy in the Treatment of Nasal Polyposis: A Systematic Review and Meta-analysis. Ann Otol Rhinol Laryngol. 2017 Nov;126(11):739-747. | CrossRef | PubMed |
- Bachert C, Sousa AR, Lund VJ, Scadding GK, Gevaert P, Nasser S, Durham SR, Cornet ME, Kariyawasam HH, Gilbert J, Austin D, Maxwell AC, Marshall RP, Fokkens WJ. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J Allergy Clin Immunol. 2017 Oct;140(4):1024-1031.e14. | CrossRef | PubMed |
- Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, Holtappels G, Tavernier J, van Cauwenberge P, Bachert C. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006 Nov;118(5):1133-41. Epub 2006 Sep 26. | PubMed |
- Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, De Ruyck N, Blomme K, Sousa AR, Marshall RP, Bachert C. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011 Nov;128(5):989-95.e1-8. | CrossRef | PubMed |
- Fokkens W, Lund V, Mullol J; European Position Paper on Rhinosinusitis and Nasal Polyps group. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl. 2007;20:1-136. | PubMed |
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, Georgalas C, Goossens H, Harvey R, Hellings P, Hopkins C, Jones N, Joos G, Kalogjera L, Kern B, Kowalski M, Price D, Riechelmann H, Schlosser R, Senior B, Thomas M, Toskala E, Voegels R, Wang de Y, Wormald PJ. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012 Mar;50(1):1-12. | CrossRef | PubMed |
 Hirsch AG, Stewart WF, Sundaresan AS, Young AJ, Kennedy TL, Scott Greene J,
Feng W, Tan BK, Schleimer RP, Kern RC, Lidder A, Schwartz BS. Nasal and sinus
symptoms and chronic rhinosinusitis in a population-based sample. Allergy. 2017
Feb;72(2):274-281
| CrossRef | PubMed | PMC |
Hirsch AG, Stewart WF, Sundaresan AS, Young AJ, Kennedy TL, Scott Greene J,
Feng W, Tan BK, Schleimer RP, Kern RC, Lidder A, Schwartz BS. Nasal and sinus
symptoms and chronic rhinosinusitis in a population-based sample. Allergy. 2017
Feb;72(2):274-281
| CrossRef | PubMed | PMC | Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, Bousquet PJ, Brozek G, Bruno A, Dahlén SE, Forsberg B, Gunnbjörnsdóttir M, Kasper L, Krämer U, Kowalski ML, Lange B, Lundbäck B, Salagean E, Todo-Bom A, Tomassen P, Toskala E, van Drunen CM, Bousquet J, Zuberbier T, Jarvis D, Burney P. Chronic rhinosinusitis in European underestimated disease. A GA²LEN study. Allergy.
2011 Sep;66(9):1216-23.
| CrossRef | PubMed |
Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, Bousquet PJ, Brozek G, Bruno A, Dahlén SE, Forsberg B, Gunnbjörnsdóttir M, Kasper L, Krämer U, Kowalski ML, Lange B, Lundbäck B, Salagean E, Todo-Bom A, Tomassen P, Toskala E, van Drunen CM, Bousquet J, Zuberbier T, Jarvis D, Burney P. Chronic rhinosinusitis in European underestimated disease. A GA²LEN study. Allergy.
2011 Sep;66(9):1216-23.
| CrossRef | PubMed | Gevaert P, Bachert C, Holtappels G, Novo CP, Van der Heyden J, Fransen L, Depraetere S, Walter H, van Cauwenberge P, Tavernier J. Enhanced soluble interleukin-5 receptor alpha expression in nasal polyposis. Allergy. 2003. May;58(5):371-9. | PubMed |
Gevaert P, Bachert C, Holtappels G, Novo CP, Van der Heyden J, Fransen L, Depraetere S, Walter H, van Cauwenberge P, Tavernier J. Enhanced soluble interleukin-5 receptor alpha expression in nasal polyposis. Allergy. 2003. May;58(5):371-9. | PubMed | Bachert C, Gevaert P, Holtappels G, Cuvelier C, van Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinol. 2000 Sep-Oct;14(5):279-90. | PubMed |
Bachert C, Gevaert P, Holtappels G, Cuvelier C, van Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinol. 2000 Sep-Oct;14(5):279-90. | PubMed | Tsetsos N, Goudakos JK, Daskalakis D, Konstantinidis I, Markou K. Monoclonal antibodies for the treatment of chronic rhinosinusitis with nasal polyposis: a systematic review. Rhinology. 2018 Mar 1;56(1):11-21. | CrossRef | PubMed |
Tsetsos N, Goudakos JK, Daskalakis D, Konstantinidis I, Markou K. Monoclonal antibodies for the treatment of chronic rhinosinusitis with nasal polyposis: a systematic review. Rhinology. 2018 Mar 1;56(1):11-21. | CrossRef | PubMed | Rudmik L, Soler ZM. Medical Therapies for Adult Chronic Sinusitis: A Systematic Review. JAMA. 2015 Sep 1;314(9):926-39. | PubMed |
Rudmik L, Soler ZM. Medical Therapies for Adult Chronic Sinusitis: A Systematic Review. JAMA. 2015 Sep 1;314(9):926-39. | PubMed | Rivero A, Liang J. Anti-IgE and Anti-IL5 Biologic Therapy in the Treatment of Nasal Polyposis: A Systematic Review and Meta-analysis. Ann Otol Rhinol Laryngol.
2017 Nov;126(11):739-747.
| CrossRef | PubMed |
Rivero A, Liang J. Anti-IgE and Anti-IL5 Biologic Therapy in the Treatment of Nasal Polyposis: A Systematic Review and Meta-analysis. Ann Otol Rhinol Laryngol.
2017 Nov;126(11):739-747.
| CrossRef | PubMed | Bachert C, Sousa AR, Lund VJ, Scadding GK, Gevaert P, Nasser S, Durham SR, Cornet ME, Kariyawasam HH, Gilbert J, Austin D, Maxwell AC, Marshall RP, Fokkens WJ. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J Allergy Clin Immunol. 2017 Oct;140(4):1024-1031.e14.
| CrossRef | PubMed |
Bachert C, Sousa AR, Lund VJ, Scadding GK, Gevaert P, Nasser S, Durham SR, Cornet ME, Kariyawasam HH, Gilbert J, Austin D, Maxwell AC, Marshall RP, Fokkens WJ. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J Allergy Clin Immunol. 2017 Oct;140(4):1024-1031.e14.
| CrossRef | PubMed | Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T,
Holtappels G, Tavernier J, van Cauwenberge P, Bachert C. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006 Nov;118(5):1133-41. Epub 2006 Sep 26. | PubMed |
Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T,
Holtappels G, Tavernier J, van Cauwenberge P, Bachert C. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006 Nov;118(5):1133-41. Epub 2006 Sep 26. | PubMed | Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, De Ruyck N, Blomme K, Sousa AR, Marshall RP, Bachert C. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin
Immunol. 2011 Nov;128(5):989-95.e1-8. | CrossRef | PubMed |
Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, De Ruyck N, Blomme K, Sousa AR, Marshall RP, Bachert C. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin
Immunol. 2011 Nov;128(5):989-95.e1-8. | CrossRef | PubMed | Fokkens W, Lund V, Mullol J; European Position Paper on Rhinosinusitis and Nasal Polyps group. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl. 2007;20:1-136.
| PubMed |
Fokkens W, Lund V, Mullol J; European Position Paper on Rhinosinusitis and Nasal Polyps group. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl. 2007;20:1-136.
| PubMed | Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, Georgalas C, Goossens H, Harvey R, Hellings P, Hopkins C, Jones N, Joos G, Kalogjera L, Kern B, Kowalski M, Price D, Riechelmann H, Schlosser R, Senior B, Thomas M, Toskala E, Voegels R, Wang de Y, Wormald PJ. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012 Mar;50(1):1-12. | CrossRef | PubMed |
Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, Georgalas C, Goossens H, Harvey R, Hellings P, Hopkins C, Jones N, Joos G, Kalogjera L, Kern B, Kowalski M, Price D, Riechelmann H, Schlosser R, Senior B, Thomas M, Toskala E, Voegels R, Wang de Y, Wormald PJ. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012 Mar;50(1):1-12. | CrossRef | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








