Abstract
It is postulated cannabinoids may have benefits in Parkinson's disease. However, its actual clinical effectiveness is still discussed. To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified six systematic reviews including eight studies overall, of which four were randomized trials relevant for the question of interest. We extracted data from the systematic reviews, reanalyzed data of primary studies included in these reviews, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded cannabinoids probably do not decrease symptoms in Parkinson’s disease or dyskinesia, and probably are associated to frequent adverse effects in patients with Parkinson’s disease.
Problem
The concept of cannabinoids includes organic compounds that interact with cannabinoid receptors in the body. These are in general active metabolites of the cannabis plant, and would explain its pharmacological effects in humans.
Theories to justify the effect of these drugs in Parkinson's disease point to alterations in the cerebral endocannabinoid system, at substantia nigra, caudate and putamen. However, it remains unclear how effective or well tolerated cannabinoids are in Parkinson’s disease.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1],[2],[3],[4],[5],[6] including eight studies that answer the question of interest [7],[8],[9],[10],[11],[12],[13],[14] from which four [7],[8],[12],[13] were randomized trials. This table and the summary in general are based on the latter. |
|
What types of patients were included* |
Patients with Parkinson’s disease with or without motor complications, especially dyskinesia, were included. It was not possible to obtain information about the proportion of patients with cognitive impairment or other non-motor manifestations. |
|
What types of interventions were included* |
One trial used extract of Cannabis sativa (tetrahydrocannabinol 2.5 mg with 1.25 mg cannabidiol) [7], one trial used cannabidiol 75 to 300 mg daily [8], one trial used cannabinoid receptor agonist-1 (SR141716) 20 mg daily [12] and other trial used oral nabilone 0.03 mg/kg daily [13]. |
|
What types of outcomes |
From multiple outcome measured by the trials, the systematic reviews grouped them as follows:
Mean follow-up was four weeks, with a range of two to six weeks. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
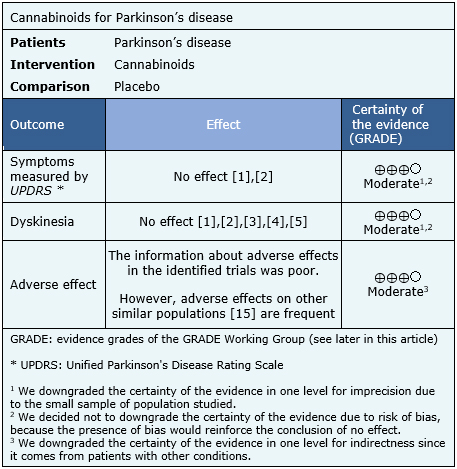
Summary of findings
The information about the effects of cannabinoids for Parkinson’s disease is based on four randomized trials [7],[8],[12],[13] including 63 patients.
None of the systematic reviews presented meta-analysis, so the information presented below corresponds to a narrative synthesis of the information obtained.
The summary of findings is the following:
- Cannabinoids probably do not decrease symptoms of Parkinson’s disease. The certainty of the evidence is moderate.
- Cannabinoids probably do not decrease dyskinesia in Parkinson’s disease. The certainty of the evidence is moderate.
- Cannabinoids are probably associated to adverse effects in patients with Parkinson’s disease. The certainty of the evidence is moderate.
|
Follow the link to access the interactive version of the Summary of Findings (iSoF) table |

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
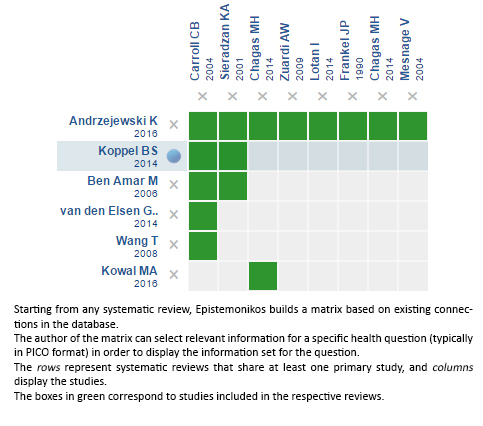
Follow the link to access the interactive version: Cannabinoids for Parkinson’s disease
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrices and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Se postula que los cannabinoides pudieran tener beneficios en la enfermedad de Parkinson. No obstante, su real efectividad clínica aún es discutida. Para responder a esta pregunta utilizamos Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante búsquedas en múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Identificamos seis revisiones sistemáticas que en conjunto incluyen ocho estudios, de los cuales cuatro corresponden a ensayos aleatorizados. Extrajimos los datos desde las revisiones identificadas, reanalizamos los datos de los estudios primarios y preparamos tablas de resumen de los resultados utilizando el método GRADE. Concluimos que los cannabinoides probablemente no disminuyen los síntomas ni las discinesias, y se asocian a efectos adversos frecuentes en pacientes con enfermedad de Parkinson.
 Authors:
Gonzalo A Bravo-Soto[1,2], Carlos Juri[2,3]
Authors:
Gonzalo A Bravo-Soto[1,2], Carlos Juri[2,3]
Affiliation:
[1] Centro evidencia UC, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Neurología, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: cajuri@gmail.com
Author address:
[1] Facultad de Medicina Pontificia Universidad Católica de Chile Lira 63 Santiago Centro Chile.

Citation: Bravo-Soto GA, Juri C. Are cannabinoids effective for Parkinson’s disease?. Medwave 2016; 17(Suppl2):e6974 doi: 10.5867/medwave.2017.6974
Submission date: 27/5/2017
Acceptance date: 5/6/2017
Publication date: 16/6/2017

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Andrzejewski K, Barbano R, Mink J. Cannabinoids in the treatment of movement disorders: A systematic review of case series and clinical trials. Basal Ganglia. 2016;6(3):173-181. | CrossRef | Link |
- Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25 | PubMed |
- Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014 Apr 29;82(17):1556-63 | CrossRef | PubMed |
- van den Elsen GA, Ahmed AI, Lammers M, Kramers C, Verkes RJ, van der Marck MA, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev. 2014 Mar;14:56-64 | CrossRef | PubMed |
- Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008 Jun 17;178(13):1669-78 | CrossRef | PubMed |
- Kowal MA, Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2010-2014. Cannabinoids. 2016;11(special issue):1-18. | Link |
- Carroll CB, Bain PG, Teare L, Liu X, Joint C, Wroath C, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology. 2004 Oct 12;63(7):1245-50. | PubMed |
- Chagas MH, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, et al. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double-blind trial. J Psychopharmacol. 2014 Nov;28(11):1088-98 | CrossRef | PubMed |
- Chagas MH, Eckeli AL, Zuardi AW, Pena-Pereira MA, Sobreira-Neto MA, Sobreira ET, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. J Clin Pharm Ther. 2014 Oct;39(5):564-6 | CrossRef | PubMed |
- Frankel JP, Hughes A, Lees AJ, Stern GM. Marijuana for parkinsonian tremor. J Neurol Neurosurg Psychiatry. 1990 May;53(5):436 | PubMed |
- Lotan I, Treves TA, Roditi Y, Djaldetti R. Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: an open-label observational study. Clin Neuropharmacol. 2014 Mar-Apr;37(2):41-4 | CrossRef | PubMed |
- Mesnage V, Houeto JL, Bonnet AM, Clavier I, Arnulf I, Cattelin F, et al. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin Neuropharmacol. 2004 May-Jun;27(3):108-10 | PubMed |
- Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson's disease: a pilot study. Neurology. 2001 Dec 11;57(11):2108-11 | PubMed |
- Zuardi AW, Crippa JA, Hallak JE, Pinto JP, Chagas MH, Rodrigues GG, et al. Cannabidiol for the treatment of psychosis in Parkinson's disease. J Psychopharmacol. 2009 Nov;23(8):979-83 | CrossRef | PubMed |
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73 | CrossRef | PubMed |
- Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M et al. (2011). The movement disorder society evidence‐based medicine review update: Treatments for the motor symptoms of Parkinson's disease. Movement Disorders, 26(S3), S2-S41. | Link |
- Grimes D, Gordon J, Snelgrove B, Lim-Carter I, Fon E, Martin W, et al. Canadian Guidelines on Parkinson's Disease. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2012. Jul;39 (4 Suppl 4):S1-30 | PubMed |
- National Collaborating Centre for Chronic Conditions (UK). Parkinson's Disease: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians (UK); 2006 | PubMed |
- Hort J, O'Brien JT, Gainotti G, Pirttila T, Popescu BO, Rektorova I, et al; EFNS Scientist Panel on Dementia. EFNS guidelines for the diagnosis and management of Alzheimer's disease. Eur J Neurol. 2010 Oct;17(10):1236-48. | CrossRef | PubMed |
- Leehey MA. Randomized, Double Blind, Placebo-controlled Crossover Study of Tolerability and Efficacy of Cannabidiol (CBD) on Tremor in Parkinson's Disease. NCT02818777. | Link |
- Danna J. Evaluation of [18F]MK-9470 as a Brain Tracer of Cannabinoid-1 Receptor in Parkinson's Disease and Healthy Subjects MK9470. NCT01462708. | Link |
- Laurie KM. Cannabis and Parkinson's Disease Tremor: A Natural History Study. NCT02028858. | Link |
 Andrzejewski K, Barbano R, Mink J. Cannabinoids in the treatment of movement disorders: A systematic review of case series and clinical trials. Basal Ganglia. 2016;6(3):173-181. | CrossRef | Link |
Andrzejewski K, Barbano R, Mink J. Cannabinoids in the treatment of movement disorders: A systematic review of case series and clinical trials. Basal Ganglia. 2016;6(3):173-181. | CrossRef | Link | Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25 | PubMed |
Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25 | PubMed | Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014 Apr 29;82(17):1556-63 | CrossRef | PubMed |
Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014 Apr 29;82(17):1556-63 | CrossRef | PubMed | van den Elsen GA, Ahmed AI, Lammers M, Kramers C, Verkes RJ, van der Marck MA, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev. 2014 Mar;14:56-64 | CrossRef | PubMed |
van den Elsen GA, Ahmed AI, Lammers M, Kramers C, Verkes RJ, van der Marck MA, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev. 2014 Mar;14:56-64 | CrossRef | PubMed | Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008 Jun 17;178(13):1669-78 | CrossRef | PubMed |
Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008 Jun 17;178(13):1669-78 | CrossRef | PubMed | Kowal MA, Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2010-2014. Cannabinoids. 2016;11(special issue):1-18. | Link |
Kowal MA, Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2010-2014. Cannabinoids. 2016;11(special issue):1-18. | Link | Carroll CB, Bain PG, Teare L, Liu X, Joint C, Wroath C, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology. 2004 Oct 12;63(7):1245-50. | PubMed |
Carroll CB, Bain PG, Teare L, Liu X, Joint C, Wroath C, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology. 2004 Oct 12;63(7):1245-50. | PubMed | Chagas MH, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, et al. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double-blind trial. J Psychopharmacol. 2014 Nov;28(11):1088-98 | CrossRef | PubMed |
Chagas MH, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, et al. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double-blind trial. J Psychopharmacol. 2014 Nov;28(11):1088-98 | CrossRef | PubMed | Chagas MH, Eckeli AL, Zuardi AW, Pena-Pereira MA, Sobreira-Neto MA, Sobreira ET, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. J Clin Pharm Ther. 2014 Oct;39(5):564-6 | CrossRef | PubMed |
Chagas MH, Eckeli AL, Zuardi AW, Pena-Pereira MA, Sobreira-Neto MA, Sobreira ET, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. J Clin Pharm Ther. 2014 Oct;39(5):564-6 | CrossRef | PubMed | Frankel JP, Hughes A, Lees AJ, Stern GM. Marijuana for parkinsonian tremor. J Neurol Neurosurg Psychiatry. 1990 May;53(5):436 | PubMed |
Frankel JP, Hughes A, Lees AJ, Stern GM. Marijuana for parkinsonian tremor. J Neurol Neurosurg Psychiatry. 1990 May;53(5):436 | PubMed | Lotan I, Treves TA, Roditi Y, Djaldetti R. Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: an open-label observational study. Clin Neuropharmacol. 2014 Mar-Apr;37(2):41-4 | CrossRef | PubMed |
Lotan I, Treves TA, Roditi Y, Djaldetti R. Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: an open-label observational study. Clin Neuropharmacol. 2014 Mar-Apr;37(2):41-4 | CrossRef | PubMed | Mesnage V, Houeto JL, Bonnet AM, Clavier I, Arnulf I, Cattelin F, et al. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin Neuropharmacol. 2004 May-Jun;27(3):108-10 | PubMed |
Mesnage V, Houeto JL, Bonnet AM, Clavier I, Arnulf I, Cattelin F, et al. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin Neuropharmacol. 2004 May-Jun;27(3):108-10 | PubMed | Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson's disease: a pilot study. Neurology. 2001 Dec 11;57(11):2108-11 | PubMed |
Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson's disease: a pilot study. Neurology. 2001 Dec 11;57(11):2108-11 | PubMed | Zuardi AW, Crippa JA, Hallak JE, Pinto JP, Chagas MH, Rodrigues GG, et al. Cannabidiol for the treatment of psychosis in Parkinson's disease. J Psychopharmacol. 2009 Nov;23(8):979-83 | CrossRef | PubMed |
Zuardi AW, Crippa JA, Hallak JE, Pinto JP, Chagas MH, Rodrigues GG, et al. Cannabidiol for the treatment of psychosis in Parkinson's disease. J Psychopharmacol. 2009 Nov;23(8):979-83 | CrossRef | PubMed | Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73 | CrossRef | PubMed |
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73 | CrossRef | PubMed | Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M et al. (2011). The movement disorder society evidence‐based medicine review update: Treatments for the motor symptoms of Parkinson's disease. Movement Disorders, 26(S3), S2-S41. | Link |
Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M et al. (2011). The movement disorder society evidence‐based medicine review update: Treatments for the motor symptoms of Parkinson's disease. Movement Disorders, 26(S3), S2-S41. | Link | Grimes D, Gordon J, Snelgrove B, Lim-Carter I, Fon E, Martin W, et al. Canadian Guidelines on Parkinson's Disease. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2012. Jul;39 (4 Suppl 4):S1-30 | PubMed |
Grimes D, Gordon J, Snelgrove B, Lim-Carter I, Fon E, Martin W, et al. Canadian Guidelines on Parkinson's Disease. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2012. Jul;39 (4 Suppl 4):S1-30 | PubMed | National Collaborating Centre for Chronic Conditions (UK). Parkinson's Disease: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians (UK); 2006 | PubMed |
National Collaborating Centre for Chronic Conditions (UK). Parkinson's Disease: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians (UK); 2006 | PubMed | Hort J, O'Brien JT, Gainotti G, Pirttila T, Popescu BO, Rektorova I, et al; EFNS Scientist Panel on Dementia. EFNS guidelines for the diagnosis and management of Alzheimer's disease. Eur J Neurol. 2010 Oct;17(10):1236-48. | CrossRef | PubMed |
Hort J, O'Brien JT, Gainotti G, Pirttila T, Popescu BO, Rektorova I, et al; EFNS Scientist Panel on Dementia. EFNS guidelines for the diagnosis and management of Alzheimer's disease. Eur J Neurol. 2010 Oct;17(10):1236-48. | CrossRef | PubMed | Leehey MA. Randomized, Double Blind, Placebo-controlled Crossover Study of Tolerability and Efficacy of Cannabidiol (CBD) on Tremor in Parkinson's Disease. NCT02818777. | Link |
Leehey MA. Randomized, Double Blind, Placebo-controlled Crossover Study of Tolerability and Efficacy of Cannabidiol (CBD) on Tremor in Parkinson's Disease. NCT02818777. | Link | Danna J. Evaluation of [18F]MK-9470 as a Brain Tracer of Cannabinoid-1 Receptor in Parkinson's Disease and Healthy Subjects MK9470. NCT01462708. | Link |
Danna J. Evaluation of [18F]MK-9470 as a Brain Tracer of Cannabinoid-1 Receptor in Parkinson's Disease and Healthy Subjects MK9470. NCT01462708. | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








