Abstract
INTRODUCTION
Wasting syndrome is a common problem in HIV. It leads to substantive morbidity and mortality. The use of cannabinoids has been suggested as a treatment for weight, but it is not clear whether they are really safe and effective.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified eight systematic reviews including ten studies overall, of which six were randomized trials. We concluded it is not clear whether cannabinoids increase appetite or weight in HIV wasting syndrome because the certainty of the evidence is very low, and they probably lead to frequent adverse effects.
Problem
The high frequency of HIV-related wasting syndrome, defined as the involuntary loss of at least 10% of standard body weight associated with chronic diarrhoea or chronic fatigue and fever for at least 30 days, was observed at the onset of the human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) epidemic [1]. This definition is no longer used, and the diagnosis is based mainly on the presence of significant, progressive or very fast weight-loss, which has led to various new definitions [2].
After the beginning of anti-retroviral therapy an important decrease of wasting syndrome cases was observed, but it still remains a common problem, estimated to affect up to 14-38% of patients [3].
Its relevance lies in its association to a greater risk of mortality [3] which makes important to look for treatment alternatives aimed to optimise the nutritional state [2].
It has been suggested the use of natural or synthetic cannabinoids would have a positive effect on appetite, weight gain and mood of adults with HIV/AIDS [4]. They would increase appetite through activation of CB1 endocannabinoid receptors at a central level. However, the real clinical effect of this treatment is still unclear [5].
Methods
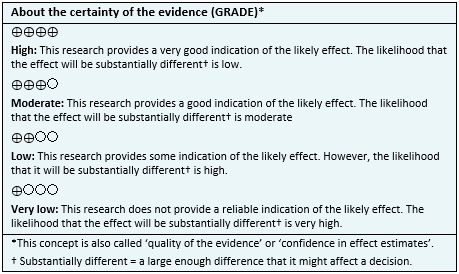
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found eight systematic reviews [4],[6],[7],[8],[9],[10],[11],[12] which include ten primary studies [13],[14],[15],[16],[17],[18],[19],[20],[21],[22] of which six are randomized controlled trials [13],[14],[15],[16],[17],[18]. This table and the summary in general are based on the latter. |
|
What types of patients were included* |
All of the trials were conducted in adults who were predominantly male. Average age ranged between 36 and 43 in the different trials. Intervention duration ranged between 2 weeks and 12 months. One of the trials was conducted in hospitalized patients [18], and the rest in outpatients. In five trials [13],[15],[16],[17],[18] certification of HIV infection was required in order to participate, and one of them included AIDS-related criteria [14]. In five trials [14],[15],[16],[17],[18] an ongoing antiretroviral treatment was required. The trials did not mention CD4 count and only one makes reference to viral count [16]. Four trials [13],[14],[15],[17] included some criteria related to weight-loss within a certain interval of time. Regarding previous cannabinoid use, two trials [17],[18] included patients that were cannabis consumers, while the other four required a given interval without cannabinoid use prior to participation [13],[14],[15],[16]. |
|
What types of interventions were included* |
All of the trials evaluated the effect of dronabinol in various doses, administered orally between one and four times a day. In three trials [16],[17],[18] dronabinol was compared to inhaled marijuana in the form of cigarette with different doses of THC (between 1,8% and 3,9% THC content) and placebo. In one trial dronabinol was compared to megestrol [15], a steroid, without comparing with a placebo. Five trials compared against placebo [13],[14],[16],[17],[18]. |
|
What types of outcomes |
Appetite increase was evaluated in all of the trials with different visual analogous scales, along with adverse effects. Five trials [13],[14],[15],[16], [18] evaluated weight gain expressed as total weight gain [kg] in a given interval. In addition, functionality scales, subjective experience and mood changes were also evaluated. Only one trial evaluated laboratory nutritional indicators [16]. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
The information on the effects of cannabinoid use in HIV wasting syndrome is based on six randomized trials [13],[14],[15],[16],[17],[18] which included 298 patients.
All of the trials reported change in appetite (298 patients) and adverse effects (298 patients). Five trials [13],[14],[15],[16],[18] measured change in weight (268 patients).
However, none of the reviews identified was able to pool data from the trials into a meta-analysis, so the results are presented in a narrative form, based on the conclusions of the individual reviews.
The summary of findings is the following:
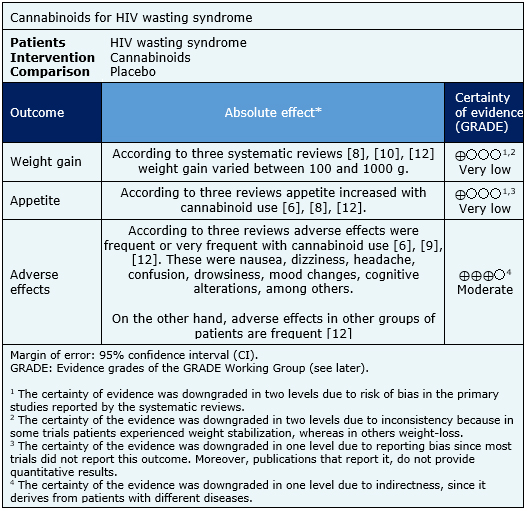
- It is not clear whether the use of cannabinoids in HIV wasting syndrome leads to weight gain because the certainty of the evidence is very low.
- It is not clear whether the use of cannabinoids in HIV wasting syndrome leads to an increase in appetite because the certainty of the evidence is very low.
- The use of cannabinoids in HIV wasting syndrome is probably associated to frequent adverse effects.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
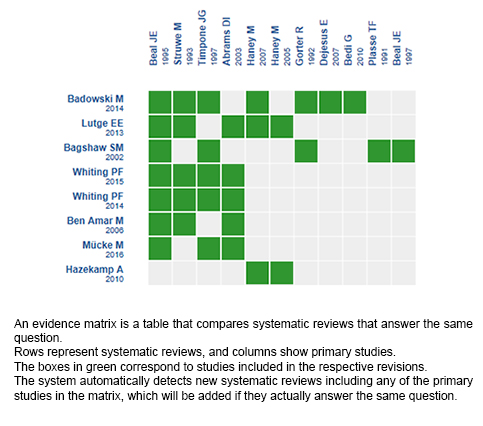
Follow the link to access the interactive version: Cannabinoids for HIV/AIDS wasting syndrome
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
El síndrome de emaciación (wasting) en VIH/SIDA aún permanece como un problema común, constituyéndose como un factor de mortalidad en esta población. Se ha postulado el uso de cannabinoides como tratamiento de la baja de peso secundaria a la infección por VIH, lo que aún es controvertido.
MÉTODOS
Para responder esta pregunta utilizamos Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante búsquedas en múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, reanalizamos los datos de los estudios primarios y preparamos tablas de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Identificamos ocho revisiones sistemáticas que en conjunto incluyen 10 estudios primarios, de los cuales, seis son ensayos aleatorizados. Concluimos que no está claro si los cannabinoides aumentan el apetito o incrementan el peso en el síndrome de wasting en pacientes con VIH, y probablemente los efectos adversos son frecuentes.
 Authors:
Alejandra Núñez[1,2], Carolina Núñez[2,3], Oscar Corsi[2,3], Gabriel Rada[2,3,4,5,6]
Authors:
Alejandra Núñez[1,2], Carolina Núñez[2,3], Oscar Corsi[2,3], Gabriel Rada[2,3,4,5,6]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Medicina Interna, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[4] Centro Evidencia UC, Pontificia Universidad Católica de Chile, Santiago, Chile
[5] GRADE working group
[6] The Cochrane Collaboration
E-mail: radagabriel@epistemonikos.org
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Centro de Innovación UC Anacleto Angelini Avda.Vicuña Mackenna 4860 Macul Santiago Chile

Citation: Núñez A, Núñez C, Corsi O, Rada G. Are cannabinoids effective for HIV wasting syndrome?. Medwave 2017 Nov-Dic;17(9):e7107 doi: 10.5867/medwave.2017.09.7107
Submission date: 15/11/2017
Acceptance date: 1/12/2017
Publication date: 22/12/2017
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Centers for Disease Control (CDC). Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Council of State and Territorial Epidemiologists; AIDS Program, Center for Infectious Diseases. MMWR Suppl. 1987 Aug 14;36(1):1S-15S. | PubMed |
- Mangili A, Murman DH, Zampini AM, Wanke CA. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis. 2006 Mar 15;42(6):836-42. | PubMed |
- Tang AM, Jacobson DL, Spiegelman D, Knox TA, Wanke C. Increasing risk of 5% or greater unintentional weight loss in a cohort of HIV-infected patients, 1995 to 2003. J Acquir Immune Defic Syndr. 2005 Sep 1;40(1):70-6. | PubMed |
- Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013 Apr 30;(4):CD005175. | CrossRef | PubMed |
- Badowski ME, Perez SE. Clinical utility of dronabinol in the treatment of weight loss associated with HIV and AIDS. HIV AIDS (Auckl). 2016 Feb 10;8:37-45. | CrossRef | PubMed | PMC |
- Mücke M, Carter C, Cuhls H, Prüß M, Radbruch L, Häuser W. [Cannabinoids in palliative care: Systematic review and meta-analysis of efficacy, tolerability and safety]. Schmerz. 2016 Feb;30(1):25-36. | CrossRef | PubMed |
- Bagshaw SM, Hagen NA. Medical efficacy of cannabinoids and marijuana: a comprehensive review of the literature. J Palliat Care. 2002 Summer;18(2):111-22. | PubMed |
- Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25. | PubMed |
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. Review. Erratum in: JAMA. 2016 Apr 12;315(14):1522. JAMA. 2015 Dec 1;314(21):2308. JAMA. 2015 Aug 4;314(5):520. JAMA. 2015 Aug 25;314(8):837. | CrossRef | PubMed |
- Badowski M, Pandit NS. Pharmacologic management of human immunodeficiency virus wasting syndrome. Pharmacotherapy. 2014 Aug;34(8):868-81. | CrossRef | PubMed |
- Hazekamp, A , Grotenhermen, F. Review on clinical studies with cannabis and cannabinoids 2005-2009. Cannabinoids. 2010 Feb 13; 5 (special issue):1-21. | Link |
- Whiting P, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes C, et al. Systematic Review of Cannabis for Medical Use. N.p., 2014. [on line] | Link |
- Struwe M, Kaempfer SH, Geiger CJ, Pavia AT, Plasse TF, Shepard KV, Ries K, Evans TG. Effect of dronabinol on nutritional status in HIV infection. Ann Pharmacother. 1993 Jul-Aug;27(7-8):827-31. | PubMed |
- Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995 Feb;10(2):89-97. | PubMed |
- Timpone JG, Wright DJ, Li N, Egorin MJ, Enama ME, Mayers J, Galetto G. The safety and pharmacokinetics of single-agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome. The DATRI 004 Study Group. Division of AIDS Treatment Research Initiative. AIDS Res Hum Retroviruses. 1997 Mar 1;13(4):305-15. | PubMed |
- Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med. 2003 Aug 19;139(4):258-66. | PubMed |
- Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology (Berl). 2005 Aug;181(1):170-8. | PubMed |
- Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr. 2007 Aug 15;45(5):545-54. | PubMed |
- Plasse TF, Gorter RW, Krasnow SH, Lane M, Shepard KV, Wadleigh RG. Recent clinical experience with dronabinol. Pharmacol Biochem Behav. 1991 Nov;40(3):695-700. | PubMed |
- Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD, Vosburg SK, Haney M. Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study. Psychopharmacology (Berl). 2010 Dec;212(4):675-86. | CrossRef | PubMed | PMC |
- Gorter R, Seefried M, Volberding P. Dronabinol effects on weight in patients with HIV infection. AIDS. 1992 Jan;6(1):127. | PubMed |
- DeJesus E, Rodwick BM, Bowers D, Cohen CJ, Pearce D. Use of Dronabinol Improves Appetite and Reverses Weight Loss in HIV/AIDS-Infected Patients. J Int Assoc Physicians AIDS Care (Chic). 2007 Jun;6(2):95-100. | PubMed |
- Watson SJ, Benson JA Jr, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000 Jun;57(6):547-52. | PubMed |
- Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations – 2016 Update. Geneva: World Health Organization; 2016. | PubMed |
- Nemechek PM, Polsky B, Gottlieb MS. Treatment guidelines for HIV-associated wasting. Mayo Clin Proc. 2000 Apr;75(4):386-94. | PubMed |
 Centers for Disease Control (CDC). Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Council of State and Territorial Epidemiologists; AIDS Program, Center for Infectious Diseases. MMWR Suppl. 1987 Aug 14;36(1):1S-15S. | PubMed |
Centers for Disease Control (CDC). Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Council of State and Territorial Epidemiologists; AIDS Program, Center for Infectious Diseases. MMWR Suppl. 1987 Aug 14;36(1):1S-15S. | PubMed | Mangili A, Murman DH, Zampini AM, Wanke CA. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis. 2006 Mar 15;42(6):836-42. | PubMed |
Mangili A, Murman DH, Zampini AM, Wanke CA. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis. 2006 Mar 15;42(6):836-42. | PubMed | Tang AM, Jacobson DL, Spiegelman D, Knox TA, Wanke C. Increasing risk of 5% or greater unintentional weight loss in a cohort of HIV-infected patients, 1995 to 2003. J Acquir Immune Defic Syndr. 2005 Sep 1;40(1):70-6. | PubMed |
Tang AM, Jacobson DL, Spiegelman D, Knox TA, Wanke C. Increasing risk of 5% or greater unintentional weight loss in a cohort of HIV-infected patients, 1995 to 2003. J Acquir Immune Defic Syndr. 2005 Sep 1;40(1):70-6. | PubMed | Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013 Apr 30;(4):CD005175. | CrossRef | PubMed |
Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013 Apr 30;(4):CD005175. | CrossRef | PubMed | Badowski ME, Perez SE. Clinical utility of dronabinol in the treatment of weight loss associated with HIV and AIDS. HIV AIDS (Auckl). 2016 Feb 10;8:37-45. | CrossRef | PubMed | PMC |
Badowski ME, Perez SE. Clinical utility of dronabinol in the treatment of weight loss associated with HIV and AIDS. HIV AIDS (Auckl). 2016 Feb 10;8:37-45. | CrossRef | PubMed | PMC | Mücke M, Carter C, Cuhls H, Prüß M, Radbruch L, Häuser W. [Cannabinoids in palliative care: Systematic review and meta-analysis of efficacy, tolerability and safety]. Schmerz. 2016 Feb;30(1):25-36. | CrossRef | PubMed |
Mücke M, Carter C, Cuhls H, Prüß M, Radbruch L, Häuser W. [Cannabinoids in palliative care: Systematic review and meta-analysis of efficacy, tolerability and safety]. Schmerz. 2016 Feb;30(1):25-36. | CrossRef | PubMed | Bagshaw SM, Hagen NA. Medical efficacy of cannabinoids and marijuana: a comprehensive review of the literature. J Palliat Care. 2002 Summer;18(2):111-22. | PubMed |
Bagshaw SM, Hagen NA. Medical efficacy of cannabinoids and marijuana: a comprehensive review of the literature. J Palliat Care. 2002 Summer;18(2):111-22. | PubMed | Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25. | PubMed |
Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25. | PubMed | Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. Review. Erratum in: JAMA. 2016 Apr 12;315(14):1522. JAMA. 2015 Dec 1;314(21):2308. JAMA. 2015 Aug 4;314(5):520. JAMA. 2015 Aug 25;314(8):837. | CrossRef | PubMed |
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. Review. Erratum in: JAMA. 2016 Apr 12;315(14):1522. JAMA. 2015 Dec 1;314(21):2308. JAMA. 2015 Aug 4;314(5):520. JAMA. 2015 Aug 25;314(8):837. | CrossRef | PubMed | Badowski M, Pandit NS. Pharmacologic management of human immunodeficiency virus wasting syndrome. Pharmacotherapy. 2014 Aug;34(8):868-81. | CrossRef | PubMed |
Badowski M, Pandit NS. Pharmacologic management of human immunodeficiency virus wasting syndrome. Pharmacotherapy. 2014 Aug;34(8):868-81. | CrossRef | PubMed | Hazekamp, A , Grotenhermen, F. Review on clinical studies with cannabis and cannabinoids 2005-2009. Cannabinoids. 2010 Feb 13; 5 (special issue):1-21. | Link |
Hazekamp, A , Grotenhermen, F. Review on clinical studies with cannabis and cannabinoids 2005-2009. Cannabinoids. 2010 Feb 13; 5 (special issue):1-21. | Link | Whiting P, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes C, et al. Systematic Review of Cannabis for Medical Use. N.p., 2014. [on line] | Link |
Whiting P, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes C, et al. Systematic Review of Cannabis for Medical Use. N.p., 2014. [on line] | Link | Struwe M, Kaempfer SH, Geiger CJ, Pavia AT, Plasse TF, Shepard KV, Ries K, Evans TG. Effect of dronabinol on nutritional status in HIV infection. Ann Pharmacother. 1993 Jul-Aug;27(7-8):827-31. | PubMed |
Struwe M, Kaempfer SH, Geiger CJ, Pavia AT, Plasse TF, Shepard KV, Ries K, Evans TG. Effect of dronabinol on nutritional status in HIV infection. Ann Pharmacother. 1993 Jul-Aug;27(7-8):827-31. | PubMed | Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995 Feb;10(2):89-97. | PubMed |
Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995 Feb;10(2):89-97. | PubMed | Timpone JG, Wright DJ, Li N, Egorin MJ, Enama ME, Mayers J, Galetto G. The safety and pharmacokinetics of single-agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome. The DATRI 004 Study Group. Division of AIDS Treatment Research Initiative. AIDS Res Hum Retroviruses. 1997 Mar 1;13(4):305-15. | PubMed |
Timpone JG, Wright DJ, Li N, Egorin MJ, Enama ME, Mayers J, Galetto G. The safety and pharmacokinetics of single-agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome. The DATRI 004 Study Group. Division of AIDS Treatment Research Initiative. AIDS Res Hum Retroviruses. 1997 Mar 1;13(4):305-15. | PubMed | Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med. 2003 Aug 19;139(4):258-66. | PubMed |
Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med. 2003 Aug 19;139(4):258-66. | PubMed | Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology (Berl). 2005 Aug;181(1):170-8. | PubMed |
Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology (Berl). 2005 Aug;181(1):170-8. | PubMed | Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr. 2007 Aug 15;45(5):545-54. | PubMed |
Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr. 2007 Aug 15;45(5):545-54. | PubMed | Plasse TF, Gorter RW, Krasnow SH, Lane M, Shepard KV, Wadleigh RG. Recent clinical experience with dronabinol. Pharmacol Biochem Behav. 1991 Nov;40(3):695-700. | PubMed |
Plasse TF, Gorter RW, Krasnow SH, Lane M, Shepard KV, Wadleigh RG. Recent clinical experience with dronabinol. Pharmacol Biochem Behav. 1991 Nov;40(3):695-700. | PubMed | Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD, Vosburg SK, Haney M. Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study. Psychopharmacology (Berl). 2010 Dec;212(4):675-86. | CrossRef | PubMed | PMC |
Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD, Vosburg SK, Haney M. Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study. Psychopharmacology (Berl). 2010 Dec;212(4):675-86. | CrossRef | PubMed | PMC | Gorter R, Seefried M, Volberding P. Dronabinol effects on weight in patients with HIV infection. AIDS. 1992 Jan;6(1):127. | PubMed |
Gorter R, Seefried M, Volberding P. Dronabinol effects on weight in patients with HIV infection. AIDS. 1992 Jan;6(1):127. | PubMed | DeJesus E, Rodwick BM, Bowers D, Cohen CJ, Pearce D. Use of Dronabinol Improves Appetite and Reverses Weight Loss in HIV/AIDS-Infected Patients. J Int Assoc Physicians AIDS Care (Chic). 2007 Jun;6(2):95-100. | PubMed |
DeJesus E, Rodwick BM, Bowers D, Cohen CJ, Pearce D. Use of Dronabinol Improves Appetite and Reverses Weight Loss in HIV/AIDS-Infected Patients. J Int Assoc Physicians AIDS Care (Chic). 2007 Jun;6(2):95-100. | PubMed | Watson SJ, Benson JA Jr, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000 Jun;57(6):547-52. | PubMed |
Watson SJ, Benson JA Jr, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000 Jun;57(6):547-52. | PubMed | Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations – 2016 Update. Geneva: World Health Organization; 2016. | PubMed |
Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations – 2016 Update. Geneva: World Health Organization; 2016. | PubMed | Nemechek PM, Polsky B, Gottlieb MS. Treatment guidelines for HIV-associated wasting. Mayo Clin Proc. 2000 Apr;75(4):386-94. | PubMed |
Nemechek PM, Polsky B, Gottlieb MS. Treatment guidelines for HIV-associated wasting. Mayo Clin Proc. 2000 Apr;75(4):386-94. | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








