Abstract
INTRODUCTION
Increasing rates of HIV infection remain of concern, especially for high-risk groups such as men who have sex with men. Oral pre-exposure prophylaxis has emerged as an alternative to prevention. However, doubts persist in patients and physicians about its effectiveness.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified six systematic reviews including twelve studies overall, of which six were randomized trials. We concluded the use of oral pre-exposure prophylaxis reduces the probability of HIV infection in men who have sex with men, has few or no adverse effects, and is a measure with a good balance between benefits, risks and costs.
Problem
Despite increased community awareness, HIV infection rates continue to rise. One of the high-risk group is men who have sex with men, so better prevention strategies are required. The use of antiretroviral drugs in uninfected individuals, or pre-exposure prophylaxis (PrEP), has emerged as a promising tool for prevention in individuals at high risk of HIV infection. The most commonly used combination of PrEP has been oral emtricitabine with tenofovir disoproxil fumarate, and to a lesser extent tenofovir disoproxil fumarate alone. Despite the approval of the first as PrEP by the FDA in 2012, physicians and patients still question the effectiveness and safety of this measure.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1],[2],[3],[4],[5],[6] |
|
What types of patients were included* |
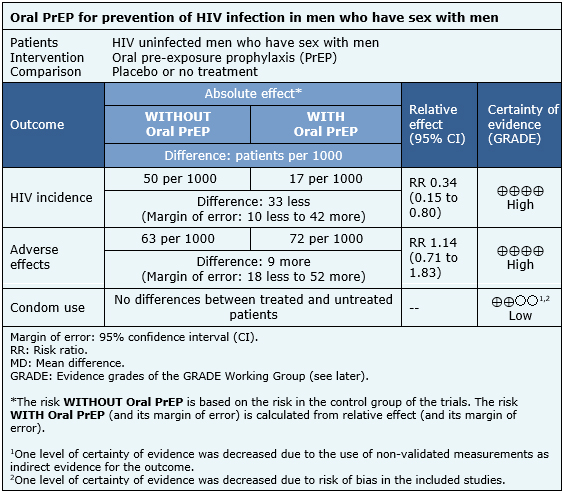
The patients included in the trials were HIV uninfected men who have sex with men, over 18 years of age, and considered at high risk of HIV infection (due to a history of high number of sexual partners, sexual intercourse without condom use, sex with people with sexually transmitted diseases, or sex in exchange for money). |
|
What types of interventions were included* |
All trials evaluated the use of oral PrEP in conjunction with standard prevention (including education and provision of condoms). Five evaluated the use of emtricitabine/tenofovir [11],[14],[20],[23],[29] and one the use of tenofovir alone [25]. Five trials evaluated against placebo [11],[14],[23],[25],[29] and three against a non-treatment group [14],[20],[25]. Five used PrEP on a daily basis [11],[14],[20],[25],[29] and one intermittently, before and after having sex [23]. One trial used behavioral therapy as a co-intervention [14] and one trial was pragmatic and open-label [20]. One trial included a small group of women [29], but since the vast majority of the participants were men who had sex with men, it was included in the analysis. |
|
What types of outcomes |
The main outcomes analyzed were the rate of HIV infection and adverse effects. Qualitatively analyzed outcomes included changes in risk behaviors, mainly condom use objectified by interviews [14],[25] or indirectly by incidence of other sexually transmitted diseases [20]. The average follow-up of the trials was 15 months, with a range between 4 and 33 months. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
The information on the effects of oral PrEP in men who have sex with men is based on six trials [11],[14],[20],[23],[25],[29] which included 3974 patients.
It was not possible to extract enough information from the reviews identified to reconstruct the meta-analysis of HIV incidence. Therefore, the information presented is based on the results of the meta-analysis of a systematic review [1] that is based on four trials [11],[23],[25],[29] that included 3371 patients.
The information on adverse events is based on three trials [11],[14],[25] whose data were reusable from systematic reviews and included 2957 patients. The information on changes in condom use is based on three trials [14],[20],[25] that compared against no treatment, whose data were described qualitatively in the systematic reviews and included 1003 patients.
The summary of findings is as follows:
- Pre-exposure prophylaxis reduces HIV infection in men who have sex with men. The certainty of the evidence is high.
- Pre-exposure prophylaxis reduces leads to minimal or no increase in adverse effects. The certainty of the evidence is high.
- Pre-exposure prophylaxis might result in little or no difference in terms of condom use in men who have sex with men. The certainty of the evidence is low.
| Follow the link to access the interactive version of this table (Interactive Summary of Findings - iSoF) |

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
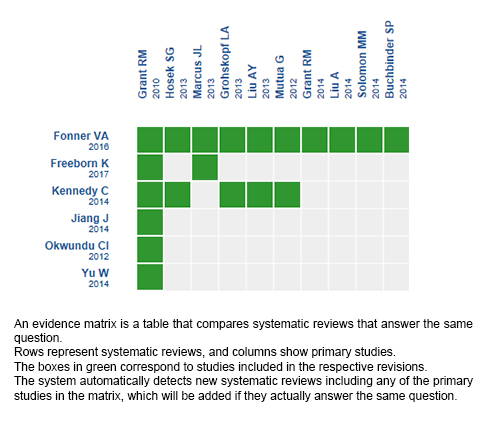
Follow the link to access the interactive version: Oral PrEP for prevention of HIV infection in men who have sex with men
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
Las altas tasas de infección por VIH siguen siendo de preocupación, especialmente para grupos de riesgo como hombres que tienen sexo con hombres. La profilaxis pre-exposición oral se ha perfilado como una alternativa de prevención. Sin embargo, aún persisten dudas en pacientes y tratantes sobre su efectividad.
MÉTODOS
Para responder a esta pregunta utilizamos Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante búsquedas en múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, reanalizamos los datos de los estudios primarios, realizamos un metanálisis y preparamos tablas de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Identificamos seis revisiones sistemáticas que en conjunto incluyen doce estudios primarios, de los cuales seis son ensayos aleatorizados. Concluimos que el uso de profilaxis pre-exposición oral disminuye la probabilidad de infección por VIH en hombres que tienen sexo con hombres, tiene pocos o nulos efectos adversos, y se trataría de una medida con un buen balance entre beneficios, riesgos y costos.
 Authors:
Rubén Allende[1,2], María Paz Acuña[2,3]
Authors:
Rubén Allende[1,2], María Paz Acuña[2,3]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Infectología, Hospital Dr. Sótero del Río, Santiago, Chile
E-mail: doctorapazacuna@gmail.com
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Centro de Innovación UC Anacleto Angelini Avda.Vicuña Mackenna 4860 Macul Santiago Chile

Citation: Allende R, Acuña M. Is pre-exposure prophylaxis effective for preventing HIV infection in men who have sex with men?. Medwave 2017 Nov-Dic;17(9):e7117 doi: 10.5867/medwave.2017.09.7117
Submission date: 18/12/2017
Acceptance date: 27/12/2017
Publication date: 27/12/2017
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016 Jul;30(12):1973–83.
- Freeborn K, Portillo CJ. Does Pre-exposure prophylaxis (PrEP) for HIV prevention in men who have sex with men (MSM) change risk behavior? A systematic review. J Clin Nurs. 2017 Aug.
- Jiang J, Yang X, Ye L, Zhou B, Ning C, Huang J, et al. Pre-exposure prophylaxis for the prevention of HIV infection in high risk populations: a meta-analysis of randomized controlled trials. PLoS One. 2014;9(2):e87674.
- Kennedy CE, Fonner VA, World Health Organization. Pre-exposure prophylaxis for men who have sex with men: a systematic review. 2014.
- Okwundu CI, Uthman OA, Okoromah CA. Antiretroviral pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals. Cochrane database Syst Rev. 2012 Jul;(7):CD007189.
- Yu W, Wang L, Han N, Zhang X, Mahapatra T, Mahapatra S, et al. Pre-exposure prophylaxis of HIV: A right way to go or a long way to go? Artif cells, nanomedicine, Biotechnol. 2016;44(1):201–8.
- Amico KR, Marcus JL, McMahan V, Liu A, Koester KA, Goicochea P, et al. Study product adherence measurement in the iPrEx placebo-controlled trial: concordance with drug detection. J Acquir Immune Defic Syndr. 2014 Aug;66(5):530–7.
- Buchbinder SP, Glidden D V, Liu AY, McMahan V, Guanira J V, Mayer KH, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis. 2014 Jun;14(6):468–75.
- Carlo Hojilla J, Koester KA, Cohen SE, Buchbinder S, Ladzekpo D, Matheson T, et al. Sexual Behavior, Risk Compensation, and HIV Prevention Strategies Among Participants in the San Francisco PrEP Demonstration Project: A Qualitative Analysis of Counseling Notes. AIDS Behav. 2016 Jul;20(7):1461–9.
- Golub SA, Kowalczyk W, Weinberger CL, Parsons JT. Preexposure prophylaxis and predicted condom use among high-risk men who have sex with men. J Acquir Immune Defic Syndr. 2010 Aug;54(5):548–55.
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec;363(27):2587–99.
- Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014 Sep;14(9):820–9.
- Hoff CC, Chakravarty D, Bircher AE, Campbell CK, Grisham K, Neilands TB, et al. Attitudes Towards PrEP and Anticipated Condom Use Among Concordant HIV-Negative and HIV-Discordant Male Couples. AIDS Patient Care STDS. 2015 Jul;29(7):408–17.
- Hosek SG, Siberry G, Bell M, Lally M, Kapogiannis B, Green K, et al. The acceptability and feasibility of an HIV preexposure prophylaxis (PrEP) trial with young men who have sex with men. J Acquir Immune Defic Syndr. 2013 Apr;62(4):447–56.
- Liegler T, Abdel-Mohsen M, Bentley LG, Atchison R, Schmidt T, Javier J, et al. HIV-1 drug resistance in the iPrEx preexposure prophylaxis trial. J Infect Dis. 2014 Oct;210(8):1217–27.
- Liu A, Glidden D V, Anderson PL, Amico KR, McMahan V, Mehrotra M, et al. Patterns and correlates of PrEP drug detection among MSM and transgender women in the Global iPrEx Study. J Acquir Immune Defic Syndr. 2014 Dec;67(5):528–37.
- Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med. 2016 Jan;176(1):75–84.
- Marcus JL, Glidden D V, Mayer KH, Liu AY, Buchbinder SP, Amico KR, et al. No evidence of sexual risk compensation in the iPrEx trial of daily oral HIV preexposure prophylaxis. PLoS One. 2013;8(12):e81997.
- McCormack S, Dunn DT. Pragmatic open-label randomised trial of preexposure prophylaxis: The PROUD Study. In: CROI. Seattle, Washington; 2015.
- McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet (London, England). 2016 Jan;387(10013):53–60.
- Solomon MM, Lama JR, Glidden D V, Mulligan K, McMahan V, Liu AY, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS. 2014 Mar;28(6):851–9.
- Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, et al. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin Infect Dis. 2015 Nov;61(10):1601–3.
- Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med. 2015 Dec;373(23):2237–46.
- Molina J-M, Capitant C, Charreau I. On demand PrEP with oral TDF-FTC in MSM: Results of the ANRS Ipergay Trial. In: CROI. Seattle, Washington; 2015.
- Grohskopf LA, Chillag KL, Gvetadze R, Liu AY, Thompson M, Mayer KH, et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013 Sep;64(1):79–86.
- Liu AY, Vittinghoff E, Gandhi M, Huang Y, Chillag K, Wiegand R. Validating measures of tenofovir drug exposure in a U.S. pre-exposure prophylaxis trial. In: CROI. Seattle, Washington; 2015.
- Liu AY, Vittinghoff E, Chillag K, Mayer K, Thompson M, Grohskopf L, et al. Sexual risk behavior among HIV-uninfected men who have sex with men participating in a tenofovir preexposure prophylaxis randomized trial in the United States. J Acquir Immune Defic Syndr. 2013 Sep;64(1):87–94.
- Liu AY, Vittinghoff E, Sellmeyer DE, Irvin R, Mulligan K, Mayer K, et al. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One. 2011;6(8):e23688.
- Mutua G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One. 2012;7(4):e33103.
- Food and Drug Administration. First Generic Drug Approvals [Internet]. 2017 [cited 2017 Dec 10]. | Link |
- Centers for Disease Control and Prevention. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States [Internet]. 2014. | Link |
- World Health Organization. WHO implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection [Internet]. 2017. | Link |
- U.S. Preventive Services Task Force. Draft Update Summary: Prevention of Human Immunodeficiency Virus (HIV) Infection: Pre-Exposure Prophylaxis [Internet]. 2017 [cited 2017 Jan 12]. | Link |
- Gilead Sciences. Safety and Efficacy of Emtricitabine and Tenofovir Alafenamide (F/TAF) Fixed-Dose Combination Once Daily for Pre-Exposure Prophylaxis in Men and Transgender Women Who Have Sex With Men and Are At Risk of HIV-1 Infection (DISCOVER) [Internet]. Report No.: NCT02842086. | Link |
- National Institute of Allergy and Infectious Diseases. Safety and Efficacy Study of Injectable Cabotegravir Compared to Daily Oral Tenofovir Disoproxil Fumarate/Emtricitabine (TDF/FTC), For Pre-Exposure Prophylaxis in HIV-Uninfected Cisgender Men and Transgender Women Who Have Sex With Men [Internet]. Report No.: NCT02720094. | Link |
- Murchu EO. Systematic review of the clinical effectiveness and cost-effectiveness of various dosing schedules of pre-exposure prophylaxis (PrEP) for the prevention of HIV in males who have sex with males (MSM). [Internet]. Report No.: CRD42017065937. | Link |
- Thavorn K, Mishra S, Tan D, Kugathasan H, Moqueet N, McFadden D. Cost-effectiveness of HIV Pre-exposure Prophylaxis (PrEP) strategies: a methodological systematic review [Internet]. Report No.: CRD42016038440. | Link |
- Traeger M, Schröder S, Doyle J, Stoové M. Effects of pre-exposure prophylaxis (PrEP) on sexual risk taking behaviour in men who have sex with men: a systematic review and meta-analysis. Report No.: CRD42017059674.
- Baranek B, Wang S, Cheung A, Mishra S, Tan D. A systematic review and meta-analysis of the effect of oral tenofovir disoproxil fumarate-containing HIV pre-exposure prophylaxis on bone mineral density [Internet]. Report No.: CRD42017070552. | Link |
 Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016 Jul;30(12):1973–83.
Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016 Jul;30(12):1973–83.  Freeborn K, Portillo CJ. Does Pre-exposure prophylaxis (PrEP) for HIV prevention in men who have sex with men (MSM) change risk behavior? A systematic review. J Clin Nurs. 2017 Aug.
Freeborn K, Portillo CJ. Does Pre-exposure prophylaxis (PrEP) for HIV prevention in men who have sex with men (MSM) change risk behavior? A systematic review. J Clin Nurs. 2017 Aug.  Jiang J, Yang X, Ye L, Zhou B, Ning C, Huang J, et al. Pre-exposure prophylaxis for the prevention of HIV infection in high risk populations: a meta-analysis of randomized controlled trials. PLoS One. 2014;9(2):e87674.
Jiang J, Yang X, Ye L, Zhou B, Ning C, Huang J, et al. Pre-exposure prophylaxis for the prevention of HIV infection in high risk populations: a meta-analysis of randomized controlled trials. PLoS One. 2014;9(2):e87674.  Kennedy CE, Fonner VA, World Health Organization. Pre-exposure prophylaxis for men who have sex with men: a systematic review. 2014.
Kennedy CE, Fonner VA, World Health Organization. Pre-exposure prophylaxis for men who have sex with men: a systematic review. 2014.  Okwundu CI, Uthman OA, Okoromah CA. Antiretroviral pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals. Cochrane database Syst Rev. 2012 Jul;(7):CD007189.
Okwundu CI, Uthman OA, Okoromah CA. Antiretroviral pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals. Cochrane database Syst Rev. 2012 Jul;(7):CD007189.  Yu W, Wang L, Han N, Zhang X, Mahapatra T, Mahapatra S, et al. Pre-exposure prophylaxis of HIV: A right way to go or a long way to go? Artif cells, nanomedicine, Biotechnol. 2016;44(1):201–8.
Yu W, Wang L, Han N, Zhang X, Mahapatra T, Mahapatra S, et al. Pre-exposure prophylaxis of HIV: A right way to go or a long way to go? Artif cells, nanomedicine, Biotechnol. 2016;44(1):201–8.  Amico KR, Marcus JL, McMahan V, Liu A, Koester KA, Goicochea P, et al. Study product adherence measurement in the iPrEx placebo-controlled trial: concordance with drug detection. J Acquir Immune Defic Syndr. 2014 Aug;66(5):530–7.
Amico KR, Marcus JL, McMahan V, Liu A, Koester KA, Goicochea P, et al. Study product adherence measurement in the iPrEx placebo-controlled trial: concordance with drug detection. J Acquir Immune Defic Syndr. 2014 Aug;66(5):530–7.  Buchbinder SP, Glidden D V, Liu AY, McMahan V, Guanira J V, Mayer KH, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis. 2014 Jun;14(6):468–75.
Buchbinder SP, Glidden D V, Liu AY, McMahan V, Guanira J V, Mayer KH, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis. 2014 Jun;14(6):468–75.  Carlo Hojilla J, Koester KA, Cohen SE, Buchbinder S, Ladzekpo D, Matheson T, et al. Sexual Behavior, Risk Compensation, and HIV Prevention Strategies Among Participants in the San Francisco PrEP Demonstration Project: A Qualitative Analysis of Counseling Notes. AIDS Behav. 2016 Jul;20(7):1461–9.
Carlo Hojilla J, Koester KA, Cohen SE, Buchbinder S, Ladzekpo D, Matheson T, et al. Sexual Behavior, Risk Compensation, and HIV Prevention Strategies Among Participants in the San Francisco PrEP Demonstration Project: A Qualitative Analysis of Counseling Notes. AIDS Behav. 2016 Jul;20(7):1461–9.  Golub SA, Kowalczyk W, Weinberger CL, Parsons JT. Preexposure prophylaxis and predicted condom use among high-risk men who have sex with men. J Acquir Immune Defic Syndr. 2010 Aug;54(5):548–55.
Golub SA, Kowalczyk W, Weinberger CL, Parsons JT. Preexposure prophylaxis and predicted condom use among high-risk men who have sex with men. J Acquir Immune Defic Syndr. 2010 Aug;54(5):548–55.  Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec;363(27):2587–99.
Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec;363(27):2587–99.  Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014 Sep;14(9):820–9.
Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014 Sep;14(9):820–9.  Hoff CC, Chakravarty D, Bircher AE, Campbell CK, Grisham K, Neilands TB, et al. Attitudes Towards PrEP and Anticipated Condom Use Among Concordant HIV-Negative and HIV-Discordant Male Couples. AIDS Patient Care STDS. 2015 Jul;29(7):408–17.
Hoff CC, Chakravarty D, Bircher AE, Campbell CK, Grisham K, Neilands TB, et al. Attitudes Towards PrEP and Anticipated Condom Use Among Concordant HIV-Negative and HIV-Discordant Male Couples. AIDS Patient Care STDS. 2015 Jul;29(7):408–17.  Hosek SG, Siberry G, Bell M, Lally M, Kapogiannis B, Green K, et al. The acceptability and feasibility of an HIV preexposure prophylaxis (PrEP) trial with young men who have sex with men. J Acquir Immune Defic Syndr. 2013 Apr;62(4):447–56.
Hosek SG, Siberry G, Bell M, Lally M, Kapogiannis B, Green K, et al. The acceptability and feasibility of an HIV preexposure prophylaxis (PrEP) trial with young men who have sex with men. J Acquir Immune Defic Syndr. 2013 Apr;62(4):447–56.  Liegler T, Abdel-Mohsen M, Bentley LG, Atchison R, Schmidt T, Javier J, et al. HIV-1 drug resistance in the iPrEx preexposure prophylaxis trial. J Infect Dis. 2014 Oct;210(8):1217–27.
Liegler T, Abdel-Mohsen M, Bentley LG, Atchison R, Schmidt T, Javier J, et al. HIV-1 drug resistance in the iPrEx preexposure prophylaxis trial. J Infect Dis. 2014 Oct;210(8):1217–27.  Liu A, Glidden D V, Anderson PL, Amico KR, McMahan V, Mehrotra M, et al. Patterns and correlates of PrEP drug detection among MSM and transgender women in the Global iPrEx Study. J Acquir Immune Defic Syndr. 2014 Dec;67(5):528–37.
Liu A, Glidden D V, Anderson PL, Amico KR, McMahan V, Mehrotra M, et al. Patterns and correlates of PrEP drug detection among MSM and transgender women in the Global iPrEx Study. J Acquir Immune Defic Syndr. 2014 Dec;67(5):528–37.  Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med. 2016 Jan;176(1):75–84.
Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med. 2016 Jan;176(1):75–84.  Marcus JL, Glidden D V, Mayer KH, Liu AY, Buchbinder SP, Amico KR, et al. No evidence of sexual risk compensation in the iPrEx trial of daily oral HIV preexposure prophylaxis. PLoS One. 2013;8(12):e81997.
Marcus JL, Glidden D V, Mayer KH, Liu AY, Buchbinder SP, Amico KR, et al. No evidence of sexual risk compensation in the iPrEx trial of daily oral HIV preexposure prophylaxis. PLoS One. 2013;8(12):e81997.  McCormack S, Dunn DT. Pragmatic open-label randomised trial of preexposure prophylaxis: The PROUD Study. In: CROI. Seattle, Washington; 2015.
McCormack S, Dunn DT. Pragmatic open-label randomised trial of preexposure prophylaxis: The PROUD Study. In: CROI. Seattle, Washington; 2015.  McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet (London, England). 2016 Jan;387(10013):53–60.
McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet (London, England). 2016 Jan;387(10013):53–60.  Solomon MM, Lama JR, Glidden D V, Mulligan K, McMahan V, Liu AY, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS. 2014 Mar;28(6):851–9.
Solomon MM, Lama JR, Glidden D V, Mulligan K, McMahan V, Liu AY, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS. 2014 Mar;28(6):851–9.  Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, et al. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin Infect Dis. 2015 Nov;61(10):1601–3.
Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, et al. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin Infect Dis. 2015 Nov;61(10):1601–3.  Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med. 2015 Dec;373(23):2237–46.
Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med. 2015 Dec;373(23):2237–46.  Molina J-M, Capitant C, Charreau I. On demand PrEP with oral TDF-FTC in MSM: Results of the ANRS Ipergay Trial. In: CROI. Seattle, Washington; 2015.
Molina J-M, Capitant C, Charreau I. On demand PrEP with oral TDF-FTC in MSM: Results of the ANRS Ipergay Trial. In: CROI. Seattle, Washington; 2015.  Grohskopf LA, Chillag KL, Gvetadze R, Liu AY, Thompson M, Mayer KH, et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013 Sep;64(1):79–86.
Grohskopf LA, Chillag KL, Gvetadze R, Liu AY, Thompson M, Mayer KH, et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013 Sep;64(1):79–86.  Liu AY, Vittinghoff E, Gandhi M, Huang Y, Chillag K, Wiegand R. Validating measures of tenofovir drug exposure in a U.S. pre-exposure prophylaxis trial. In: CROI. Seattle, Washington; 2015.
Liu AY, Vittinghoff E, Gandhi M, Huang Y, Chillag K, Wiegand R. Validating measures of tenofovir drug exposure in a U.S. pre-exposure prophylaxis trial. In: CROI. Seattle, Washington; 2015.  Liu AY, Vittinghoff E, Chillag K, Mayer K, Thompson M, Grohskopf L, et al. Sexual risk behavior among HIV-uninfected men who have sex with men participating in a tenofovir preexposure prophylaxis randomized trial in the United States. J Acquir Immune Defic Syndr. 2013 Sep;64(1):87–94.
Liu AY, Vittinghoff E, Chillag K, Mayer K, Thompson M, Grohskopf L, et al. Sexual risk behavior among HIV-uninfected men who have sex with men participating in a tenofovir preexposure prophylaxis randomized trial in the United States. J Acquir Immune Defic Syndr. 2013 Sep;64(1):87–94.  Liu AY, Vittinghoff E, Sellmeyer DE, Irvin R, Mulligan K, Mayer K, et al. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One. 2011;6(8):e23688.
Liu AY, Vittinghoff E, Sellmeyer DE, Irvin R, Mulligan K, Mayer K, et al. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One. 2011;6(8):e23688.  Mutua G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One. 2012;7(4):e33103.
Mutua G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One. 2012;7(4):e33103.  Food and Drug Administration. First Generic Drug Approvals [Internet]. 2017 [cited 2017 Dec 10]. | Link |
Food and Drug Administration. First Generic Drug Approvals [Internet]. 2017 [cited 2017 Dec 10]. | Link | Centers for Disease Control and Prevention. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States [Internet]. 2014. | Link |
Centers for Disease Control and Prevention. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States [Internet]. 2014. | Link | World Health Organization. WHO implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection [Internet]. 2017. | Link |
World Health Organization. WHO implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection [Internet]. 2017. | Link | U.S. Preventive Services Task Force. Draft Update Summary: Prevention of Human Immunodeficiency Virus (HIV) Infection: Pre-Exposure Prophylaxis [Internet]. 2017 [cited 2017 Jan 12]. | Link |
U.S. Preventive Services Task Force. Draft Update Summary: Prevention of Human Immunodeficiency Virus (HIV) Infection: Pre-Exposure Prophylaxis [Internet]. 2017 [cited 2017 Jan 12]. | Link | Gilead Sciences. Safety and Efficacy of Emtricitabine and Tenofovir Alafenamide (F/TAF) Fixed-Dose Combination Once Daily for Pre-Exposure Prophylaxis in Men and Transgender Women Who Have Sex With Men and Are At Risk of HIV-1 Infection (DISCOVER) [Internet]. Report No.: NCT02842086. | Link |
Gilead Sciences. Safety and Efficacy of Emtricitabine and Tenofovir Alafenamide (F/TAF) Fixed-Dose Combination Once Daily for Pre-Exposure Prophylaxis in Men and Transgender Women Who Have Sex With Men and Are At Risk of HIV-1 Infection (DISCOVER) [Internet]. Report No.: NCT02842086. | Link | National Institute of Allergy and Infectious Diseases. Safety and Efficacy Study of Injectable Cabotegravir Compared to Daily Oral Tenofovir Disoproxil Fumarate/Emtricitabine (TDF/FTC), For Pre-Exposure Prophylaxis in HIV-Uninfected Cisgender Men and Transgender Women Who Have Sex With Men [Internet]. Report No.: NCT02720094. | Link |
National Institute of Allergy and Infectious Diseases. Safety and Efficacy Study of Injectable Cabotegravir Compared to Daily Oral Tenofovir Disoproxil Fumarate/Emtricitabine (TDF/FTC), For Pre-Exposure Prophylaxis in HIV-Uninfected Cisgender Men and Transgender Women Who Have Sex With Men [Internet]. Report No.: NCT02720094. | Link | Murchu EO. Systematic review of the clinical effectiveness and cost-effectiveness of various dosing schedules of pre-exposure prophylaxis (PrEP) for the prevention of HIV in males who have sex with males (MSM). [Internet]. Report No.: CRD42017065937. | Link |
Murchu EO. Systematic review of the clinical effectiveness and cost-effectiveness of various dosing schedules of pre-exposure prophylaxis (PrEP) for the prevention of HIV in males who have sex with males (MSM). [Internet]. Report No.: CRD42017065937. | Link | Thavorn K, Mishra S, Tan D, Kugathasan H, Moqueet N, McFadden D. Cost-effectiveness of HIV Pre-exposure Prophylaxis (PrEP) strategies: a methodological systematic review [Internet]. Report No.: CRD42016038440. | Link |
Thavorn K, Mishra S, Tan D, Kugathasan H, Moqueet N, McFadden D. Cost-effectiveness of HIV Pre-exposure Prophylaxis (PrEP) strategies: a methodological systematic review [Internet]. Report No.: CRD42016038440. | Link | Traeger M, Schröder S, Doyle J, Stoové M. Effects of pre-exposure prophylaxis (PrEP) on sexual risk taking behaviour in men who have sex with men: a systematic review and meta-analysis. Report No.: CRD42017059674.
Traeger M, Schröder S, Doyle J, Stoové M. Effects of pre-exposure prophylaxis (PrEP) on sexual risk taking behaviour in men who have sex with men: a systematic review and meta-analysis. Report No.: CRD42017059674.  Baranek B, Wang S, Cheung A, Mishra S, Tan D. A systematic review and meta-analysis of the effect of oral tenofovir disoproxil fumarate-containing HIV pre-exposure prophylaxis on bone mineral density [Internet]. Report No.: CRD42017070552. | Link |
Baranek B, Wang S, Cheung A, Mishra S, Tan D. A systematic review and meta-analysis of the effect of oral tenofovir disoproxil fumarate-containing HIV pre-exposure prophylaxis on bone mineral density [Internet]. Report No.: CRD42017070552. | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








