Abstract
INTRODUCTION
Antidepressant treatment does not lead to a satisfactory response in a significant proportion of patients with depression. It has been postulated that co-administration of pharmacologically standardized nutrients (nutraceuticals), such as folate, would potentiate the effect of antidepressants.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified four systematic reviews including nine studies overall, of which eight were randomized trials. We concluded augmentation with folate for the treatment of major depressive disorder probably results in little or no difference in depressive symptoms. It would be interesting to evaluate the effects of specific presentation forms of folate or in population with objective folate deficit.
Problem
After the introduction of antidepressants in the 1950s, the number of pharmacological treatments for major depressive disorder has increased, but the efficacy of these has remained largely unchanged. Approximately 50% of patients who initiate antidepressant treatment show little or no response after a first trial. Moreover, after several therapeutic approaches, non-remission rates are still around 30% [1],[3]. Adding a drug of a different pharmacological class to the current antidepressant treatment has showed an enhancing effect. Most of the evidence is focused in lithium and atypical antipsychotics. New approaches, such as co-administration of nutraceuticals, in particular folate, as both folic acid and its active presentation (methylfolate), could provide a novel and safer alternative for the treatment of depression [1],[2],[3].
Folate deficiency is a common finding in psychiatric patients and low folate levels have been associated with a worse response to pharmacological treatment. In addition, an association between folate and serotonin metabolism has been observed in patients with congenital defects of the metabolism of the former and in patients with neuropsychiatric disorders. This association could be explained by the role that folate plays in the methylation of homocysteine, necessary for its conversion into s-adenosyl methionine, which has been shown to influence the metabolism of serotonin. Another hypothesis is folate is involved in the methylation reactions of tetrahydrobiopterin, an essential cofactor for the hydroxylation enzymes involved in the synthesis of serotonin [1],[4]. However, the clinical impact of the use of folate as an augmentation strategy in depressive disorders is still controversial.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found four systematic reviews [1],[2],[3],[4], which included nine primary studies overall [5],[6],[7],[8],[9],[10],[11],[12],[13], of which eight were randomized trials [6],[7],[8],[9],[10],[11],[12],[13]. This table and the summary in general are based on the latter, since the observational study did not increase the certainty of the existing evidence or provide relevant additional information. |
|
What types of patients were included* |
Six trials [6],8],[9],[10],[12] included patients diagnosed with major depressive disorder according to DSM III or IV criteria, and two trials included patients with the same diagnosis according to ICD-10 [7],[13]. Of the eight trials selected, only one [9] exclusively included patients with low folate levels. On the contrary, two trials [6],[7] only included patients without baseline folate or B12 deficiency, and one trial [8] excluded patients with altered laboratory tests (including megaloblastic anemia). The rest of the trials [10],[12],[13] did not report this data. |
|
What types of interventions were included* |
All of the trials used selective serotonin reuptake inhibitors (SSRIs) as antidepressant, except two trials that did not report which antidepressant was used [7],[9]. Methylfolate was used as supplementation in three trials [9],[10] and folic acid in five trials [6],[7],[8],[12],[13]. Six trials compared against placebo [7],[8],[9],[10],[12], one trial compared against antidepressant monotherapy [6] and another trial compared different ranges of folate doses [13]. |
|
What types of outcomes |
The trials measured multiple outcomes, which were grouped by the systematic reviews identified as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
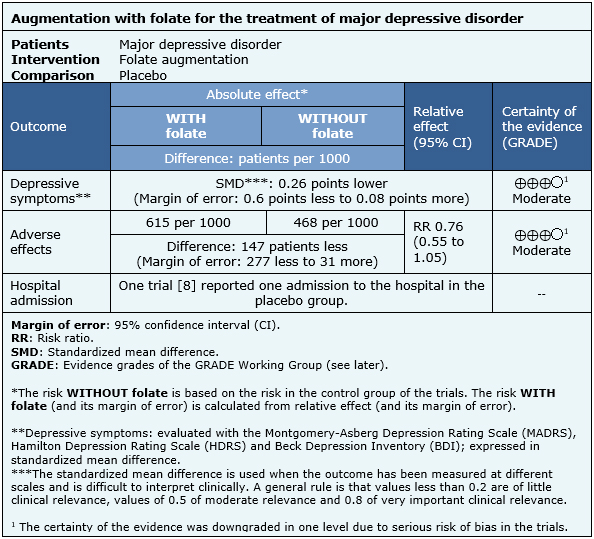
The information on the effects of folate augmentation for depression is based in four randomized trials [7],[8],[9],[12] that included 591 patients.
Four trials [7],[8],[9],[12] measured the outcome depressive symptoms at the end of the trial (591 patients), one trial [8] reported hospital admission (127 patients) and only one trial [8] reported the rate of adverse effects (127 patients).
The summary of findings is as follows:
- The addition of folate as augmentation in the treatment of major depressive disorder probably results in little or no difference in depressive symptoms at the end of treatment. The certainty of the evidence is moderate.
- The addition of folate as augmentation in the treatment of major depressive disorder probably results in little or no difference in adverse effects. The certainty of the evidence is moderate.
| Following the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
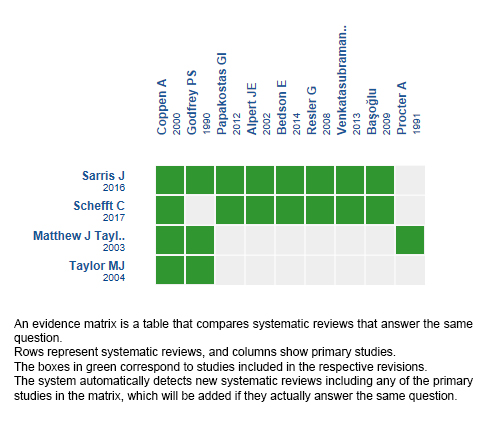
Follow the link to access the interactive version: Folate for depressive disorders
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
En una proporción importante de los pacientes con depresión, el tratamiento antidepresivo no lleva a una respuesta satisfactoria. Actualmente se postula que la coadministración de nutrientes estandarizados farmacológicamente (nutracéuticos), como el folato en este caso, podrían potenciar los efectos de los antidepresivos.
MÉTODOS
Para responder esta pregunta utilizamos Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante búsquedas en múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, reanalizamos los datos de los estudios primarios, realizamos un metanálisis y preparamos una tabla de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Identificamos cuatro revisiones sistemáticas que en conjunto incluyen nueve estudios primarios, de los cuales, ocho son ensayos aleatorizados. Concluimos que la potenciación con folato en el tratamiento del trastorno depresivo mayor probablemente resulta en poca o nula diferencia en los síntomas depresivos. Pudiese ser interesante evaluar el efecto de formas de presentación específicas del folato o en población con déficit objetivado.
 Authors:
Javier Trincado[1,2], Constanza Caneo[2,3]
Authors:
Javier Trincado[1,2], Constanza Caneo[2,3]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Psiquiatría, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: cmcaneo@uc.cl
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Centro de Innovación UC Anacleto Angelini Avda.Vicuña Mackenna 4860 Macul Santiago Chile

Citation: Trincado J, Caneo C. Is augmentation with folate effective for major depressive disorder?. Medwave 2018 Ene-Feb;18(1):e7155 doi: 10.5867/medwave.2018.01.7155
Submission date: 4/12/2017
Acceptance date: 21/12/2017
Publication date: 25/2/2018
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Taylor, M. J., Carney, S. M., Goodwin, G. M., & Geddes, J. R. (2004). Folate for Depressive Disorders: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Psychopharmacology, 18(2), 251–256. | CrossRef |
- Schefft, C., Kilarski, L. L., Bschor, T., & Köhler, S. (2017). Efficacy of adding nutritional supplements in unipolar depression: A systematic review and meta-analysis. European Neuropsychopharmacology, 27(11), 1090–1109. | CrossRef |
- Sarris, J., Murphy, J., Mischoulon, D., Papakostas, G. I., Fava, M., Berk, M., & Ng, C. H. (2016). Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. American Journal of Psychiatry, 173(6), 575–587. | CrossRef |
- Taylor, M. J., Carney, S. M., Geddes, J., & Goodwin, G. (2003, April 22). Folate for depressive disorders. (J. Geddes, Ed.), Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd. | CrossRef |
- Alpert JE, Mischoulon D, Rubenstein GE, Bottonari K, Nierenberg AA, Fava M. Folinic acid (Leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry. 2002 Mar;14(1):33-8. | PubMed |
- BaşoğIu, C., Ateş, M. A., AIgüI, A., İpçioğIu, O. M., Geçici, Ö., Yılmaz, O., Semiz, Ü. B., Ebrinç, S., Gülsün, M., Özcan, Ö., Kılıç, S., Çetin, M. Adjuvant Folate with Escitalopram Treatment and Homocystein, Folate, Vitamin B-12 Levels in Patients with Major Depressive Disorder. Klinik Psikofarmakoloji Bulteni. 2009 Jun;19(2):135-142. | Link |
- Bedson E, Bell D, Carr D, Carter B, Hughes D, Jorgensen A, Lewis H, Lloyd K, McCaddon A, Moat S, Pink J, Pirmohamed M, Roberts S, Russell I, Sylvestre Y, Tranter R, Whitaker R, Wilkinson C, Williams N. Folate Augmentation of Treatment--Evaluation for Depression (FolATED): randomised trial and economic evaluation. Health Technol Assess. 2014 Jul; 18(48):vii-viii, 1-159. | CrossRef | PubMed | PMC |
- Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. 2000 Nov;60(2):121-30. | PubMed |
- Godfrey PS, Toone BK, Carney MW, Flynn TG, Bottiglieri T, Laundy M, Chanarin I, Reynolds EH. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990 Aug 18;336(8712):392-5. | PubMed |
- Papakostas GI, Shelton RC, Zajecka JM, Etemad B, Rickels K, Clain A, Baer L, Dalton ED, Sacco GR, Schoenfeld D, Pencina M, Meisner A, Bottiglieri T, Nelson E, Mischoulon D, Alpert JE, Barbee JG, Zisook S, Fava M. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012 Dec;169(12):1267-74. | CrossRef | PubMed |
- Procter A. Enhancement of recovery from psychiatric illness by methylfolate. Br J Psychiatry. 1991 Aug; 159:271-2. | PubMed |
- Resler G, Lavie R, Campos J, Mata S, Urbina M, García A, Apitz R, Lima L. Effect of folic acid combined with fluoxetine in patients with major depression on plasma homocysteine and vitamin B12, and serotonin levels in lymphocytes. Neuroimmunomodulation. 2008;15(3):145-52. | CrossRef | PubMed |
- Venkatasubramanian R, Kumar CN, Pandey RS. A randomized double-blind comparison of fluoxetine augmentation by high and low dosage folic acid in patients with depressive episodes. J Affect Disord. 2013 Sep 5;150(2):644-8. | CrossRef | PubMed |
- Kaptchuk TJ. The placebo effect in alternative medicine: can the performance of a healing ritual have clinical significance? Ann Intern Med. 2002 Jun 4;136(11):817-25. Review. | PubMed |
- Hansen AH, Kristoffersen AE. The use of CAM providers and psychiatric outpatient services in people with anxiety/depression: a cross-sectional survey. BMC Complement Altern Med. 2016 Nov 11;16(1):461. | PubMed | PMC |
- Kemppainen LM, Kemppainen TT, Reippainen JA, Salmenniemi ST, Vuolanto PH. Use of complementary and alternative medicine in Europe: Health-related and sociodemographic determinants. Scand J Public Health. 2017 Oct 1:1403494817733869. | CrossRef | PubMed |
- American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. APA; 2010.
- Ravindran AV, Balneaves LG, Faulkner G, Ortiz A, McIntosh D, Morehouse RL, Ravindran L, Yatham LN, Kennedy SH, Lam RW, MacQueen GM, Milev RV, Parikh SV; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and Alternative Medicine Treatments. Can J Psychiatry. 2016 Sep;61(9):576-87. | CrossRef | PubMed | PMC |
- National Institute for Health and Care Excellence. Depression in adults: recognition and management. NICE Guideline (CG90). London: NICE; 2009.
- Sandra Yvonne Goumans, Maurits van Tulder, Sidney Rubenstein. Effect of folate on depressive disorders. PROSPERO 2013 CRD42013005727. | Link |
- Chavan BS. Use of bioavailable form of Folic acid for Treatment of Depression. CTRI/2017/03/008255. | Link |
- Gholampour N. Effect of Folic acid supplementation on the therapeutic regimen for major depressive disorder. IRCT2014082518922N1. | Link |
- Tatari F. The effects of folic acid in treatment of patients with depression. IRCT2013033112825N2. | Link |
- Palatnik A. Folic Acid and Omega -3 Fatty Acid Supplementation in Depressed Older Adults. NCT00480207. | Link |
- Dunn R. Phase 4 Study: An Open Study of the Efficacy and Tolerability in Unipolar Depression of Augmentation of the One-carbon Cycle With L-methionine, Betaine and Folate. NCT00226356. | Link |
 Taylor, M. J., Carney, S. M., Goodwin, G. M., & Geddes, J. R. (2004). Folate for Depressive Disorders: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Psychopharmacology, 18(2), 251–256. | CrossRef |
Taylor, M. J., Carney, S. M., Goodwin, G. M., & Geddes, J. R. (2004). Folate for Depressive Disorders: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Psychopharmacology, 18(2), 251–256. | CrossRef | Schefft, C., Kilarski, L. L., Bschor, T., & Köhler, S. (2017). Efficacy of adding nutritional supplements in unipolar depression: A systematic review and meta-analysis. European Neuropsychopharmacology, 27(11), 1090–1109. | CrossRef |
Schefft, C., Kilarski, L. L., Bschor, T., & Köhler, S. (2017). Efficacy of adding nutritional supplements in unipolar depression: A systematic review and meta-analysis. European Neuropsychopharmacology, 27(11), 1090–1109. | CrossRef | Sarris, J., Murphy, J., Mischoulon, D., Papakostas, G. I., Fava, M., Berk, M., & Ng, C. H. (2016). Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. American Journal of Psychiatry, 173(6), 575–587. | CrossRef |
Sarris, J., Murphy, J., Mischoulon, D., Papakostas, G. I., Fava, M., Berk, M., & Ng, C. H. (2016). Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. American Journal of Psychiatry, 173(6), 575–587. | CrossRef | Taylor, M. J., Carney, S. M., Geddes, J., & Goodwin, G. (2003, April 22). Folate for depressive disorders. (J. Geddes, Ed.), Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd. | CrossRef |
Taylor, M. J., Carney, S. M., Geddes, J., & Goodwin, G. (2003, April 22). Folate for depressive disorders. (J. Geddes, Ed.), Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd. | CrossRef | Alpert JE, Mischoulon D, Rubenstein GE, Bottonari K, Nierenberg AA, Fava M. Folinic acid (Leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry. 2002 Mar;14(1):33-8. | PubMed |
Alpert JE, Mischoulon D, Rubenstein GE, Bottonari K, Nierenberg AA, Fava M. Folinic acid (Leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry. 2002 Mar;14(1):33-8. | PubMed | BaşoğIu, C., Ateş, M. A., AIgüI, A., İpçioğIu, O. M., Geçici, Ö., Yılmaz, O., Semiz, Ü. B., Ebrinç, S., Gülsün, M., Özcan, Ö., Kılıç, S., Çetin, M. Adjuvant Folate with Escitalopram Treatment and Homocystein, Folate, Vitamin B-12 Levels in Patients with Major Depressive Disorder. Klinik Psikofarmakoloji Bulteni. 2009 Jun;19(2):135-142. | Link |
BaşoğIu, C., Ateş, M. A., AIgüI, A., İpçioğIu, O. M., Geçici, Ö., Yılmaz, O., Semiz, Ü. B., Ebrinç, S., Gülsün, M., Özcan, Ö., Kılıç, S., Çetin, M. Adjuvant Folate with Escitalopram Treatment and Homocystein, Folate, Vitamin B-12 Levels in Patients with Major Depressive Disorder. Klinik Psikofarmakoloji Bulteni. 2009 Jun;19(2):135-142. | Link | Bedson E, Bell D, Carr D, Carter B, Hughes D, Jorgensen A, Lewis H, Lloyd K, McCaddon A, Moat S, Pink J, Pirmohamed M, Roberts S, Russell I, Sylvestre Y, Tranter R, Whitaker R, Wilkinson C, Williams N. Folate Augmentation of Treatment--Evaluation for Depression (FolATED): randomised trial and economic evaluation. Health Technol Assess. 2014 Jul; 18(48):vii-viii, 1-159. | CrossRef | PubMed | PMC |
Bedson E, Bell D, Carr D, Carter B, Hughes D, Jorgensen A, Lewis H, Lloyd K, McCaddon A, Moat S, Pink J, Pirmohamed M, Roberts S, Russell I, Sylvestre Y, Tranter R, Whitaker R, Wilkinson C, Williams N. Folate Augmentation of Treatment--Evaluation for Depression (FolATED): randomised trial and economic evaluation. Health Technol Assess. 2014 Jul; 18(48):vii-viii, 1-159. | CrossRef | PubMed | PMC | Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. 2000 Nov;60(2):121-30. | PubMed |
Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. 2000 Nov;60(2):121-30. | PubMed | Godfrey PS, Toone BK, Carney MW, Flynn TG, Bottiglieri T, Laundy M, Chanarin I, Reynolds EH. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990 Aug 18;336(8712):392-5. | PubMed |
Godfrey PS, Toone BK, Carney MW, Flynn TG, Bottiglieri T, Laundy M, Chanarin I, Reynolds EH. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990 Aug 18;336(8712):392-5. | PubMed | Papakostas GI, Shelton RC, Zajecka JM, Etemad B, Rickels K, Clain A, Baer L, Dalton ED, Sacco GR, Schoenfeld D, Pencina M, Meisner A, Bottiglieri T, Nelson E, Mischoulon D, Alpert JE, Barbee JG, Zisook S, Fava M. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012 Dec;169(12):1267-74. | CrossRef | PubMed |
Papakostas GI, Shelton RC, Zajecka JM, Etemad B, Rickels K, Clain A, Baer L, Dalton ED, Sacco GR, Schoenfeld D, Pencina M, Meisner A, Bottiglieri T, Nelson E, Mischoulon D, Alpert JE, Barbee JG, Zisook S, Fava M. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012 Dec;169(12):1267-74. | CrossRef | PubMed | Procter A. Enhancement of recovery from psychiatric illness by methylfolate. Br J Psychiatry. 1991 Aug; 159:271-2. | PubMed |
Procter A. Enhancement of recovery from psychiatric illness by methylfolate. Br J Psychiatry. 1991 Aug; 159:271-2. | PubMed | Resler G, Lavie R, Campos J, Mata S, Urbina M, García A, Apitz R, Lima L. Effect of folic acid combined with fluoxetine in patients with major depression on plasma homocysteine and vitamin B12, and serotonin levels in lymphocytes. Neuroimmunomodulation. 2008;15(3):145-52. | CrossRef | PubMed |
Resler G, Lavie R, Campos J, Mata S, Urbina M, García A, Apitz R, Lima L. Effect of folic acid combined with fluoxetine in patients with major depression on plasma homocysteine and vitamin B12, and serotonin levels in lymphocytes. Neuroimmunomodulation. 2008;15(3):145-52. | CrossRef | PubMed | Venkatasubramanian R, Kumar CN, Pandey RS. A randomized double-blind comparison of fluoxetine augmentation by high and low dosage folic acid in patients with depressive episodes. J Affect Disord. 2013 Sep 5;150(2):644-8. | CrossRef | PubMed |
Venkatasubramanian R, Kumar CN, Pandey RS. A randomized double-blind comparison of fluoxetine augmentation by high and low dosage folic acid in patients with depressive episodes. J Affect Disord. 2013 Sep 5;150(2):644-8. | CrossRef | PubMed | Kaptchuk TJ. The placebo effect in alternative medicine: can the performance of a healing ritual have clinical significance? Ann Intern Med. 2002 Jun 4;136(11):817-25. Review. | PubMed |
Kaptchuk TJ. The placebo effect in alternative medicine: can the performance of a healing ritual have clinical significance? Ann Intern Med. 2002 Jun 4;136(11):817-25. Review. | PubMed | Hansen AH, Kristoffersen AE. The use of CAM providers and psychiatric outpatient services in people with anxiety/depression: a cross-sectional survey. BMC Complement Altern Med. 2016 Nov 11;16(1):461. | PubMed | PMC |
Hansen AH, Kristoffersen AE. The use of CAM providers and psychiatric outpatient services in people with anxiety/depression: a cross-sectional survey. BMC Complement Altern Med. 2016 Nov 11;16(1):461. | PubMed | PMC | Kemppainen LM, Kemppainen TT, Reippainen JA, Salmenniemi ST, Vuolanto PH. Use of complementary and alternative medicine in Europe: Health-related and sociodemographic determinants. Scand J Public Health. 2017 Oct 1:1403494817733869. | CrossRef | PubMed |
Kemppainen LM, Kemppainen TT, Reippainen JA, Salmenniemi ST, Vuolanto PH. Use of complementary and alternative medicine in Europe: Health-related and sociodemographic determinants. Scand J Public Health. 2017 Oct 1:1403494817733869. | CrossRef | PubMed | American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. APA; 2010.
American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. APA; 2010.  Ravindran AV, Balneaves LG, Faulkner G, Ortiz A, McIntosh D, Morehouse RL, Ravindran L, Yatham LN, Kennedy SH, Lam RW, MacQueen GM, Milev RV, Parikh SV; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and Alternative Medicine Treatments. Can J Psychiatry. 2016 Sep;61(9):576-87. | CrossRef | PubMed | PMC |
Ravindran AV, Balneaves LG, Faulkner G, Ortiz A, McIntosh D, Morehouse RL, Ravindran L, Yatham LN, Kennedy SH, Lam RW, MacQueen GM, Milev RV, Parikh SV; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and Alternative Medicine Treatments. Can J Psychiatry. 2016 Sep;61(9):576-87. | CrossRef | PubMed | PMC | National Institute for Health and Care Excellence. Depression in adults: recognition and management. NICE Guideline (CG90). London: NICE; 2009.
National Institute for Health and Care Excellence. Depression in adults: recognition and management. NICE Guideline (CG90). London: NICE; 2009.  Sandra Yvonne Goumans, Maurits van Tulder, Sidney Rubenstein. Effect of folate on depressive disorders. PROSPERO 2013 CRD42013005727. | Link |
Sandra Yvonne Goumans, Maurits van Tulder, Sidney Rubenstein. Effect of folate on depressive disorders. PROSPERO 2013 CRD42013005727. | Link | Chavan BS. Use of bioavailable form of Folic acid for Treatment of Depression. CTRI/2017/03/008255. | Link |
Chavan BS. Use of bioavailable form of Folic acid for Treatment of Depression. CTRI/2017/03/008255. | Link | Gholampour N. Effect of Folic acid supplementation on the therapeutic regimen for major depressive disorder. IRCT2014082518922N1. | Link |
Gholampour N. Effect of Folic acid supplementation on the therapeutic regimen for major depressive disorder. IRCT2014082518922N1. | Link | Tatari F. The effects of folic acid in treatment of patients with depression. IRCT2013033112825N2. | Link |
Tatari F. The effects of folic acid in treatment of patients with depression. IRCT2013033112825N2. | Link | Palatnik A. Folic Acid and Omega -3 Fatty Acid Supplementation in Depressed Older Adults. NCT00480207. | Link |
Palatnik A. Folic Acid and Omega -3 Fatty Acid Supplementation in Depressed Older Adults. NCT00480207. | Link | Dunn R. Phase 4 Study: An Open Study of the Efficacy and Tolerability in Unipolar Depression of Augmentation of the One-carbon Cycle With L-methionine, Betaine and Folate. NCT00226356. | Link |
Dunn R. Phase 4 Study: An Open Study of the Efficacy and Tolerability in Unipolar Depression of Augmentation of the One-carbon Cycle With L-methionine, Betaine and Folate. NCT00226356. | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








