Abstract
INTRODUCTION
It has been suggested that cannabinoids would constitute a therapeutic alternative for patients with insomnia.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified eight systematic reviews including three studies overall, of which two were randomized trials. We concluded it is not clear whether cannabinoids have an effect on insomnia severity or on sleep quality; that they might have no effect on sleep conciliation, sleep awakening or behavior during wakefulness, and are probably associated with frequent adverse effects.
Problem
Insomnia is the most frequent sleep disorder in general population and represents a common reason for consultation. This disorder has a great impact on the waking state, the work capacity and the quality of life of people who suffer from it.
It has been proposed cannabinoids would have an effect on the stimulation of sleep and thus could be an effective treatment for patients with insomnia and other sleep disorders. However, its clinical efficacy and safety are a matter of debate.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
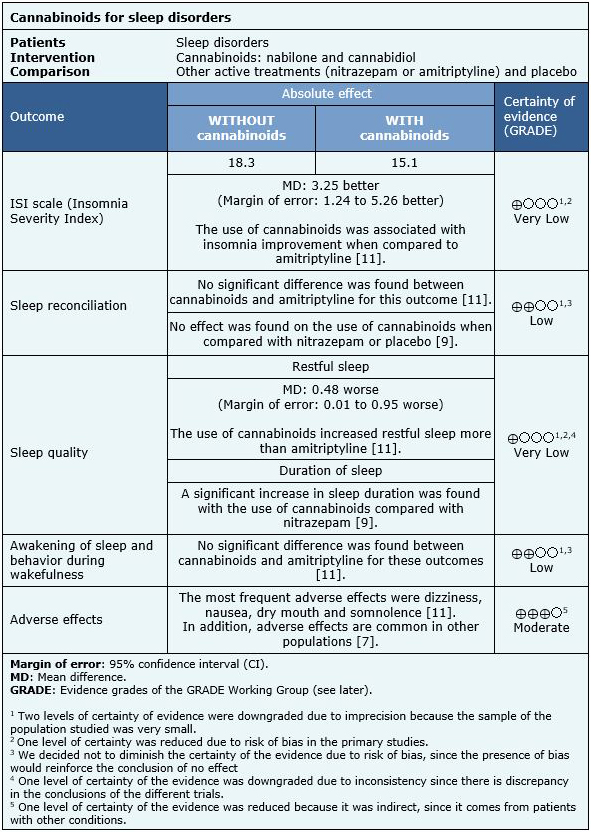
We found eight systematic reviews [1],[2],[3],[4],[5],[6],[7],[8] that included three primary studies reported in six references [9],[10],[11],[12],[13],[14], of which two correspond to randomized trials, reported in five references [9],[11],[12],[13],[14]. This table and the summary in general are based on the randomized trials, since the observational study did not increase the certainty of the existing evidence, nor did provide relevant additional information. |
|
What types of patients were included* |
The first trial [9] included 15 patients with insomnia (diagnostic criterion was not specified). The second trial [11] included 32 patients diagnosed with chronic insomnia, defined as a sleep disturbance according to self-report at least every other night alternately for a minimum of 6 months. Those who fulfilled these criteria were selected within a population of patients with fibromyalgia. In this last trial, a negative urine test for cannabinoids was also required. The average age was 49.5 years and 16% were men. |
|
What types of interventions were included* |
The first trial [9] compared cannabidiol at doses of 40, 80 and 160 mg versus nitrazepam 5 mg and versus placebo. The second trial [11] compared the effect of nabilone between 0.5 to 1 mg versus amitriptyline between 10 to 20 mg. All these drugs were administered orally once a day, before going to sleep. |
|
What types of outcomes |
The first trial [9] measured the duration of sleep and the induction of sleep. The second trial [11] measured the results according to the ISI (Insomnia Severity Index) and LSEQ (Leeds Sleep Evaluation Questionnaire) scales. LSEQ evaluates the reconciliation of sleep, quality of sleep, awakening from sleep and behavior during wakefulness. In addition, the occurrence of adverse effects associated with the use of cannabinoids was evaluated. The follow-up of the first trial [11] was 6 weeks (2 weeks each period separated by 2 weeks), the second trial [9] did not specify the duration of follow-up, only that it was "acute". |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
The information on the effects of cannabinoids on insomnia is based on two randomized trials [9],[11] that included 47 patients.
The first trial [9] measured the duration of sleep and the induction of sleep, the second trial [11] measured the results according to the ISI (Insomnia Severity Index) and LSEQ (Leeds Sleep Evaluation Questionnaire) scales. LSEQ evaluates the reconciliation of sleep, quality of sleep, awakening from sleep and behavior during wakefulness.
The summary of findings is as follows:
- It is not clear whether cannabinoids have an effect on insomnia severity because the certainty of the evidence is very low.
- Cannabinoids might have no effect on sleep conciliation, but the certainty of the evidence is low.
- It is not clear whether cannabinoids have an effect on sleep quality because the certainty of the evidence is very low.
- Cannabinoids might have no effect on waking sleep and waking behavior, but the certainty of the evidence is low.
- Cannabinoids are probably associated with frequent adverse effects in patients with insomnia. The certainty of the evidence is moderate.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
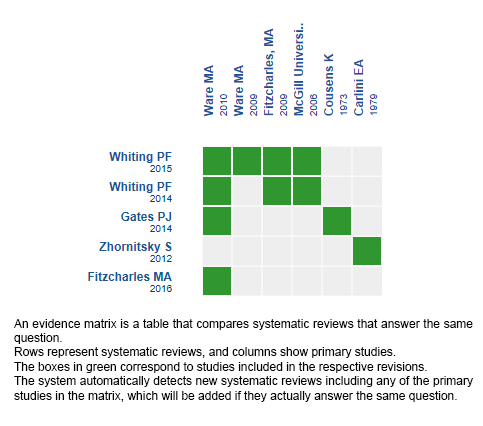
Follow the link to access the interactive version: Cannabinoids for insomnia
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
Se ha planteado que la estimulación del sueño con cannabinoides podría constituir una alternativa terapéutica en pacientes con insomnio.
MÉTODOS
Para responder esta pregunta utilizamos Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante búsquedas en múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, reanalizamos los datos de los estudios primarios y preparamos una tabla de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Identificamos ocho revisiones sistemáticas que en conjunto incluyen tres estudios primarios, de los cuales dos corresponden a ensayos aleatorizados. Concluimos que no está claro si los cannabinoides tienen un efecto en la severidad del insomnio o en la calidad del sueño; que podrían no tener efecto en la conciliación del sueño, despertar del sueño ni comportamiento durante vigilia, y probablemente se asocian a efectos adversos frecuentes.
 Authors:
Tania Contreras[1,2], Gonzalo Bravo-Soto[2,4], Gabriel Rada[2,3,4,5,6]
Authors:
Tania Contreras[1,2], Gonzalo Bravo-Soto[2,4], Gabriel Rada[2,3,4,5,6]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Medicina Interna, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[4] Centro Evidencia UC, Escuela de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[5] The Cochrane Collaboration
[6] GRADE working group
E-mail: radagabriel@epistemonikos.org
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Centro de Innovación UC Anacleto Angelini Avda.Vicuña Mackenna 4860 Macul Santiago Chile

Citation: Contreras T, Bravo-Soto GA, Rada G. Do cannabinoids constitute a therapeutic alternative for insomnia?. Medwave 2018 Ene-Feb;18(1):e7151 doi: 10.5867/medwave.2018.01.7151
Submission date: 29/12/2017
Acceptance date: 31/12/2017
Publication date: 13/2/2018
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- de Souza Nascimento S, Desantana JM, Nampo FK, Ribeiro EA, da Silva DL, Araújo-Júnior JX, da Silva Almeida JR, Bonjardim LR, de Souza Araújo AA, Quintans-Júnior LJ. Efficacy and safety of medicinal plants or related natural products for fibromyalgia: a systematic review. Evid Based Complement Alternat Med. 2013;2013:149468. | CrossRef | PubMed | PMC |
- Fitzcharles MA, Ste-Marie PA, Häuser W, Clauw DJ, Jamal S, Karsh J, Landry T, Leclercq S, Mcdougall JJ, Shir Y, Shojania K, Walsh Z. Efficacy, Tolerability, and Safety of Cannabinoid Treatments in the Rheumatic Diseases: A Systematic Review of Randomized Controlled Trials. Arthritis Care Res (Hoboken). 2016 May;68(5):681-8. | CrossRef | PubMed |
- Gates PJ, Albertella L, Copeland J. The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep Med Rev. 2014 Dec;18(6):477-87. | CrossRef | PubMed |
- Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016 Jul-Aug;12(4):638-54. | CrossRef | PubMed |
- Walitt B, Klose P, Fitzcharles MA, Phillips T, Häuser W. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016 Jul 18;7:CD011694. | CrossRef | PubMed |
- Whiting PF, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes C, Lang S, Harker J, Despande S, Ryder S, Di Nisio M, Hernández AV, Schmidlkofer S, Kleijnen J. Systematic review of cannabis for medical use. Kleijnen Systematic Reviews Ltd. 2014.
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. Review. Erratum in: JAMA. 2016 Apr 12;315(14):1522. JAMA. 2015 Dec 1;314(21):2308. JAMA. 2015 Aug 4;314(5):520. JAMA. 2015 Aug 25;314(8):837. | CrossRef | PubMed |
- Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 2012 May 21;5(5):529-52. | CrossRef | PubMed | PMC |
- Carlini EA, Masur J, Magalhaes CCPB. Possible hypnotic effect of cannabidiol on human. preliminary study. Ciência e Cultura. 1979;31:315-322.
- Cousens K, DiMascio A. (-) Delta 9 THC as an hypnotic. An experimental study of three dose levels. Psychopharmacologia. 1973 Dec 20;33(4):355-64. | PubMed |
- Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg. 2010 Feb 1;110(2):604-10. | CrossRef | PubMed |
- Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Canadian Rheumatology Association Meeting. 2009.
- Fitzcharles, MA, Shir, Y, Joseph, L, Ware, MA. The effects of nabilone on insomnia in fibromyalgia: results of a randomized controlled trial. American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting. 2009.
- McGill University Health Center. Nabilone Versus Amitriptyline in Improving Quality of Sleep in Patients With Fibromyalgia. clinicaltrials.gov. 2006.
- National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press. 2017. | CrossRef |
- University Health Network Toronto, Valeant Pharmaceuticals International Inc. Use of the cannabinoid nabilone for the promotion of sleep in chronic, non-malignant pain patients. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2006- [cited 2014 Apr 7]. | Link |
 de Souza Nascimento S, Desantana JM, Nampo FK, Ribeiro EA, da Silva DL, Araújo-Júnior JX, da Silva Almeida JR, Bonjardim LR, de Souza Araújo AA, Quintans-Júnior LJ. Efficacy and safety of medicinal plants or related natural products for fibromyalgia: a systematic review. Evid Based Complement Alternat Med. 2013;2013:149468. | CrossRef | PubMed | PMC |
de Souza Nascimento S, Desantana JM, Nampo FK, Ribeiro EA, da Silva DL, Araújo-Júnior JX, da Silva Almeida JR, Bonjardim LR, de Souza Araújo AA, Quintans-Júnior LJ. Efficacy and safety of medicinal plants or related natural products for fibromyalgia: a systematic review. Evid Based Complement Alternat Med. 2013;2013:149468. | CrossRef | PubMed | PMC | Fitzcharles MA, Ste-Marie PA, Häuser W, Clauw DJ, Jamal S, Karsh J, Landry T, Leclercq S, Mcdougall JJ, Shir Y, Shojania K, Walsh Z. Efficacy, Tolerability, and Safety of Cannabinoid Treatments in the Rheumatic Diseases: A Systematic Review of Randomized Controlled Trials. Arthritis Care Res (Hoboken). 2016 May;68(5):681-8. | CrossRef | PubMed |
Fitzcharles MA, Ste-Marie PA, Häuser W, Clauw DJ, Jamal S, Karsh J, Landry T, Leclercq S, Mcdougall JJ, Shir Y, Shojania K, Walsh Z. Efficacy, Tolerability, and Safety of Cannabinoid Treatments in the Rheumatic Diseases: A Systematic Review of Randomized Controlled Trials. Arthritis Care Res (Hoboken). 2016 May;68(5):681-8. | CrossRef | PubMed | Gates PJ, Albertella L, Copeland J. The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep Med Rev. 2014 Dec;18(6):477-87. | CrossRef | PubMed |
Gates PJ, Albertella L, Copeland J. The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep Med Rev. 2014 Dec;18(6):477-87. | CrossRef | PubMed | Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016 Jul-Aug;12(4):638-54. | CrossRef | PubMed |
Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016 Jul-Aug;12(4):638-54. | CrossRef | PubMed | Walitt B, Klose P, Fitzcharles MA, Phillips T, Häuser W. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016 Jul 18;7:CD011694. | CrossRef | PubMed |
Walitt B, Klose P, Fitzcharles MA, Phillips T, Häuser W. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016 Jul 18;7:CD011694. | CrossRef | PubMed | Whiting PF, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes C, Lang S, Harker J, Despande S, Ryder S, Di Nisio M, Hernández AV, Schmidlkofer S, Kleijnen J. Systematic review of cannabis for medical use. Kleijnen Systematic Reviews Ltd. 2014.
Whiting PF, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes C, Lang S, Harker J, Despande S, Ryder S, Di Nisio M, Hernández AV, Schmidlkofer S, Kleijnen J. Systematic review of cannabis for medical use. Kleijnen Systematic Reviews Ltd. 2014.  Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. Review. Erratum in: JAMA. 2016 Apr 12;315(14):1522. JAMA. 2015 Dec 1;314(21):2308. JAMA. 2015 Aug 4;314(5):520. JAMA. 2015 Aug 25;314(8):837. | CrossRef | PubMed |
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. Review. Erratum in: JAMA. 2016 Apr 12;315(14):1522. JAMA. 2015 Dec 1;314(21):2308. JAMA. 2015 Aug 4;314(5):520. JAMA. 2015 Aug 25;314(8):837. | CrossRef | PubMed | Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 2012 May 21;5(5):529-52. | CrossRef | PubMed | PMC |
Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 2012 May 21;5(5):529-52. | CrossRef | PubMed | PMC | Carlini EA, Masur J, Magalhaes CCPB. Possible hypnotic effect of cannabidiol on human. preliminary study. Ciência e Cultura. 1979;31:315-322.
Carlini EA, Masur J, Magalhaes CCPB. Possible hypnotic effect of cannabidiol on human. preliminary study. Ciência e Cultura. 1979;31:315-322.  Cousens K, DiMascio A. (-) Delta 9 THC as an hypnotic. An experimental study of three dose levels. Psychopharmacologia. 1973 Dec 20;33(4):355-64. | PubMed |
Cousens K, DiMascio A. (-) Delta 9 THC as an hypnotic. An experimental study of three dose levels. Psychopharmacologia. 1973 Dec 20;33(4):355-64. | PubMed | Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg. 2010 Feb 1;110(2):604-10. | CrossRef | PubMed |
Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg. 2010 Feb 1;110(2):604-10. | CrossRef | PubMed | Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Canadian Rheumatology Association Meeting. 2009.
Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Canadian Rheumatology Association Meeting. 2009.  Fitzcharles, MA, Shir, Y, Joseph, L, Ware, MA. The effects of nabilone on insomnia in fibromyalgia: results of a randomized controlled trial. American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting. 2009.
Fitzcharles, MA, Shir, Y, Joseph, L, Ware, MA. The effects of nabilone on insomnia in fibromyalgia: results of a randomized controlled trial. American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting. 2009.  McGill University Health Center. Nabilone Versus Amitriptyline in Improving Quality of Sleep in Patients With Fibromyalgia. clinicaltrials.gov. 2006.
McGill University Health Center. Nabilone Versus Amitriptyline in Improving Quality of Sleep in Patients With Fibromyalgia. clinicaltrials.gov. 2006.  National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press. 2017. | CrossRef |
National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press. 2017. | CrossRef | University Health Network Toronto, Valeant Pharmaceuticals International Inc. Use of the cannabinoid nabilone for the promotion of sleep in chronic, non-malignant pain patients. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2006- [cited 2014 Apr 7]. | Link |
University Health Network Toronto, Valeant Pharmaceuticals International Inc. Use of the cannabinoid nabilone for the promotion of sleep in chronic, non-malignant pain patients. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2006- [cited 2014 Apr 7]. | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








