Abstract
INTRODUCTION: Many osteoarthritis patients continue to present symptoms despite nonsurgical treatment. Duloxetine might be a viable alternative for such cases, but real clinical relevance remains unclear. METHODS: A literature review was conducted in Epistemonikos, the largest database for systematic reviews in health that compiles multiple sources, including MEDLINE, EMBASE, and Cochrane, among others. Relevant data were extracted, and information from the primary studies was reanalyzed. A subsequent meta-analysis was conducted, and summary of findings tables were constructed using the GRADE methodology. RESULTS AND CONCLUSIONS: Four systematic reviews including four randomized trials, were identified. In conclusion, while duloxetine slightly improves pain and functionality in osteoarthritis patients, its use is associated with frequent adverse side effects. Therefore, the benefit/risk balance appears unfavorable.
Problem
Osteoarthritis is a highly prevalent disease worldwide and a frequent cause for visits to both primary care and specialists. The persistence of pain despite nonsurgical treatment is one reason for such consults, and no clearly established alternatives exist for pain management.
Duloxetine has been used to treat various conditions with chronic pain, since it exerts central inhibitory effects, so it might be a therapeutic alternative for osteoarthritis with persistent pain after regular treatment. However, duloxetine is associated with adverse side effects, including fatigue, drowsiness, constipation, and high blood pressure.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found four systematic reviews [1],[2],[3],[4] including four primary studies, reported in six references [5],[6],[7],[8],[9],[10]. All correspond to randomized controlled trials. |
|
What types of patients were included* |
Three trials focused on osteoarthritis of the knee [5],[6],[8] and one did not specify it [7]. Average WOMAC (The Western Ontario and McMaster Universities Osteoarthritis Index) ranged between 50 and 57 in three trials [5],[6],[8] and one did not report it [7]. Average age of the participants ranged between 61 and 69 years in the different trials. The proportion of women was between 16 and 77% in the different trials. |
|
What types of interventions were included* |
All of the trials used duloxetine in oral presentation. One trial used 60 mg/day as starting dose, which was increased to 120 mg/day at the seventh week in patients that reported a reduction in pain less than 30% [5]. One trial used 60 mg [6]. One trial used a flexible dosage between 60 and 120 mg per day, keeping a baseline treatment with NSAIDs at therapeutical dose [7]. One trial had two active arms, with 60 mg and 120 mg per day [8].** All trials allowed the use of concomitant analgesia. All trials compared against placebo. |
|
What types of outcomes |
The outcomes, as classified in the identified systematic reviews, were as follows: effect on the intensity of pain (analyzed as a decrease or improvement); overall impression of the patient-reported improvement; sub-scale of physical functionality established by the Western Ontario and McMaster Universities Arthritis Index (WOMAC); and adverse side effects. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
** This information was directly obtained from the primary studies.
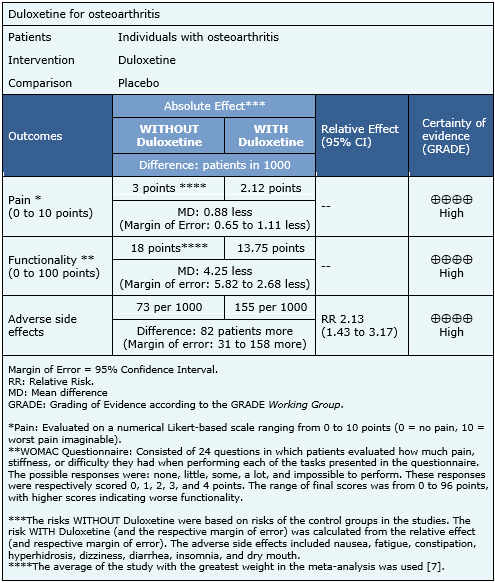
Summary of Findings
The information on the effects of duloxetine was based on three randomized trials that included 1011 patients [5],[7],[8]. The remaining trials were not included in the meta-analysis as none of the identified systematic reviews extracted sufficient trial data. The three evaluated trials reported improvements in pain and functionality, and adverse side effects. The summary of findings is as follows:
- Duloxetine slightly decreases pain. The certainty of the evidence is high.
- Duloxetine slightly improves functionality in osteoarthritis. The certainty of the evidence is high.
- Duloxetine is associated with frequent side effects. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
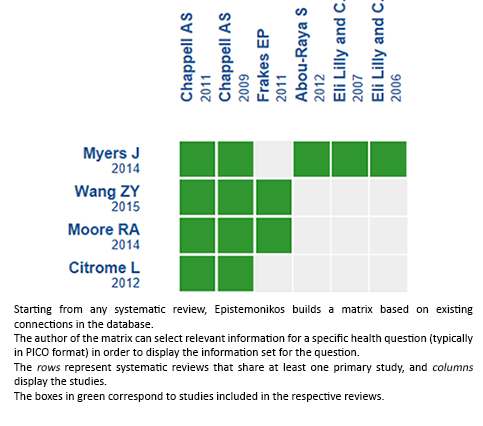
Follow the link to access the interactive version: Duloxetine for osteoarthritis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN: Muchos pacientes con artrosis persisten con síntomas a pesar del tratamiento no quirúrgico. Se ha planteado el uso de duloxetina en estos casos, sin embargo, no está clara su real utilidad clínica. MÉTODOS: Para responder esta pregunta utilizamos Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante búsquedas en múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, reanalizamos los datos de los estudios primarios, realizamos un metanálisis y preparamos tablas de resumen de los resultados utilizando el método GRADE. RESULTADOS Y CONCLUSIONES: Identificamos cuatro revisiones sistemáticas que en conjunto incluyen cuatro estudios aleatorizados. Concluimos que la duloxetina produce una leve mejoría del dolor y la funcionalidad en pacientes con artrosis, pero se asocia a efectos adversos frecuentes, por lo que el balance entre beneficios y riesgos probablemente no es favorable.
 Authors:
Joaquín Ananías[1,2], Sebastián Irarrázaval[2,3]
Authors:
Joaquín Ananías[1,2], Sebastián Irarrázaval[2,3]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Traumatología y Ortopedia, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: sirarraz@med.puc.cl
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Centro de Innovación UC Anacleto Angelini Avda.Vicuña Mackenna 4860 Macul Santiago Chile

Citation: Ananías J, Irarrázaval S. Is duloxetine an alternative in the treatment of osteoarthritis?. Medwave 2017 Sep-Oct; 17(8):e7063 doi: 10.5867/medwave.2017.08.7063
Submission date: 5/9/2017
Acceptance date: 11/10/2017
Publication date: 18/10/2017
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Myers J, Wielage RC, Han B, Price K, Gahn J, Paget MA, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014 Mar 11;15:76. | CrossRef | PubMed |
- Wang ZY, Shi SY, Li SJ, Chen F, Chen H, Lin HZ, et al. Efficacy and Safety of Duloxetine on Osteoarthritis Knee Pain: A Meta-Analysis of Randomized Controlled Trials. Pain Med. 2015 Jul;16(7):1373-85. | CrossRef | PubMed |
- Citrome L, Weiss-Citrome A. A systematic review of duloxetine for osteoarthritic pain: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed? Postgrad Med. 2012 Jan;124(1):83-93. | CrossRef | PubMed |
- Moore RA, Cai N, Skljarevski V, Tölle TR. Duloxetine use in chronic painful conditions--individual patient data responder analysis. Eur J Pain. 2014 Jan;18(1):67-75. | CrossRef | PubMed |
- Chappell AS, Desaiah D, Liu-Seifert H, Zhang S, Skljarevski V, Belenkov Y, et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Pract. 2011 Jan-Feb;11(1):33-41. | CrossRef | PubMed |
- Abou-Raya S, Abou-Raya A, Helmii M. Duloxetine for the management of pain in older adults with knee osteoarthritis: randomised placebo-controlled trial. Age Ageing. 2012 Sep;41(5):646-52. | CrossRef | PubMed |
- Frakes EP, Risser RC, Ball TD, Hochberg MC, Wohlreich MM. Duloxetine added to oral nonsteroidal anti-inflammatory drugs for treatment of knee pain due to osteoarthritis: results of a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin. 2011 Dec;27(12):2361-72. | CrossRef | PubMed |
- Chappell AS, Ossanna MJ, Liu-Seifert H, Iyengar S, Skljarevski V, Li LC, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009 Dec;146(3):253-60. | CrossRef | PubMed |
- Eli Lilly and Company. Duloxetine vs. Placebo in the Treatment of Osteoarthritis Knee Pain. 2007 | Link |
- Eli Lilly and Company. Duloxetine Versus Placebo for Osteoarthritis Knee Pain. 2006 | Link |
- COMET Initiative. Defining an International Standard Set of Outcome Measures for Patients With Hip or Knee Osteoarthritis: Consensus of the International Consortium for Health Outcomes Measurement Hip and Knee Osteoarthritis Working Group. Arthritis Care and Research. 2016;68(11):1631-39. | CrossRef |
- Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, Bombardier C, Felson D, Hochberg M, van der Heijde D, Dougados M. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005 Jan;64(1):29-33.
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014 Mar;22(3):363-88.
- Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013 Sep;21(9):577-9.
- Wang G, Bi L, Li X, Li Z, Zhao D, Chen J, He D, Wang CN, Dueñas H, Skljarevski V, Yue L. Efficacy and safety of duloxetine in Chinese patients with chronic pain due to osteoarthritis: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage. 2017 Jun;25(6):832-838. | CrossRef |
 Myers J, Wielage RC, Han B, Price K, Gahn J, Paget MA, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014 Mar 11;15:76. | CrossRef | PubMed |
Myers J, Wielage RC, Han B, Price K, Gahn J, Paget MA, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014 Mar 11;15:76. | CrossRef | PubMed | Wang ZY, Shi SY, Li SJ, Chen F, Chen H, Lin HZ, et al. Efficacy and Safety of Duloxetine on Osteoarthritis Knee Pain: A Meta-Analysis of Randomized Controlled Trials. Pain Med. 2015 Jul;16(7):1373-85. | CrossRef | PubMed |
Wang ZY, Shi SY, Li SJ, Chen F, Chen H, Lin HZ, et al. Efficacy and Safety of Duloxetine on Osteoarthritis Knee Pain: A Meta-Analysis of Randomized Controlled Trials. Pain Med. 2015 Jul;16(7):1373-85. | CrossRef | PubMed | Citrome L, Weiss-Citrome A. A systematic review of duloxetine for osteoarthritic pain: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed? Postgrad Med. 2012 Jan;124(1):83-93. | CrossRef | PubMed |
Citrome L, Weiss-Citrome A. A systematic review of duloxetine for osteoarthritic pain: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed? Postgrad Med. 2012 Jan;124(1):83-93. | CrossRef | PubMed | Moore RA, Cai N, Skljarevski V, Tölle TR. Duloxetine use in chronic painful conditions--individual patient data responder analysis. Eur J Pain. 2014 Jan;18(1):67-75. | CrossRef | PubMed |
Moore RA, Cai N, Skljarevski V, Tölle TR. Duloxetine use in chronic painful conditions--individual patient data responder analysis. Eur J Pain. 2014 Jan;18(1):67-75. | CrossRef | PubMed | Chappell AS, Desaiah D, Liu-Seifert H, Zhang S, Skljarevski V, Belenkov Y, et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Pract. 2011 Jan-Feb;11(1):33-41. | CrossRef | PubMed |
Chappell AS, Desaiah D, Liu-Seifert H, Zhang S, Skljarevski V, Belenkov Y, et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Pract. 2011 Jan-Feb;11(1):33-41. | CrossRef | PubMed | Abou-Raya S, Abou-Raya A, Helmii M. Duloxetine for the management of pain in older adults with knee osteoarthritis: randomised placebo-controlled trial. Age Ageing. 2012 Sep;41(5):646-52. | CrossRef | PubMed |
Abou-Raya S, Abou-Raya A, Helmii M. Duloxetine for the management of pain in older adults with knee osteoarthritis: randomised placebo-controlled trial. Age Ageing. 2012 Sep;41(5):646-52. | CrossRef | PubMed | Frakes EP, Risser RC, Ball TD, Hochberg MC, Wohlreich MM. Duloxetine added to oral nonsteroidal anti-inflammatory drugs for treatment of knee pain due to osteoarthritis: results of a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin. 2011 Dec;27(12):2361-72. | CrossRef | PubMed |
Frakes EP, Risser RC, Ball TD, Hochberg MC, Wohlreich MM. Duloxetine added to oral nonsteroidal anti-inflammatory drugs for treatment of knee pain due to osteoarthritis: results of a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin. 2011 Dec;27(12):2361-72. | CrossRef | PubMed | Chappell AS, Ossanna MJ, Liu-Seifert H, Iyengar S, Skljarevski V, Li LC, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009 Dec;146(3):253-60. | CrossRef | PubMed |
Chappell AS, Ossanna MJ, Liu-Seifert H, Iyengar S, Skljarevski V, Li LC, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009 Dec;146(3):253-60. | CrossRef | PubMed | Eli Lilly and Company. Duloxetine vs. Placebo in the Treatment of Osteoarthritis Knee Pain. 2007 | Link |
Eli Lilly and Company. Duloxetine vs. Placebo in the Treatment of Osteoarthritis Knee Pain. 2007 | Link | COMET Initiative. Defining an International Standard Set of Outcome Measures for Patients With Hip or Knee Osteoarthritis: Consensus of the International Consortium for Health Outcomes Measurement Hip and Knee Osteoarthritis Working Group. Arthritis Care and Research. 2016;68(11):1631-39. | CrossRef |
COMET Initiative. Defining an International Standard Set of Outcome Measures for Patients With Hip or Knee Osteoarthritis: Consensus of the International Consortium for Health Outcomes Measurement Hip and Knee Osteoarthritis Working Group. Arthritis Care and Research. 2016;68(11):1631-39. | CrossRef | Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, Bombardier C, Felson D, Hochberg M, van der Heijde D, Dougados M. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005 Jan;64(1):29-33.
Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, Bombardier C, Felson D, Hochberg M, van der Heijde D, Dougados M. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005 Jan;64(1):29-33.  McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014 Mar;22(3):363-88.
McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014 Mar;22(3):363-88.  Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013 Sep;21(9):577-9.
Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013 Sep;21(9):577-9.  Wang G, Bi L, Li X, Li Z, Zhao D, Chen J, He D, Wang CN, Dueñas H, Skljarevski V, Yue L. Efficacy and safety of duloxetine in Chinese patients with chronic pain due to osteoarthritis: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage. 2017 Jun;25(6):832-838. | CrossRef |
Wang G, Bi L, Li X, Li Z, Zhao D, Chen J, He D, Wang CN, Dueñas H, Skljarevski V, Yue L. Efficacy and safety of duloxetine in Chinese patients with chronic pain due to osteoarthritis: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage. 2017 Jun;25(6):832-838. | CrossRef |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








