Abstract
Psoriasis is a frequent chronic inflammatory disease. The plaque variant being its most common form of presentation. Although there is still no cure, treatment alternatives that induce remission and reduce lesions are available. Topical therapies, particularly corticosteroids and vitamin D analogues, are considered effective, but it is still not clear which would be the best alternative. To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified eight systematic reviews including 26 studies overall, of which 22 were randomized trials relevant for the question of interest. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded there might be little or no difference in clinical response between topical corticosteroids and topical vitamin D analogues, but topical corticosteroids are less irritating at the site of application. No studies evaluating their long term adverse effects were found.
Problem
Psoriasis is a systemic inflammatory disease, where skin is the most commonly affected organ. Although there is still no cure, there is a wide variety of treatments available that can induce remission and reduce lesions. In daily practice different alternatives are used, and therapy is adjusted according to clinical response. The most commonly used drugs, due to their greater availability, ease of application and lower costs are topical medications. Within these, corticosteroids are the most used. Even though there are many available alternatives such as tazarotene, tacrolimus, anthralin, UV light, among others, vitamin D analogues are frequently chosen. Despite vast experience with these topical therapies, there is stilll controversy about their effects.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found eight systematic reviews, reported in nine references [1],[2],[3],[4],[5],[6],[7],[8],[9], that include 26 primary studies, reported in 32 references [10],[11], |
|
What types of patients were included* |
All of the trials included adult patients (15 to 90 years) with plaque psoriasis in trunk and limbs, not including scalp. Trials includes patients with low, moderate and severe disease. |
|
What types of interventions were included* |
All trials used topical corticosteroids as intervention. Within them, some of high potency were used such as fluocinonide 0.05% twice a day [11], betamethasone dipropionate 0.05% once, or twice a day [13],[16],[32], [40], betamethasone 17-valerate 0.1% once [37],[39] or twice a day [15],[23],[26],[29],[41] and desoxymethasone 0.25% twice a day [21]. Also, some corticosteroids of very high potency were used such as clobetasol propionate 0.05% twice a day [22],[24] and diflorasone diacetate 0.05% twice a day [28]. As a comparison, topical treatment with a vitamin D analogue was used, including calcipotriol 50 mcg/g once [17],[20] or twice a day [11],[15],[16],[21],[22],[23],[24],[28],[29],[32],[40],[41], calcitriol 3 mcg/g twice a day [13],[26] and tacalcitol |
|
What types of outcomes |
The outcomes were pooled by the different systematic reviews as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
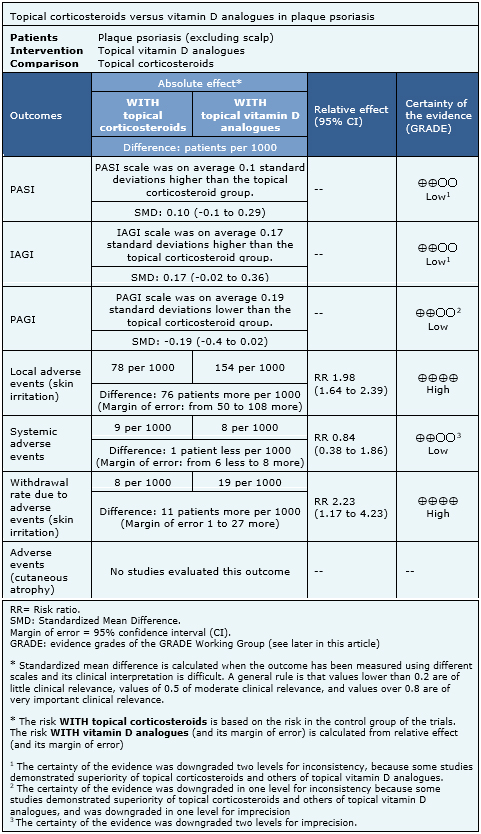
Information on the effects of topical corticosteroids versus vitamin D analogues is based on 15 randomized trials [11],[13],[15],[17],[20],[22],[23],[24],[26],[28],[29],[32],[37],[39],[41] that included 4238 patients overall. Nine trials reported PASI [13],[15],[17],[20],[23],[24],[29],[32],[41], nine reported IAGI [11],[13],[17],[20],[22],
- There might be little or no difference in PASI score between topical corticosteroids and topical vitamin D analogues. The certainty of the evidence is low.
- There might be little or no difference IAGI score between topical corticosteroids and topical vitamin D analogues. The certainty of the evidence is low.
- There might be little or no difference in PAGI score between topical corticosteroids and topical vitamin D analogues. The certainty of the evidence is low.
- Topical corticosteroids lead to fewer local adverse events (skin irritation) than topical vitamin D analogues. The certainty of the evidence is high.
- There might be little or no difference in systemic adverse events between topical corticosteroids and topical vitamin D analogues. The certainty of the evidence is low.
- Topical corticosteroids lead to fewer withdrawals due to adverse events than topical vitamin D analogues. The certainty of the evidence is high.
- No studies were found that evaluated the impact of topical corticosteroids and topical vitamin D analogues in cutaneous atrophy.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
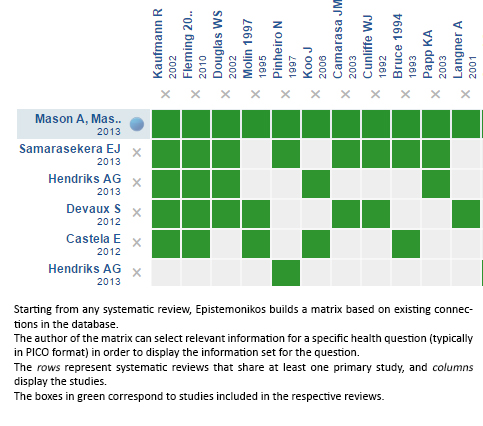
Follow the link to access the interactive version: Topical corticosteroids versus vitamin D analogues in plaque psoriasis excluding scalp psoriasis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrices and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

La psoriasis es una enfermedad inflamatoria crónica frecuente, siendo la variante en placa su forma de presentación más común. Si bien aún no existe una cura, se dispone de medicamentos que inducen remisión y disminuyen las lesiones. Las terapias tópicas, en particular los corticoides y los análogos de vitamina D, se consideran efectivos, pero no está claro cuál de ellos constituiría la mejor alternativa. Para responder esta pregunta utilizamos Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante búsquedas en múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Identificamos ocho revisiones sistemáticas que en conjunto incluyen 26 estudios pertinentes a esta pregunta, entre ellos 22 ensayos aleatorizados. Extrajimos los datos desde las revisiones identificadas, reanalizamos los datos de los estudios primarios, realizamos un metanálisis y preparamos tablas de resumen de los resultados utilizando el método GRADE. Concluimos que podría existir poca o nula diferencia en la respuesta clínica entre corticoides tópicos y análogos de vitamina D tópicos. Por otra parte, los corticoides tópicos producen menos irritación en el sitio de aplicación, pero no se encontraron estudios evaluando sus efectos adversos a largo plazo.
 Authors:
Soledad Venegas-Iribarren[1,2], Romina Andino[2,3]
Authors:
Soledad Venegas-Iribarren[1,2], Romina Andino[2,3]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Dermatología, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: rominaandino@gmail.com
Author address:
[1] Facultad de Medicina Pontificia Universidad Católica de Chile Diagonal Paraguay 362 Santiago Centro Chile.

Citation: Venegas-Iribarren S, Andino R. Topical corticosteroids or vitamin D analogues for plaque psoriasis?. Medwave 2016; 16(Suppl2):e6981 doi: 10.5867/medwave.2017.6981
Submission date: 5/6/2017
Acceptance date: 19/6/2017
Publication date: 27/6/2017

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Castela E, Archier E, Devaux S, Gallini A, Aractingi S, Cribier B, et al. Topical corticosteroids in plaque psoriasis: a systematic review of efficacy and treatment modalities. J Eur Acad Dermatol Venereol. 2012 May;26 Suppl 3:36-46 | CrossRef | PubMed |
- Castela E, Archier E, Devaux S, Gallini A, Aractingi S, Cribier B, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012 May;26 Suppl 3:47-51 | CrossRef | PubMed |
- Devaux S, Castela A, Archier E, Gallini A, Joly P, Misery L, et al. Topical vitamin D analogues alone or in association with topical steroids for psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2012 May;26 Suppl 3:52-60 | CrossRef | PubMed |
- Hendriks AG, Keijsers RR, de Jong EM, Seyger MM, van de Kerkhof PC. Combinations of classical time-honoured topicals in plaque psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2013 Apr;27(4):399-410 | CrossRef | PubMed |
- Hendriks AG, Keijsers RR, de Jong EM, Seyger MM, van de Kerkhof PC. Efficacy and safety of combinations of first-line topical treatments in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013 Aug;27(8):931-51 | CrossRef | PubMed |
- Mason AR, Mason J, Cork M, Dooley G, Hancock H. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2013 Mar 28;(3):CD005028 | CrossRef | PubMed |
- Mason A, Mason J, Cork M, Hancock H, Dooley G. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013 Nov;69(5):799-807 | CrossRef | PubMed |
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002 Mar;146(3):351-64 | PubMed |
- Samarasekera EJ, Sawyer L, Wonderling D, Tucker R, Smith CH. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br J Dermatol. 2013 May;168(5):954-67 | CrossRef | PubMed |
- Bowman PH, Maloney JE, Koo JY. Combination of calcipotriene (Dovonex) ointment and tazarotene (Tazorac) gel versus clobetasol ointment in the treatment of plaque psoriasis: a pilot study. J Am Acad Dermatol. 2002 Jun;46(6):907-13. | PubMed |
- Bruce S, Epinette WW, Funicella T, Ison A, Jones EL, Loss R Jr, et al. Comparative study of calcipotriene (MC 903) ointment and fluocinonide ointment in the treatment of psoriasis. J Am Acad Dermatol. 1994 Nov;31(5 Pt1):755-9 | PubMed |
- Siskin SB. Efficacy and Safety of Calcipotriol Ointment Compared to Fluocinonide. 2nd International Symposium on Calcipotriol, Monaco. 1993 | Link |
- Camarasa JM, Ortonne JP, Dubertret L. Calcitriol shows greater persistence of treatment effect than betamethasone dipropionate in topical psoriasis therapy. J Dermatolog Treat. 2003 Jan;14(1):8-13 | PubMed |
- Crosti C, Finzi AF, Mian E, Scarpa C. Calcipotriol in psoriasis vulgaris: a controlled trial comparing betamethasone dipropionate + salicylic acid. Int J Dermatol. 1997 Jul;36(7):537-9 | PubMed |
- Cunliffe WJ, Berth-Jones J, Claudy A, Fairiss G, Goldin D, Gratton D, et al. Comparative study of calcipotriol (MC 903) ointment and betamethasone 17-valerate ointment in patients with psoriasis vulgaris. J Am Acad Dermatol. 1992 May;26(5 Pt 1):736-43 | PubMed |
- Douglas WS, Poulin Y, Decroix J, Ortonne JP, Mrowietz U, Gulliver W, et al. A new calcipotriol/betamethasone formulation with rapid onset of action was superior to monotherapy with betamethasone dipropionate or calcipotriol in psoriasis vulgaris. Acta Derm Venereol. 2002;82(2):131-5 | PubMed |
- Fleming C, Ganslandt C, Guenther L, Johannesson A, Buckley C, Simon JC, et al. Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: a randomised, parallel group, double-blind, exploratory study. Eur J Dermatol. 2010 Jul-Aug;20(4):465-71 | CrossRef | PubMed |
- Fleming C, Ganslandt C, Leese GP. Short- and long-term safety assessment of a two-compound ointment containing calcipotriene/betamethasone dipropionate (Taclonex/Daivobet/Dovobet ointment): hypothalamic-pituitary-adrenal axis function in patients with psoriasis vulgaris. J Drugs Dermatol. 2010 Aug;9(8):969-74 | PubMed |
- Guenther LC, Poulin YP, Pariser DM. A comparison of tazarotene 0.1% gel once daily plus mometasone furoate 0.1% cream once daily versus calcipotriene 0.005% ointment twice daily in the treatment of plaque psoriasis. Clin Ther. 2000 Oct;22(10):1225-38 | PubMed |
- Kaufmann R, Bibby AJ, Bissonnette R, Cambazard F, Chu AC, Decroix J, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205(4):389-93 | PubMed |
- Kim Jo Yong, You Young Ho, Kim Tae Yoon, Kim Chung Won. Comparative study of calcipotriol and desoxymethasone ointments in the treatment of psoriasis vulgaris: the clinical effect and immunohistochemical change. Korean Journal of Dermatology. 1994;32(6):1054-1063. | Link |
- https://www.epistemonikos.org/en/documents/d4a2ed5c12e8c842534a3f26099609978d857529 | PubMed |
- Kragballe K, Gjertsen BT, De Hoop D, Karlsmark T, van de Kerkhof PC, Larkö O, et al. Double-blind, right/left comparison of calcipotriol and betamethasone valerate in treatment of psoriasis vulgaris. Lancet. 1991 Jan 26;337(8735):193-6 | PubMed |
- Landi G, PierleoniM, Polverelli M, Fioravanti F. Calcipotriol, a New Topical Product in the Therapy of Psoriasis: Controlled Study Versus Clobetasol. Giornale Italiano di Dermatologia e Venereologia. 1993;128(9):89-93. | Link |
- Landi G. Efficacy and safety of calcipotriol ointment compared to clobetasol ointment in psoriasis vulgaris. 3rd Congress of the European Academy of Dermatology & Venereology. Copenhagen, Denmark. 1993;199 | Link |
- Langner A, Stapór W, Ambroziak M. Efficacy and tolerance of topical calcitriol 3 microg g(-1) in psoriasis treatment: a review of our experience in Poland. Br J Dermatol. 2001 Apr;144 Suppl 58:11-6 | PubMed |
- Lebwohl M, Siskin SB, Epinette W, Breneman D, Funicella T, Kalb R, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996 Aug;35(2 Pt 1):268-9 | PubMed |
- Medansky RS, Greenspan A, Kraus SJ, Todd Plott R. The Comparative Efficacy of Diflorasone Diacetate Ointment 0.05% (Psorcon®) vs Calcipotriene Ointment (Dovonex®) in the Treatment of Psoriasis. Journal of Geriatric Dermatology 1. 1996;4(1):20-24. | Link |
- Molin L, Cutler TP, Helander I, Nyfors B, Downes N. Comparative efficacy of calcipotriol (MC903) cream and betamethasone 17-valerate cream in the treatment of chronic plaque psoriasis. A randomized, double-blind, parallel group multicentre study. Calcipotriol Study Group. Br J Dermatol. 1997 Jan;136(1):89-93 | PubMed |
- Molin L, Cutler TP, Helander I, Nyfors B. Comparative Efficacy of Calcipotriol Cream and Betamethasone Valerate Cream in the Treatment of Psoriasis. Journal of Investigative Dermatology. Symposium Proceedings. 1996;1(1):110. | Link |
- Molin L, Cutler TP, Helander I, Nyfors B. Calcipotriol cream and betamethasone valerate cream of equal efficacy in psoriasis. Journal of the European Academy of Dermatology and Venereology. 1995;5:S92. | Link |
- Papp KA, Guenther L, Boyden B, Larsen FG, Harvima RJ, Guilhou JJ, et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. J Am Acad Dermatol. 2003 Jan;48(1):48-54 | PubMed |
- Pinheiro N. Comparative effects of calcipotriol ointment (50 micrograms/g) and 5% coal tar/2% allantoin/0.5% hydrocortisone cream in treating plaque psoriasis. Br J Clin Pract. 1997 Jan-Feb;51(1):16-9 | PubMed |
- Sander P, Stücker M, Hermes N, Hoffmann K, Altmeyer P. [Mometasone and calcipotriol optimize the initial therapeutic effect of dithranol in chronic persistent psoriasis]. Hautarzt. 1998 Apr;49(4):291-4 | PubMed |
- Sander P, Stücker M, Hoffmann M, Hoffmann K, Altmeyer P. Topical Triple-Therapy of Chronic Stationary Psoriasis (CSP). Increased Healing Effectiveness Compared with a Dithranol Monotherapy. Aktuelle Dermatologie. 2001;27(06):173-177. | Link |
- Scarpa C. Calcipotriol: clinical trial versus betamethasone dipropionate + salicylic acid. Acta Derm Venereol Suppl (Stockh). 1994;186:47 | PubMed |
- Scarpa C. Tacalcitol ointment is an efficacious and well tolerated treatment for psoriasis. Journal of the European Academy of Dermatology and Venereology. 1996;6(2):142-146. | Link |
- Scarpa C, Kokelj F, Plozzer C, Lavaroni G. Efficacy and Tolerability of Tacalcitol Ointment on Psoriatic Skin: Study in 63 Patients. Journal of Investigative Dermatology. Symposium Proceedings 1996. 1996;1(1):110. | Link |
- Seidenari S, Magni R, Giannetti A. Assessment of the Activity of Tacalcitol on Psoriatic Plaques by Means of Colorimetry and High-Frequency Ultrasound: A Double-Blind Intrasubject Half-Side Right-Left Comparison with Betamethasone Valerate and Placebo. Skin Pharmacology and Physiology. 1997;10(1):40-47. | Link |
- van der Velden HM, Pasch MC, van Erp PE, van Lingen RG, Otero ME, de Boer-van Huizen RT, et al. Treatment of plaque psoriasis with the two-compound product calcipotriol/betamethasone dipropionate versus both monotherapies: an immunohistochemical study. J Dermatolog Treat. 2010 Jan;21(1):13-22 | CrossRef | PubMed |
- Vladimirov VV, Tcherjomukchina IG, Kurjanova ON. Efficacy of calcipotriol ointment compared to betamethasone 17-valerate ointment in the treatment of psoriasis. International Meeting Skin Therapy Update. 1994 | Link |
- Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009 Apr;60(4):643-59 | CrossRef |
 Castela E, Archier E, Devaux S, Gallini A, Aractingi S, Cribier B, et al. Topical corticosteroids in plaque psoriasis: a systematic review of efficacy and treatment modalities. J Eur Acad Dermatol Venereol. 2012 May;26 Suppl 3:36-46 | CrossRef | PubMed |
Castela E, Archier E, Devaux S, Gallini A, Aractingi S, Cribier B, et al. Topical corticosteroids in plaque psoriasis: a systematic review of efficacy and treatment modalities. J Eur Acad Dermatol Venereol. 2012 May;26 Suppl 3:36-46 | CrossRef | PubMed | Castela E, Archier E, Devaux S, Gallini A, Aractingi S, Cribier B, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012 May;26 Suppl 3:47-51 | CrossRef | PubMed |
Castela E, Archier E, Devaux S, Gallini A, Aractingi S, Cribier B, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012 May;26 Suppl 3:47-51 | CrossRef | PubMed | Devaux S, Castela A, Archier E, Gallini A, Joly P, Misery L, et al. Topical vitamin D analogues alone or in association with topical steroids for psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2012 May;26 Suppl 3:52-60 | CrossRef | PubMed |
Devaux S, Castela A, Archier E, Gallini A, Joly P, Misery L, et al. Topical vitamin D analogues alone or in association with topical steroids for psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2012 May;26 Suppl 3:52-60 | CrossRef | PubMed | Hendriks AG, Keijsers RR, de Jong EM, Seyger MM, van de Kerkhof PC. Combinations of classical time-honoured topicals in plaque psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2013 Apr;27(4):399-410 | CrossRef | PubMed |
Hendriks AG, Keijsers RR, de Jong EM, Seyger MM, van de Kerkhof PC. Combinations of classical time-honoured topicals in plaque psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2013 Apr;27(4):399-410 | CrossRef | PubMed | Hendriks AG, Keijsers RR, de Jong EM, Seyger MM, van de Kerkhof PC. Efficacy and safety of combinations of first-line topical treatments in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013 Aug;27(8):931-51 | CrossRef | PubMed |
Hendriks AG, Keijsers RR, de Jong EM, Seyger MM, van de Kerkhof PC. Efficacy and safety of combinations of first-line topical treatments in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013 Aug;27(8):931-51 | CrossRef | PubMed | Mason AR, Mason J, Cork M, Dooley G, Hancock H. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2013 Mar 28;(3):CD005028 | CrossRef | PubMed |
Mason AR, Mason J, Cork M, Dooley G, Hancock H. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2013 Mar 28;(3):CD005028 | CrossRef | PubMed | Mason A, Mason J, Cork M, Hancock H, Dooley G. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013 Nov;69(5):799-807 | CrossRef | PubMed |
Mason A, Mason J, Cork M, Hancock H, Dooley G. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013 Nov;69(5):799-807 | CrossRef | PubMed | Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002 Mar;146(3):351-64 | PubMed |
Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002 Mar;146(3):351-64 | PubMed | Samarasekera EJ, Sawyer L, Wonderling D, Tucker R, Smith CH. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br J Dermatol. 2013 May;168(5):954-67 | CrossRef | PubMed |
Samarasekera EJ, Sawyer L, Wonderling D, Tucker R, Smith CH. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br J Dermatol. 2013 May;168(5):954-67 | CrossRef | PubMed | Bowman PH, Maloney JE, Koo JY. Combination of calcipotriene (Dovonex) ointment and tazarotene (Tazorac) gel versus clobetasol ointment in the treatment of plaque psoriasis: a pilot study. J Am Acad Dermatol. 2002 Jun;46(6):907-13. | PubMed |
Bowman PH, Maloney JE, Koo JY. Combination of calcipotriene (Dovonex) ointment and tazarotene (Tazorac) gel versus clobetasol ointment in the treatment of plaque psoriasis: a pilot study. J Am Acad Dermatol. 2002 Jun;46(6):907-13. | PubMed | Bruce S, Epinette WW, Funicella T, Ison A, Jones EL, Loss R Jr, et al. Comparative study of calcipotriene (MC 903) ointment and fluocinonide ointment in the treatment of psoriasis. J Am Acad Dermatol. 1994 Nov;31(5 Pt1):755-9 | PubMed |
Bruce S, Epinette WW, Funicella T, Ison A, Jones EL, Loss R Jr, et al. Comparative study of calcipotriene (MC 903) ointment and fluocinonide ointment in the treatment of psoriasis. J Am Acad Dermatol. 1994 Nov;31(5 Pt1):755-9 | PubMed | Siskin SB. Efficacy and Safety of Calcipotriol Ointment Compared to Fluocinonide. 2nd International Symposium on Calcipotriol, Monaco. 1993 | Link |
Siskin SB. Efficacy and Safety of Calcipotriol Ointment Compared to Fluocinonide. 2nd International Symposium on Calcipotriol, Monaco. 1993 | Link | Camarasa JM, Ortonne JP, Dubertret L. Calcitriol shows greater persistence of treatment effect than betamethasone dipropionate in topical psoriasis therapy. J Dermatolog Treat. 2003 Jan;14(1):8-13 | PubMed |
Camarasa JM, Ortonne JP, Dubertret L. Calcitriol shows greater persistence of treatment effect than betamethasone dipropionate in topical psoriasis therapy. J Dermatolog Treat. 2003 Jan;14(1):8-13 | PubMed | Crosti C, Finzi AF, Mian E, Scarpa C. Calcipotriol in psoriasis vulgaris: a controlled trial comparing betamethasone dipropionate + salicylic acid. Int J Dermatol. 1997 Jul;36(7):537-9 | PubMed |
Crosti C, Finzi AF, Mian E, Scarpa C. Calcipotriol in psoriasis vulgaris: a controlled trial comparing betamethasone dipropionate + salicylic acid. Int J Dermatol. 1997 Jul;36(7):537-9 | PubMed | Cunliffe WJ, Berth-Jones J, Claudy A, Fairiss G, Goldin D, Gratton D, et al. Comparative study of calcipotriol (MC 903) ointment and betamethasone 17-valerate ointment in patients with psoriasis vulgaris. J Am Acad Dermatol. 1992 May;26(5 Pt 1):736-43 | PubMed |
Cunliffe WJ, Berth-Jones J, Claudy A, Fairiss G, Goldin D, Gratton D, et al. Comparative study of calcipotriol (MC 903) ointment and betamethasone 17-valerate ointment in patients with psoriasis vulgaris. J Am Acad Dermatol. 1992 May;26(5 Pt 1):736-43 | PubMed | Douglas WS, Poulin Y, Decroix J, Ortonne JP, Mrowietz U, Gulliver W, et al. A new calcipotriol/betamethasone formulation with rapid onset of action was superior to monotherapy with betamethasone dipropionate or calcipotriol in psoriasis vulgaris. Acta Derm Venereol. 2002;82(2):131-5 | PubMed |
Douglas WS, Poulin Y, Decroix J, Ortonne JP, Mrowietz U, Gulliver W, et al. A new calcipotriol/betamethasone formulation with rapid onset of action was superior to monotherapy with betamethasone dipropionate or calcipotriol in psoriasis vulgaris. Acta Derm Venereol. 2002;82(2):131-5 | PubMed | Fleming C, Ganslandt C, Guenther L, Johannesson A, Buckley C, Simon JC, et al. Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: a randomised, parallel group, double-blind, exploratory study. Eur J Dermatol. 2010 Jul-Aug;20(4):465-71 | CrossRef | PubMed |
Fleming C, Ganslandt C, Guenther L, Johannesson A, Buckley C, Simon JC, et al. Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: a randomised, parallel group, double-blind, exploratory study. Eur J Dermatol. 2010 Jul-Aug;20(4):465-71 | CrossRef | PubMed | Fleming C, Ganslandt C, Leese GP. Short- and long-term safety assessment of a two-compound ointment containing calcipotriene/betamethasone dipropionate (Taclonex/Daivobet/Dovobet ointment): hypothalamic-pituitary-adrenal axis function in patients with psoriasis vulgaris. J Drugs Dermatol. 2010 Aug;9(8):969-74 | PubMed |
Fleming C, Ganslandt C, Leese GP. Short- and long-term safety assessment of a two-compound ointment containing calcipotriene/betamethasone dipropionate (Taclonex/Daivobet/Dovobet ointment): hypothalamic-pituitary-adrenal axis function in patients with psoriasis vulgaris. J Drugs Dermatol. 2010 Aug;9(8):969-74 | PubMed | Guenther LC, Poulin YP, Pariser DM. A comparison of tazarotene 0.1% gel once daily plus mometasone furoate 0.1% cream once daily versus calcipotriene 0.005% ointment twice daily in the treatment of plaque psoriasis. Clin Ther. 2000 Oct;22(10):1225-38 | PubMed |
Guenther LC, Poulin YP, Pariser DM. A comparison of tazarotene 0.1% gel once daily plus mometasone furoate 0.1% cream once daily versus calcipotriene 0.005% ointment twice daily in the treatment of plaque psoriasis. Clin Ther. 2000 Oct;22(10):1225-38 | PubMed | Kaufmann R, Bibby AJ, Bissonnette R, Cambazard F, Chu AC, Decroix J, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205(4):389-93 | PubMed |
Kaufmann R, Bibby AJ, Bissonnette R, Cambazard F, Chu AC, Decroix J, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205(4):389-93 | PubMed | Kim Jo Yong, You Young Ho, Kim Tae Yoon, Kim Chung Won. Comparative study of calcipotriol and desoxymethasone ointments in the treatment of psoriasis vulgaris: the clinical effect and immunohistochemical change. Korean Journal of Dermatology. 1994;32(6):1054-1063. | Link |
Kim Jo Yong, You Young Ho, Kim Tae Yoon, Kim Chung Won. Comparative study of calcipotriol and desoxymethasone ointments in the treatment of psoriasis vulgaris: the clinical effect and immunohistochemical change. Korean Journal of Dermatology. 1994;32(6):1054-1063. | Link | Kragballe K, Gjertsen BT, De Hoop D, Karlsmark T, van de Kerkhof PC, Larkö O, et al. Double-blind, right/left comparison of calcipotriol and betamethasone valerate in treatment of psoriasis vulgaris. Lancet. 1991 Jan 26;337(8735):193-6 | PubMed |
Kragballe K, Gjertsen BT, De Hoop D, Karlsmark T, van de Kerkhof PC, Larkö O, et al. Double-blind, right/left comparison of calcipotriol and betamethasone valerate in treatment of psoriasis vulgaris. Lancet. 1991 Jan 26;337(8735):193-6 | PubMed | Landi G, PierleoniM, Polverelli M, Fioravanti F. Calcipotriol, a New Topical Product in the Therapy of Psoriasis: Controlled Study Versus Clobetasol. Giornale Italiano di Dermatologia e Venereologia. 1993;128(9):89-93. | Link |
Landi G, PierleoniM, Polverelli M, Fioravanti F. Calcipotriol, a New Topical Product in the Therapy of Psoriasis: Controlled Study Versus Clobetasol. Giornale Italiano di Dermatologia e Venereologia. 1993;128(9):89-93. | Link | Landi G. Efficacy and safety of calcipotriol ointment compared to clobetasol ointment in psoriasis vulgaris. 3rd Congress of the European Academy of Dermatology & Venereology. Copenhagen, Denmark. 1993;199 | Link |
Landi G. Efficacy and safety of calcipotriol ointment compared to clobetasol ointment in psoriasis vulgaris. 3rd Congress of the European Academy of Dermatology & Venereology. Copenhagen, Denmark. 1993;199 | Link | Langner A, Stapór W, Ambroziak M. Efficacy and tolerance of topical calcitriol 3 microg g(-1) in psoriasis treatment: a review of our experience in Poland. Br J Dermatol. 2001 Apr;144 Suppl 58:11-6 | PubMed |
Langner A, Stapór W, Ambroziak M. Efficacy and tolerance of topical calcitriol 3 microg g(-1) in psoriasis treatment: a review of our experience in Poland. Br J Dermatol. 2001 Apr;144 Suppl 58:11-6 | PubMed | Lebwohl M, Siskin SB, Epinette W, Breneman D, Funicella T, Kalb R, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996 Aug;35(2 Pt 1):268-9 | PubMed |
Lebwohl M, Siskin SB, Epinette W, Breneman D, Funicella T, Kalb R, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996 Aug;35(2 Pt 1):268-9 | PubMed | Medansky RS, Greenspan A, Kraus SJ, Todd Plott R. The Comparative Efficacy of Diflorasone Diacetate Ointment 0.05% (Psorcon®) vs Calcipotriene Ointment (Dovonex®) in the Treatment of Psoriasis. Journal of Geriatric Dermatology 1. 1996;4(1):20-24. | Link |
Medansky RS, Greenspan A, Kraus SJ, Todd Plott R. The Comparative Efficacy of Diflorasone Diacetate Ointment 0.05% (Psorcon®) vs Calcipotriene Ointment (Dovonex®) in the Treatment of Psoriasis. Journal of Geriatric Dermatology 1. 1996;4(1):20-24. | Link | Molin L, Cutler TP, Helander I, Nyfors B, Downes N. Comparative efficacy of calcipotriol (MC903) cream and betamethasone 17-valerate cream in the treatment of chronic plaque psoriasis. A randomized, double-blind, parallel group multicentre study. Calcipotriol Study Group. Br J Dermatol. 1997 Jan;136(1):89-93 | PubMed |
Molin L, Cutler TP, Helander I, Nyfors B, Downes N. Comparative efficacy of calcipotriol (MC903) cream and betamethasone 17-valerate cream in the treatment of chronic plaque psoriasis. A randomized, double-blind, parallel group multicentre study. Calcipotriol Study Group. Br J Dermatol. 1997 Jan;136(1):89-93 | PubMed | Molin L, Cutler TP, Helander I, Nyfors B. Comparative Efficacy of Calcipotriol Cream and Betamethasone Valerate Cream in the Treatment of Psoriasis. Journal of Investigative Dermatology. Symposium Proceedings. 1996;1(1):110. | Link |
Molin L, Cutler TP, Helander I, Nyfors B. Comparative Efficacy of Calcipotriol Cream and Betamethasone Valerate Cream in the Treatment of Psoriasis. Journal of Investigative Dermatology. Symposium Proceedings. 1996;1(1):110. | Link | Molin L, Cutler TP, Helander I, Nyfors B. Calcipotriol cream and betamethasone valerate cream of equal efficacy in psoriasis. Journal of the European Academy of Dermatology and Venereology. 1995;5:S92. | Link |
Molin L, Cutler TP, Helander I, Nyfors B. Calcipotriol cream and betamethasone valerate cream of equal efficacy in psoriasis. Journal of the European Academy of Dermatology and Venereology. 1995;5:S92. | Link | Papp KA, Guenther L, Boyden B, Larsen FG, Harvima RJ, Guilhou JJ, et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. J Am Acad Dermatol. 2003 Jan;48(1):48-54 | PubMed |
Papp KA, Guenther L, Boyden B, Larsen FG, Harvima RJ, Guilhou JJ, et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. J Am Acad Dermatol. 2003 Jan;48(1):48-54 | PubMed | Pinheiro N. Comparative effects of calcipotriol ointment (50 micrograms/g) and 5% coal tar/2% allantoin/0.5% hydrocortisone cream in treating plaque psoriasis. Br J Clin Pract. 1997 Jan-Feb;51(1):16-9 | PubMed |
Pinheiro N. Comparative effects of calcipotriol ointment (50 micrograms/g) and 5% coal tar/2% allantoin/0.5% hydrocortisone cream in treating plaque psoriasis. Br J Clin Pract. 1997 Jan-Feb;51(1):16-9 | PubMed | Sander P, Stücker M, Hermes N, Hoffmann K, Altmeyer P. [Mometasone and calcipotriol optimize the initial therapeutic effect of dithranol in chronic persistent psoriasis]. Hautarzt. 1998 Apr;49(4):291-4 | PubMed |
Sander P, Stücker M, Hermes N, Hoffmann K, Altmeyer P. [Mometasone and calcipotriol optimize the initial therapeutic effect of dithranol in chronic persistent psoriasis]. Hautarzt. 1998 Apr;49(4):291-4 | PubMed | Sander P, Stücker M, Hoffmann M, Hoffmann K, Altmeyer P. Topical Triple-Therapy of Chronic Stationary Psoriasis (CSP). Increased Healing Effectiveness Compared with a Dithranol Monotherapy. Aktuelle Dermatologie. 2001;27(06):173-177. | Link |
Sander P, Stücker M, Hoffmann M, Hoffmann K, Altmeyer P. Topical Triple-Therapy of Chronic Stationary Psoriasis (CSP). Increased Healing Effectiveness Compared with a Dithranol Monotherapy. Aktuelle Dermatologie. 2001;27(06):173-177. | Link | Scarpa C. Calcipotriol: clinical trial versus betamethasone dipropionate + salicylic acid. Acta Derm Venereol Suppl (Stockh). 1994;186:47 | PubMed |
Scarpa C. Calcipotriol: clinical trial versus betamethasone dipropionate + salicylic acid. Acta Derm Venereol Suppl (Stockh). 1994;186:47 | PubMed | Scarpa C. Tacalcitol ointment is an efficacious and well tolerated treatment for psoriasis. Journal of the European Academy of Dermatology and Venereology. 1996;6(2):142-146. | Link |
Scarpa C. Tacalcitol ointment is an efficacious and well tolerated treatment for psoriasis. Journal of the European Academy of Dermatology and Venereology. 1996;6(2):142-146. | Link | Scarpa C, Kokelj F, Plozzer C, Lavaroni G. Efficacy and Tolerability of Tacalcitol Ointment on Psoriatic Skin: Study in 63 Patients. Journal of Investigative Dermatology. Symposium Proceedings 1996. 1996;1(1):110. | Link |
Scarpa C, Kokelj F, Plozzer C, Lavaroni G. Efficacy and Tolerability of Tacalcitol Ointment on Psoriatic Skin: Study in 63 Patients. Journal of Investigative Dermatology. Symposium Proceedings 1996. 1996;1(1):110. | Link | Seidenari S, Magni R, Giannetti A. Assessment of the Activity of Tacalcitol on Psoriatic Plaques by Means of Colorimetry and High-Frequency Ultrasound: A Double-Blind Intrasubject Half-Side Right-Left Comparison with Betamethasone Valerate and Placebo. Skin Pharmacology and Physiology. 1997;10(1):40-47. | Link |
Seidenari S, Magni R, Giannetti A. Assessment of the Activity of Tacalcitol on Psoriatic Plaques by Means of Colorimetry and High-Frequency Ultrasound: A Double-Blind Intrasubject Half-Side Right-Left Comparison with Betamethasone Valerate and Placebo. Skin Pharmacology and Physiology. 1997;10(1):40-47. | Link | van der Velden HM, Pasch MC, van Erp PE, van Lingen RG, Otero ME, de Boer-van Huizen RT, et al. Treatment of plaque psoriasis with the two-compound product calcipotriol/betamethasone dipropionate versus both monotherapies: an immunohistochemical study. J Dermatolog Treat. 2010 Jan;21(1):13-22 | CrossRef | PubMed |
van der Velden HM, Pasch MC, van Erp PE, van Lingen RG, Otero ME, de Boer-van Huizen RT, et al. Treatment of plaque psoriasis with the two-compound product calcipotriol/betamethasone dipropionate versus both monotherapies: an immunohistochemical study. J Dermatolog Treat. 2010 Jan;21(1):13-22 | CrossRef | PubMed | Vladimirov VV, Tcherjomukchina IG, Kurjanova ON. Efficacy of calcipotriol ointment compared to betamethasone 17-valerate ointment in the treatment of psoriasis. International Meeting Skin Therapy Update. 1994 | Link |
Vladimirov VV, Tcherjomukchina IG, Kurjanova ON. Efficacy of calcipotriol ointment compared to betamethasone 17-valerate ointment in the treatment of psoriasis. International Meeting Skin Therapy Update. 1994 | Link | Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009 Apr;60(4):643-59 | CrossRef |
Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009 Apr;60(4):643-59 | CrossRef |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








