Abstract
INTRODUCTION
Flumazenil is an antagonist of the GABA/benzodiazepines receptor complex that might play a role in the treatment of hepatic encephalopathy. However, its efficacy and safety are a matter of debate.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified two systematic reviews including fourteen randomized trials. We concluded flumazenil does not reduce mortality in patients with hepatic encephalopathy and it is not clear whether it leads to any clinical improvement because the certainty of the evidence is very low.
Problem
Hepatic encephalopathy is one of the most frequent complications of chronic liver disease [1]. Among multiple mechanisms to explain its causes, the role of sensorineural pathways involving GABA receptors has been proposed. For this reason, the use of an antagonist of the GABA/benzodiazepine receptor complex, flumazenil, has been posed as an alternative for this complication. However, some gastrointestinal, cardiac and neurological adverse effects have been described with the use of this intervention and it is not clear what is its real efficacy and safety in the management of hepatic encephalopathy.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found two systematics reviews [2],[3] including fourteen primary studies reported in 25 references [4],[5],[6],[7],[8], |
|
What types of patients were included* |
All of the trials included patients with liver cirrhosis [4],[8], Six trials evaluated patients with acute hepatic encephalopathy [11],[17],[21],[24],[27],[28] and in two trials patients with chronic hepatic encephalopathy [4],[22]. In the rest of the trials [8],[14],[19],[23],[25],[26] the timing of hepatic encephalopathy was not specified. In relation to the severity of hepatic encephalopathy, four trials included patients with minimal hepatic encephalopathy [14],[19],[23],[25], two with grade I hepatic encephalopathy [4],[25], four with grade II [4],[11],[25],[26], nine with grade III [4],[11],[17],[22],[24],[25],[26],[27],[28] and six with grade IV [11],[17],[21],[24],[26],[28]. In addition, patients with abnormal trunk evoked potentials were included in one trial [14], impaired visual evoked potentials in one trial [19], abnormal electroencephalography in two trials [8],[27], abnormal Number Connection Test in two trials [14],[19], abnormal Digit Symbol Substitution Test in one trial [23] and ammonium levels over 30 µmol/L in one trial [8]. |
|
What types of interventions were included* |
Flumazenil was used intravenously in all of the trials. In two [17],[21] it was used with 20 cc of saline solution, in one [24] with 50 cc of saline solution and in one [26] with 19 cc of saline solution. Regarding dosification, the most frequent was 1 mg single dose in five trials [11],[17],[19],[22],[26] and 2 mg single dose in three trials [4],[21],[24]. The rest of the trials used 6.5 mg per day for three days and 1 mg in a fourth day, with a total of 20.5 mg in a first part and 1 mg in 10 minutes in a second part [8], 1 mg/hour for five hours with a total of 5 mg [25], 0.2 mg once [23], 0.2 mg/kg once [27], 0.5 mg and subsequently 1 mg in 30 minutes [28] and 1 mg of loading together with 0.5 mg every 30 minutes until completing 3 mg [14]. Continuous intravenous infusion was used in four trials [4],[8],[25],[28]. In three trials [4],[8],[28] intravenous loading doses were used. One of them [8], used 0.5 mg in the first part and 1 mg in the second part of the intervention. Another trial [4], used three sequential boluses of 0.4, 0.8 and 1 mg in one minute, each prior to the use of continuous infusion. The last trial [28] used an intravenous bolus of 0.5 mg. In the remaining ten trials [11],[14],[17],[19],[21],[22],[23],[24],[26],[27] intravenous infusion was used in bolus. All the trials compared against placebo. |
|
What types of outcomes |
The main outcomes according to the systematics reviews were:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
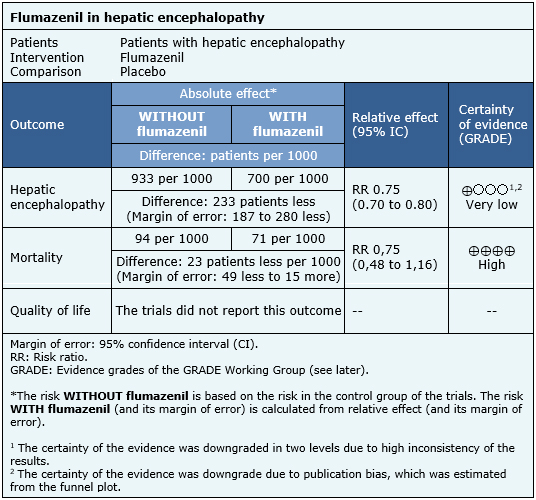
The information on the effects of flumazenil in hepatic encephalopathy is based on eleven randomized trials [4],[8],[11],[17],[21],[22],[23],[24],[25],[26],[28] including 872 patients. The rest of the trials did not report the outcomes of interest, or none of the identified reviews could extract the data so they could be incorporated into a meta-analysis.
Nine trials measured the outcome hepatic encephalopathy [4],[8],[11],[17],[21],[24],[25],[26],[28] and eleven trials reported mortality [4],[8],[11],[17],[21],[22],[23],[24],[25],[26],[28].
The summary of findings is the following:
- It is not clear whether flumazenil improves hepatic encephalopathy, because the certainty of the evidence is very low.
- Flumazenil does not reduce mortality in patients with hepatic encephalopathy. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
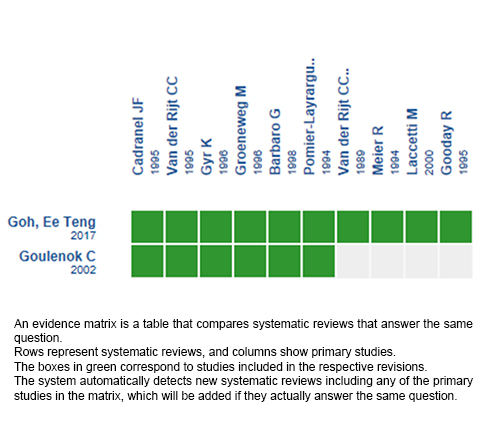
Follow the link to access the interactive version: Flumazenil for hepatic encephalopathy
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
El flumazenil es un antagonista del complejo receptor GABA/benzodiacepinas que podría tener un rol en el manejo de la encefalopatía hepática. Sin embargo, existe controversia sobre su eficacia y seguridad.
MÉTODOS
Para responder esta pregunta utilizamos Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante búsquedas en múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, reanalizamos los datos de los estudios primarios, realizamos un metanálisis y preparamos tablas de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Identificamos dos revisiones sistemáticas que en conjunto incluyen catorce estudios aleatorizados. Concluimos que el uso de flumazenil no disminuye la mortalidad en pacientes con encefalopatía hepática, y no está claro si produce alguna mejoría clínica porque la certeza de la evidencia es muy baja.
 Authors:
Diego Reyes[1,2], Francisco Barrera[2,3]
Authors:
Diego Reyes[1,2], Francisco Barrera[2,3]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Gastroenterología, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: fjbarrer@gmail.com
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Centro de Innovación UC Anacleto Angelini Avda.Vicuña Mackenna 4860 Macul Santiago Chile

Citation: Reyes D, Barrera F. Is flumazenil an alternative for the treatment of hepatic encephalopathy?. Medwave 2017 Nov-Dic;17(9):e7113 doi: 10.5867/medwave.2017.09.7113
Submission date: 18/11/2017
Acceptance date: 4/12/2017
Publication date: 26/12/2017
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014 Aug;60(2):715-35. | CrossRef | PubMed |
- Goulenok C, Bernard B, Cadranel JF, Thabut D, Di Martino V, Opolon P, et al. Flumazenil vs. placebo in hepatic encephalopathy in patients with cirrhosis: a meta-analysis. Aliment Pharmacol Ther. 2002 Mar;16(3):361-72. | PubMed |
- Goh ET, Andersen ML, Morgan MY, Gluud LL. Flumazenil versus placebo or no intervention for people with cirrhosis and hepatic encephalopathy. Cochrane Database Syst Rev. 2017 Jul 26;7:CD002798. | CrossRef | PubMed |
- Gyr K, Meier R, Häussler J, Boulétreau P, Fleig WE, Gatta A, et al. Evaluation of the efficacy and safety of flumazenil in the treatment of portal systemic encephalopathy: a double blind, randomised, placebo controlled multicentre study. Gut. 1996 Aug;39(2):319-24. | PubMed |
- Lotterer E, Hoppe M, Balzer C, Fleig WE.. Short-term effects of flumazenil in early stage of portosystemic encephalopathy (PSE): a placebo-controlled, prospective, randomized study. Gastroenterology. 2001;120:376-77. | Link |
- Groeneweg M, Gyr K, Amrein R, Scollo-Lavizzari G, Williams R, Yoo JY, et al. Effect of flumazenil on the electroencephalogram of patients with portosystemic encephalopathy. Results of a double blind, randomised, placebo-controlled multicentre trial. Electroencephalogr Clin Neurophysiol. 1996 Jan;98(1):29-34. | PubMed |
- Meier R, Gyr K, Häussler R, and the PSE-Study Group. Treatment of portosystemic encephalopathy with the benzodiazepine-receptor antagonist flumazenil (a randomized, double-blind, placebo-controlled, multicenter study). Gastroenterology 1994;106 Suppl(4):A942. | Link |
- Van der Rijt CC, Schalm SW, Meulstee J, Stijnen T. Flumazenil therapy for hepatic encephalopathy. A double-blind cross over study. Gastroenterol Clin Biol. 1995 Jun-Jul;19(6-7):572-80. | PubMed |
- Van der Rijt CCD, Schalm SW, Meulstee J, Stijnen T.. Flumazenil therapy for hepatic encephalopathy: a double-blind cross-over study. Hepatology (Baltimore, Md.). 1989;10:4. | Link |
- Van der Rijt CCD.. Hepatic Encephalopathy: Clinical and Experimental Studies. Alblasserdam, Netherlands: Offsetdrukkerij Haveka BV. 1991. | Link |
- Cadranel JF, el Younsi M, Pidoux B, Zylberberg P, Benhamou Y, Valla D, et al. Flumazenil therapy for hepatic encephalopathy in cirrhotic patients: a double-blind pragmatic randomized, placebo study. Eur J Gastroenterol Hepatol. 1995 Apr;7(4):325-9. | PubMed |
- Cadranel JF, El Younsi M, Pidoux B, Zylberberg P, Benhamou Y, et al.. Immediate improvement of hepatic encephalopathy (HE) in cirrhotic patients by flumazenil. Journal of Hepatology. 1991;13(Suppl(2)):104. | Link |
- El Younsi M, Cadranel JF, Pidoux B, Zylberberg P, Valla D, et al. The immediate effect on the clinical grade and electroencephalogram of cirrhotic patients with hepatic encephalopathy [Effets immediats du flumazenil sur le degre clinique et electroencephalographique de l’encephalopathie hepatique chez le cirrhotique [abstract]]. Gastroenterologie Clinique et Biologique 1991; Vol. 15, issue 2:A216. | Link |
- Amodio P, Marchetti P, Comacchio F, Beghi A, Del Piccolo F, Merkel C, et al. Effects of flumazenil on subclinical hepatic encephalopathy (SHE): preliminary data. Italian Journal of Gastroenterology. 1993;23:179. | Link |
- Amodio P, Marchetti P, Del Piccolo F, Beghi A, Comacchio F, Carraro P, et al. The effect of flumazenil on subclinical psychometric or neurophysiological alterations in cirrhotic patients: a double-blind placebo-controlled study. Clin Physiol. 1997 Sep;17(5):533-9. | PubMed |
- Amodio P, Marchetti P, Comacchio F, Beghi A, del Piccolo F, et al. Effects of flumazenil on subclinical hepatic encephalopathy (SHE) [EASL abstract]. Journal of Hepatology 1993;18 Suppl(1):88. | Link |
- Barbaro G, Di Lorenzo G, Soldini M, Giancaspro G, Bellomo G, Belloni G, et al. Flumazenil for hepatic encephalopathy grade III and IVa in patients with cirrhosis: an Italian multicenter double-blind, placebo-controlled, cross-over study. Hepatology. 1998 Aug;28(2):374-8. | PubMed |
- Barbaro G, Di Lorenzo G, Soldini M, Marziali M, Bellomo G, Belloni G, et al. Flumazenil for hepatic coma in patients with liver cirrhosis: an Italian multicentre double-blind, placebo-controlled, crossover study. Eur J Emerg Med. 1998 Jun;5(2):213-8. | PubMed |
- Giger-Mateeva VI, Reits D, Liberov B, Jones EA, Spekreijse H. The effect of flumazenil on visual event-related potentials of clinically non-encephalopathic patients with cirrhosis. Neurosci Lett. 1999 Dec 10;276(3):173-6. | PubMed |
- Jones EA, Giger-Mateeva VI, Reits D, Riemslag FC, Liberov B, Spekrijse H. Visual event-related potentials in cirrhotic patients without overt encephalopathy: the effects of flumazenil. Metab Brain Dis. 2001 Jun;16(1-2):43-53. | PubMed |
- Pomier-Layrargues G, Giguère JF, Lavoie J, Perney P, Gagnon S, D'Amour M, et al. Flumazenil in cirrhotic patients in hepatic coma: a randomized double-blind placebo-controlled crossover trial. Hepatology. 1994 Jan;19(1):32-7. | PubMed |
- Klotz U, Walker S. Flumazenil and hepatic encephalopathy. Lancet. 1989 Jan 21;1(8630):155-6. | PubMed |
- Gooday R, Hayes PC, Bzeizi K, O'Carroll RE. Benzodiazepine receptor antagonism improves reaction time in latent hepatic encephalopathy. Psychopharmacology (Berl). 1995 Jun;119(3):295-8. | PubMed |
- Laccetti M, Manes G, Uomo G, Lioniello M, Rabitti PG, Balzano A. Flumazenil in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double blind randomized placebo controlled study. Dig Liver Dis. 2000 May;32(4):335-8. | PubMed |
- Dursun M, Caliskan M, Canoruc F, Aluclu U, Canoruc N, Tuzcu A, et al. The efficacy of flumazenil in subclinical to mild hepatic encephalopathic ambulatory patients. A prospective, randomised, double-blind, placebo-controlled study. Swiss Med Wkly. 2003 Feb 22;133(7-8):118-23. | PubMed |
- Zhu C, Wang J, Liu T. Flumazenil in the treatment of cirrhotic patients with hepatic encephalopathy: A randomized doubled-blind clinical trial. Chinese Journal of Digestion 1998;18:355–8. | Link |
- Hermant JL, Levacher S, Frenkel AL, Blaise M, Volter F, et al. The clinical and electroencephalographic effects of flumazenil in acute hepatic encephalopathy [Effets cliniques et electroencephalographiques du flumazenil dans l’encephalopathie hepatique aigue]. Annales Francaises d’Anesthesie et de Reanimation 1991;10 Suppl:R172. | Link |
- Li F, Lei L, Wei L.. Clinical study of flumazenil on severe hepatic encephalopathy. Practical Pharmacy and Clinical Remedies. 2009;12:79-81. | Link |
- Treatment of Hepatic Encephalopathy with Flumazenil and change in Cortical GABA levels in MRS. NCT02048969. | Link |
 Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014 Aug;60(2):715-35. | CrossRef | PubMed |
Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014 Aug;60(2):715-35. | CrossRef | PubMed | Goulenok C, Bernard B, Cadranel JF, Thabut D, Di Martino V, Opolon P, et al. Flumazenil vs. placebo in hepatic encephalopathy in patients with cirrhosis: a meta-analysis. Aliment Pharmacol Ther. 2002 Mar;16(3):361-72. | PubMed |
Goulenok C, Bernard B, Cadranel JF, Thabut D, Di Martino V, Opolon P, et al. Flumazenil vs. placebo in hepatic encephalopathy in patients with cirrhosis: a meta-analysis. Aliment Pharmacol Ther. 2002 Mar;16(3):361-72. | PubMed | Goh ET, Andersen ML, Morgan MY, Gluud LL. Flumazenil versus placebo or no intervention for people with cirrhosis and hepatic encephalopathy. Cochrane Database Syst Rev. 2017 Jul 26;7:CD002798. | CrossRef | PubMed |
Goh ET, Andersen ML, Morgan MY, Gluud LL. Flumazenil versus placebo or no intervention for people with cirrhosis and hepatic encephalopathy. Cochrane Database Syst Rev. 2017 Jul 26;7:CD002798. | CrossRef | PubMed | Gyr K, Meier R, Häussler J, Boulétreau P, Fleig WE, Gatta A, et al. Evaluation of the efficacy and safety of flumazenil in the treatment of portal systemic encephalopathy: a double blind, randomised, placebo controlled multicentre study. Gut. 1996 Aug;39(2):319-24. | PubMed |
Gyr K, Meier R, Häussler J, Boulétreau P, Fleig WE, Gatta A, et al. Evaluation of the efficacy and safety of flumazenil in the treatment of portal systemic encephalopathy: a double blind, randomised, placebo controlled multicentre study. Gut. 1996 Aug;39(2):319-24. | PubMed | Lotterer E, Hoppe M, Balzer C, Fleig WE.. Short-term effects of flumazenil in early stage of portosystemic encephalopathy (PSE): a placebo-controlled, prospective, randomized study. Gastroenterology. 2001;120:376-77. | Link |
Lotterer E, Hoppe M, Balzer C, Fleig WE.. Short-term effects of flumazenil in early stage of portosystemic encephalopathy (PSE): a placebo-controlled, prospective, randomized study. Gastroenterology. 2001;120:376-77. | Link | Groeneweg M, Gyr K, Amrein R, Scollo-Lavizzari G, Williams R, Yoo JY, et al. Effect of flumazenil on the electroencephalogram of patients with portosystemic encephalopathy. Results of a double blind, randomised, placebo-controlled multicentre trial. Electroencephalogr Clin Neurophysiol. 1996 Jan;98(1):29-34. | PubMed |
Groeneweg M, Gyr K, Amrein R, Scollo-Lavizzari G, Williams R, Yoo JY, et al. Effect of flumazenil on the electroencephalogram of patients with portosystemic encephalopathy. Results of a double blind, randomised, placebo-controlled multicentre trial. Electroencephalogr Clin Neurophysiol. 1996 Jan;98(1):29-34. | PubMed | Meier R, Gyr K, Häussler R, and the PSE-Study Group. Treatment of portosystemic encephalopathy with the benzodiazepine-receptor antagonist flumazenil (a randomized, double-blind, placebo-controlled, multicenter study). Gastroenterology 1994;106 Suppl(4):A942. | Link |
Meier R, Gyr K, Häussler R, and the PSE-Study Group. Treatment of portosystemic encephalopathy with the benzodiazepine-receptor antagonist flumazenil (a randomized, double-blind, placebo-controlled, multicenter study). Gastroenterology 1994;106 Suppl(4):A942. | Link | Van der Rijt CC, Schalm SW, Meulstee J, Stijnen T. Flumazenil therapy for hepatic encephalopathy. A double-blind cross over study. Gastroenterol Clin Biol. 1995 Jun-Jul;19(6-7):572-80. | PubMed |
Van der Rijt CC, Schalm SW, Meulstee J, Stijnen T. Flumazenil therapy for hepatic encephalopathy. A double-blind cross over study. Gastroenterol Clin Biol. 1995 Jun-Jul;19(6-7):572-80. | PubMed | Van der Rijt CCD, Schalm SW, Meulstee J, Stijnen T.. Flumazenil therapy for hepatic encephalopathy: a double-blind cross-over study. Hepatology (Baltimore, Md.). 1989;10:4. | Link |
Van der Rijt CCD, Schalm SW, Meulstee J, Stijnen T.. Flumazenil therapy for hepatic encephalopathy: a double-blind cross-over study. Hepatology (Baltimore, Md.). 1989;10:4. | Link | Van der Rijt CCD.. Hepatic Encephalopathy: Clinical and Experimental Studies. Alblasserdam, Netherlands: Offsetdrukkerij Haveka BV. 1991. | Link |
Van der Rijt CCD.. Hepatic Encephalopathy: Clinical and Experimental Studies. Alblasserdam, Netherlands: Offsetdrukkerij Haveka BV. 1991. | Link | Cadranel JF, el Younsi M, Pidoux B, Zylberberg P, Benhamou Y, Valla D, et al. Flumazenil therapy for hepatic encephalopathy in cirrhotic patients: a double-blind pragmatic randomized, placebo study. Eur J Gastroenterol Hepatol. 1995 Apr;7(4):325-9. | PubMed |
Cadranel JF, el Younsi M, Pidoux B, Zylberberg P, Benhamou Y, Valla D, et al. Flumazenil therapy for hepatic encephalopathy in cirrhotic patients: a double-blind pragmatic randomized, placebo study. Eur J Gastroenterol Hepatol. 1995 Apr;7(4):325-9. | PubMed | Cadranel JF, El Younsi M, Pidoux B, Zylberberg P, Benhamou Y, et al.. Immediate improvement of hepatic encephalopathy (HE) in cirrhotic patients by flumazenil. Journal of Hepatology. 1991;13(Suppl(2)):104. | Link |
Cadranel JF, El Younsi M, Pidoux B, Zylberberg P, Benhamou Y, et al.. Immediate improvement of hepatic encephalopathy (HE) in cirrhotic patients by flumazenil. Journal of Hepatology. 1991;13(Suppl(2)):104. | Link | El Younsi M, Cadranel JF, Pidoux B, Zylberberg P, Valla D, et al. The immediate effect on the clinical grade and electroencephalogram of cirrhotic patients with hepatic encephalopathy [Effets immediats du flumazenil sur le degre clinique et electroencephalographique de l’encephalopathie hepatique chez le cirrhotique [abstract]]. Gastroenterologie Clinique et Biologique 1991; Vol. 15, issue 2:A216. | Link |
El Younsi M, Cadranel JF, Pidoux B, Zylberberg P, Valla D, et al. The immediate effect on the clinical grade and electroencephalogram of cirrhotic patients with hepatic encephalopathy [Effets immediats du flumazenil sur le degre clinique et electroencephalographique de l’encephalopathie hepatique chez le cirrhotique [abstract]]. Gastroenterologie Clinique et Biologique 1991; Vol. 15, issue 2:A216. | Link | Amodio P, Marchetti P, Comacchio F, Beghi A, Del Piccolo F, Merkel C, et al. Effects of flumazenil on subclinical hepatic encephalopathy (SHE): preliminary data. Italian Journal of Gastroenterology. 1993;23:179. | Link |
Amodio P, Marchetti P, Comacchio F, Beghi A, Del Piccolo F, Merkel C, et al. Effects of flumazenil on subclinical hepatic encephalopathy (SHE): preliminary data. Italian Journal of Gastroenterology. 1993;23:179. | Link | Amodio P, Marchetti P, Del Piccolo F, Beghi A, Comacchio F, Carraro P, et al. The effect of flumazenil on subclinical psychometric or neurophysiological alterations in cirrhotic patients: a double-blind placebo-controlled study. Clin Physiol. 1997 Sep;17(5):533-9. | PubMed |
Amodio P, Marchetti P, Del Piccolo F, Beghi A, Comacchio F, Carraro P, et al. The effect of flumazenil on subclinical psychometric or neurophysiological alterations in cirrhotic patients: a double-blind placebo-controlled study. Clin Physiol. 1997 Sep;17(5):533-9. | PubMed | Amodio P, Marchetti P, Comacchio F, Beghi A, del Piccolo F, et al. Effects of flumazenil on subclinical hepatic encephalopathy (SHE) [EASL abstract]. Journal of Hepatology 1993;18 Suppl(1):88. | Link |
Amodio P, Marchetti P, Comacchio F, Beghi A, del Piccolo F, et al. Effects of flumazenil on subclinical hepatic encephalopathy (SHE) [EASL abstract]. Journal of Hepatology 1993;18 Suppl(1):88. | Link | Barbaro G, Di Lorenzo G, Soldini M, Giancaspro G, Bellomo G, Belloni G, et al. Flumazenil for hepatic encephalopathy grade III and IVa in patients with cirrhosis: an Italian multicenter double-blind, placebo-controlled, cross-over study. Hepatology. 1998 Aug;28(2):374-8. | PubMed |
Barbaro G, Di Lorenzo G, Soldini M, Giancaspro G, Bellomo G, Belloni G, et al. Flumazenil for hepatic encephalopathy grade III and IVa in patients with cirrhosis: an Italian multicenter double-blind, placebo-controlled, cross-over study. Hepatology. 1998 Aug;28(2):374-8. | PubMed | Barbaro G, Di Lorenzo G, Soldini M, Marziali M, Bellomo G, Belloni G, et al. Flumazenil for hepatic coma in patients with liver cirrhosis: an Italian multicentre double-blind, placebo-controlled, crossover study. Eur J Emerg Med. 1998 Jun;5(2):213-8. | PubMed |
Barbaro G, Di Lorenzo G, Soldini M, Marziali M, Bellomo G, Belloni G, et al. Flumazenil for hepatic coma in patients with liver cirrhosis: an Italian multicentre double-blind, placebo-controlled, crossover study. Eur J Emerg Med. 1998 Jun;5(2):213-8. | PubMed | Giger-Mateeva VI, Reits D, Liberov B, Jones EA, Spekreijse H. The effect of flumazenil on visual event-related potentials of clinically non-encephalopathic patients with cirrhosis. Neurosci Lett. 1999 Dec 10;276(3):173-6. | PubMed |
Giger-Mateeva VI, Reits D, Liberov B, Jones EA, Spekreijse H. The effect of flumazenil on visual event-related potentials of clinically non-encephalopathic patients with cirrhosis. Neurosci Lett. 1999 Dec 10;276(3):173-6. | PubMed | Jones EA, Giger-Mateeva VI, Reits D, Riemslag FC, Liberov B, Spekrijse H. Visual event-related potentials in cirrhotic patients without overt encephalopathy: the effects of flumazenil. Metab Brain Dis. 2001 Jun;16(1-2):43-53. | PubMed |
Jones EA, Giger-Mateeva VI, Reits D, Riemslag FC, Liberov B, Spekrijse H. Visual event-related potentials in cirrhotic patients without overt encephalopathy: the effects of flumazenil. Metab Brain Dis. 2001 Jun;16(1-2):43-53. | PubMed | Pomier-Layrargues G, Giguère JF, Lavoie J, Perney P, Gagnon S, D'Amour M, et al. Flumazenil in cirrhotic patients in hepatic coma: a randomized double-blind placebo-controlled crossover trial. Hepatology. 1994 Jan;19(1):32-7. | PubMed |
Pomier-Layrargues G, Giguère JF, Lavoie J, Perney P, Gagnon S, D'Amour M, et al. Flumazenil in cirrhotic patients in hepatic coma: a randomized double-blind placebo-controlled crossover trial. Hepatology. 1994 Jan;19(1):32-7. | PubMed | Klotz U, Walker S. Flumazenil and hepatic encephalopathy. Lancet. 1989 Jan 21;1(8630):155-6. | PubMed |
Klotz U, Walker S. Flumazenil and hepatic encephalopathy. Lancet. 1989 Jan 21;1(8630):155-6. | PubMed | Gooday R, Hayes PC, Bzeizi K, O'Carroll RE. Benzodiazepine receptor antagonism improves reaction time in latent hepatic encephalopathy. Psychopharmacology (Berl). 1995 Jun;119(3):295-8. | PubMed |
Gooday R, Hayes PC, Bzeizi K, O'Carroll RE. Benzodiazepine receptor antagonism improves reaction time in latent hepatic encephalopathy. Psychopharmacology (Berl). 1995 Jun;119(3):295-8. | PubMed | Laccetti M, Manes G, Uomo G, Lioniello M, Rabitti PG, Balzano A. Flumazenil in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double blind randomized placebo controlled study. Dig Liver Dis. 2000 May;32(4):335-8. | PubMed |
Laccetti M, Manes G, Uomo G, Lioniello M, Rabitti PG, Balzano A. Flumazenil in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double blind randomized placebo controlled study. Dig Liver Dis. 2000 May;32(4):335-8. | PubMed | Dursun M, Caliskan M, Canoruc F, Aluclu U, Canoruc N, Tuzcu A, et al. The efficacy of flumazenil in subclinical to mild hepatic encephalopathic ambulatory patients. A prospective, randomised, double-blind, placebo-controlled study. Swiss Med Wkly. 2003 Feb 22;133(7-8):118-23. | PubMed |
Dursun M, Caliskan M, Canoruc F, Aluclu U, Canoruc N, Tuzcu A, et al. The efficacy of flumazenil in subclinical to mild hepatic encephalopathic ambulatory patients. A prospective, randomised, double-blind, placebo-controlled study. Swiss Med Wkly. 2003 Feb 22;133(7-8):118-23. | PubMed | Zhu C, Wang J, Liu T. Flumazenil in the treatment of cirrhotic patients with hepatic encephalopathy: A randomized doubled-blind clinical trial. Chinese Journal of Digestion 1998;18:355–8. | Link |
Zhu C, Wang J, Liu T. Flumazenil in the treatment of cirrhotic patients with hepatic encephalopathy: A randomized doubled-blind clinical trial. Chinese Journal of Digestion 1998;18:355–8. | Link | Hermant JL, Levacher S, Frenkel AL, Blaise M, Volter F, et al. The clinical and electroencephalographic effects of flumazenil in acute hepatic encephalopathy [Effets cliniques et electroencephalographiques du flumazenil dans l’encephalopathie hepatique aigue]. Annales Francaises d’Anesthesie et de Reanimation 1991;10 Suppl:R172. | Link |
Hermant JL, Levacher S, Frenkel AL, Blaise M, Volter F, et al. The clinical and electroencephalographic effects of flumazenil in acute hepatic encephalopathy [Effets cliniques et electroencephalographiques du flumazenil dans l’encephalopathie hepatique aigue]. Annales Francaises d’Anesthesie et de Reanimation 1991;10 Suppl:R172. | Link | Li F, Lei L, Wei L.. Clinical study of flumazenil on severe hepatic encephalopathy. Practical Pharmacy and Clinical Remedies. 2009;12:79-81. | Link |
Li F, Lei L, Wei L.. Clinical study of flumazenil on severe hepatic encephalopathy. Practical Pharmacy and Clinical Remedies. 2009;12:79-81. | Link | Treatment of Hepatic Encephalopathy with Flumazenil and change in Cortical GABA levels in MRS. NCT02048969. | Link |
Treatment of Hepatic Encephalopathy with Flumazenil and change in Cortical GABA levels in MRS. NCT02048969. | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








