Abstract
INTRODUCTION
Cannabinoids have been postulated as an alternative for anorexia nervosa. However, their actual clinical efficacy and safety are still discussed.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified four systematic reviews including two primary studies, both corresponding to randomized trials. We concluded cannabinoids might not increase weight or improve symptoms in anorexia nervosa, and are probably associated to frequent adverse effects.
Problem
Anorexia nervosa is an eating disorder characterized by distortion in body image perception leading to weight loss and malnutrition. It causes important work, social and family dysfunction, and in severe cases it can be fatal.
Cannabinoids, by their effects as appetite stimulants would promote weight gain and so constitute a therapeutic alternative in anorexia nervosa. However, its clinical effects and safety still generate controversy among patients and clinicians.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found four systematic reviews [1],[2],[3],[4] which included two primary studies that answered the question of interest, reported in three references [5],[6],[7]. Both studies correspond to randomized trials. |
|
What types of patients were included* |
One trial [5] included 11 women with a primary diagnosis of anorexia nervosa in a hospital setting. The average age was 23.6 years. The diagnosis of anorexia nervosa was made based on the Feighner criteria. All patients had amenorrhea and had lost at least 25% of their weight but were not below 15% of ideal weight **. The other trial [6] included 25 women aged over 18 years, diagnosed with DSM-IV anorexia nervosa for at least 5 years. Patients were considered in ambulatory and hospitalized setting. |
|
What types of interventions were included* |
In one trial [5] THC (delta-9-tetrahydrocannabinol) was used orally in doses between 2.5 and 10 mg three times daily. The control group received an active placebo (oral diazepam between 3 and 15 mg three times daily). In addition to the pharmacological treatment under study, patients received individual psychotherapy twice a week, group therapy once a week, and nasogastric tube feeding occasionally **. In another trial [6] dronabinol 2.5 mg twice daily was compared orally versus placebo.In both groups, the regular baseline treatment was maintained. |
|
What types of outcomes |
The main outcome used by systematic reviews was weight gain measured in kilograms [5],[6].
Other outcomes evaluated were the presence of adverse effects, such as sleep disturbance, paranoia and dysphoria. Follow-up was performed in one trial for 4 weeks [5] and for 8 weeks in the other trial [6]. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
** Information obtained directly from the primary study, because information provided by systematic reviews was incomplete.
Summary of findings
The information on the effects of cannabinoids for anorexia nervosa is based on two trials that included a total of 36 patients [5],[6],[7].
The trials measured weight and symptoms based on clinical efficacy scales: HSCL-90, GAAQ, SDS, and PRS. The summary of findings is the following:
- Cannabinoids might not increase weight in anorexia nervosa. The certainty of the evidence is low.
- Cannabinoids might not improve symptoms in anorexia nervosa. The certainty of the evidence is low.
- Cannabinoids are probably associated to frequent adverse effects in patients with anorexia nervosa. The certainty of the evidence is moderate.
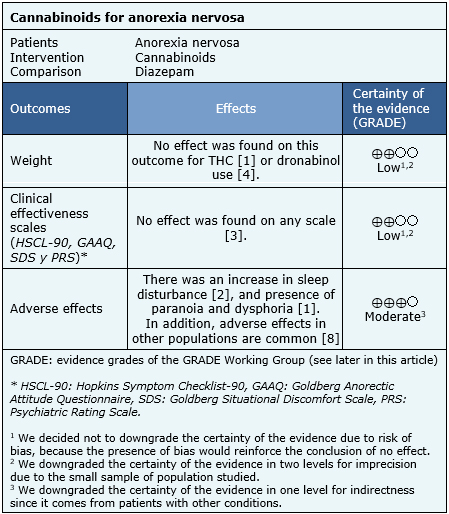
Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF)

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
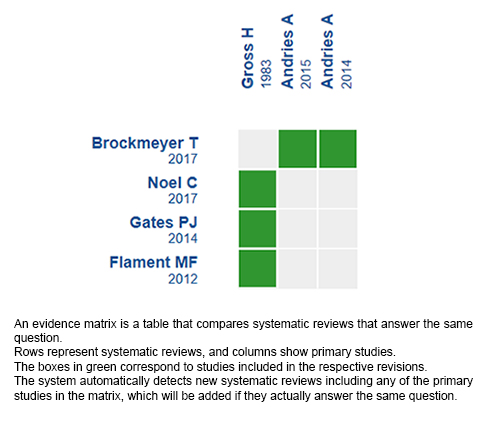
Follow the link to access the interactive version: Cannabinoids for anorexia nervosa
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
Se ha planteado que la estimulación del apetito con cannabinoides podría constituir una alternativa terapéutica en anorexia nerviosa. Sin embargo, su utilidad clínica y seguridad genera controversia.
MÉTODOS
Para responder esta pregunta utilizamos Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante búsquedas en múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, reanalizamos los datos de los estudios primarios, preparamos tablas de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Identificamos cuatro revisiones sistemáticas que en conjunto incluyen dos estudios primarios, ambos correspondientes a ensayos aleatorizados. Concluimos que los cannabinoides podrían no aumentar el peso ni mejorar la sintomatología en la anorexia nerviosa, y se asocian a efectos adversos frecuentes.
 Authors:
Tania Contreras[1,2], Gonzalo A Bravo-Soto[2,4], Gabriel Rada[2,3,4,5,6]
Authors:
Tania Contreras[1,2], Gonzalo A Bravo-Soto[2,4], Gabriel Rada[2,3,4,5,6]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Medicina Interna, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[4] Centro Evidencia UC, Escuela de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[5] The Cochrane Collaboration
[6] GRADE working group
E-mail: radagabriel@epistemonikos.org
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Centro de Innovación UC Anacleto Angelini Avda.Vicuña Mackenna 4860 Macul Santiago Chile

Citation: Contreras T, Bravo-Soto GA, Rada G. Do cannabinoids constitute a therapeutic alternative for anorexia nervosa? . Medwave 2017 Nov-Dic;17(9):e7096 doi: 10.5867/medwave.2017.09.7095
Submission date: 14/8/2017
Acceptance date: 24/11/2017
Publication date: 1/12/2017
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Flament MF, Bissada H, Spettigue W. Evidence-based pharmacotherapy of eating disorders. International Journal of Neuropsychopharmacology. 2012;15(2):189-207. | Link |
- Gates PJ, Albertella L, Copeland J. The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep medicine reviews. 2014;18(6):477-487. | Link |
- Noel C. Evidence for the use of “medical marijuana” in psychiatric and neurologic disorders. Mental Health Clinician. 2017;7(1):29-38. | Link |
- Brockmeyer T, Friederich HC, Schmidt U. Advances in the treatment of anorexia nervosa: a review of established and emerging interventions. Psychol Med. 2017 Sep 11:1-37. | CrossRef | PubMed |
- Gross H, Ebert MH, Faden VB, Goldberg SC, Kaye WH, Caine ED, Hawks R, Zinberg N. A double-blind trial of delta 9-tetrahydrocannabinol in primary anorexia nervosa. J Clin Psychopharmacol. 1983 Jun;3(3):165-71 | PubMed |
- Andries A, Frystyk J, Flyvbjerg A, Støving RK. Dronabinol in severe, enduring anorexia nervosa: a randomized controlled trial. Int J Eat Disord. 2014 Jan;47(1):18-23. | CrossRef | PubMed |
- Andries A, Gram B, Støving RK. Effect of dronabinol therapy on physical activity in anorexia nervosa: a randomised, controlled trial. Eat Weight Disord. 2015 Mar;20(1):13-21 | CrossRef | PubMed |
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. | CrossRef | PubMed |
- National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press. 2017. | CrossRef |
 Flament MF, Bissada H, Spettigue W. Evidence-based pharmacotherapy of eating disorders. International Journal of Neuropsychopharmacology. 2012;15(2):189-207. | Link |
Flament MF, Bissada H, Spettigue W. Evidence-based pharmacotherapy of eating disorders. International Journal of Neuropsychopharmacology. 2012;15(2):189-207. | Link | Gates PJ, Albertella L, Copeland J. The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep medicine reviews. 2014;18(6):477-487. | Link |
Gates PJ, Albertella L, Copeland J. The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep medicine reviews. 2014;18(6):477-487. | Link | Noel C. Evidence for the use of “medical marijuana” in psychiatric and neurologic disorders. Mental Health Clinician. 2017;7(1):29-38. | Link |
Noel C. Evidence for the use of “medical marijuana” in psychiatric and neurologic disorders. Mental Health Clinician. 2017;7(1):29-38. | Link | Brockmeyer T, Friederich HC, Schmidt U. Advances in the treatment of anorexia
nervosa: a review of established and emerging interventions. Psychol Med. 2017
Sep 11:1-37.
| CrossRef | PubMed |
Brockmeyer T, Friederich HC, Schmidt U. Advances in the treatment of anorexia
nervosa: a review of established and emerging interventions. Psychol Med. 2017
Sep 11:1-37.
| CrossRef | PubMed | Gross H, Ebert MH, Faden VB, Goldberg SC, Kaye WH, Caine ED, Hawks R, Zinberg N. A double-blind trial of delta 9-tetrahydrocannabinol in primary anorexia nervosa. J Clin Psychopharmacol. 1983 Jun;3(3):165-71 | PubMed |
Gross H, Ebert MH, Faden VB, Goldberg SC, Kaye WH, Caine ED, Hawks R, Zinberg N. A double-blind trial of delta 9-tetrahydrocannabinol in primary anorexia nervosa. J Clin Psychopharmacol. 1983 Jun;3(3):165-71 | PubMed | Andries A, Frystyk J, Flyvbjerg A, Støving RK. Dronabinol in severe, enduring anorexia nervosa: a randomized controlled trial. Int J Eat Disord. 2014 Jan;47(1):18-23.
| CrossRef | PubMed |
Andries A, Frystyk J, Flyvbjerg A, Støving RK. Dronabinol in severe, enduring anorexia nervosa: a randomized controlled trial. Int J Eat Disord. 2014 Jan;47(1):18-23.
| CrossRef | PubMed | Andries A, Gram B, Støving RK. Effect of dronabinol therapy on physical activity in anorexia nervosa: a randomised, controlled trial. Eat Weight Disord. 2015 Mar;20(1):13-21
| CrossRef | PubMed |
Andries A, Gram B, Støving RK. Effect of dronabinol therapy on physical activity in anorexia nervosa: a randomised, controlled trial. Eat Weight Disord. 2015 Mar;20(1):13-21
| CrossRef | PubMed | Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. | CrossRef | PubMed |
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. | CrossRef | PubMed | National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press. 2017. | CrossRef |
National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press. 2017. | CrossRef |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








