Abstract
Dexamethasone has been proposed as an alternative in the treatment of acute asthma exacerbation in children. It allows shortening the duration of treatment, reducing costs and adverse effects. However, it is not clear whether its efficacy is similar to the traditional steroid regimen. To answer this question, we searched in Epistemonikos database, which is maintained by screening multiple information sources. We identified six systematic reviews including 10 randomized trials. We extracted data, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded dexamethasone has probably fewer adverse effects than others corticosteroids, and might be equally effective in reducing hospitalizations and revisits.
Problem
Systemic corticosteroids, typically oral prednisone, constitute the cornerstone in the treatment of asthmatic exacerbation in children. However, there is concern about their adverse effects in both the short- and long-term. Dexamethasone allows administration for a shorter period of time, which would reduce adverse effects and costs. It is not clear, however, whether its efficacy is similar.
Methods
We used Epistemonikos database, which is maintained by screening multiple information sources, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1],[2],[3],[4],[5],[6] including 10 primary studies relevant to the question of interest, reported in 13 references [7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19]. All of them correspond to randomized controlled trials. Two trials [15],[16] were not considered for this summary since they evaluated adult population. |
|
What types of patients were included |
Regarding the definition of asthma, the different trials used different inclusion criteria: Three trials [7],[8],[19] required a history of clinical episode of wheezing, two trials [12],[17] history of two or more episodes of wheezing, three trials [11],[13],[14] used as inclusion criteria "history of previous asthma". Regarding to age, eight trials [7],[8],[11],[12],[13],[14],[17],[19] focused on pediatric population; six trials with an age range between 2 to 18 years [7],[8],[12],[14],[17],[19], one trial [11] between 1.5 to 7 years and one [13] between 0.5 to seven years. The inclusion criteria were mild or moderate acute asthma exacerbation in four trials [7],[11],[13],[14], moderate acute asthma exacerbation in one [19], three trials [8],[12],[17] excluded patients with severity factors, either life-threatening exacerbation [8], need of intubation [17], or previous history of severe acute asthma exacerbation/need of intubation [12]. Five trials excluded patients who had received corticosteroids in the last four weeks [8],[11],[12],[14], [17], two trials excluded children who received corticosteroids in the last two weeks [7],[13] and in one trial [19] this information could not be obtained from any systematic review. Six trials [7],[8],[11],[12],[14],[17] excluded patients with some chronic comorbidity, one trial [13] did not exclude for this reason, and this information could not be obtained from any systematic review for one trial [19] Other exclusion criteria were exposure to tuberculosis [8], [11], chickenpox [7],[8],[11],[12] and infection by respiratory syncytial virus [8],[13]. One trial [11] excluded patients who presented two episodes of vomiting during emergency consultation after administration of dexamethasone. |
|
What types of interventions were included |
Regarding the intervention group: All of the trials used dexamethasone. It was administered orally in four trials [7],[8],[12],[17], intramuscular in three [11],[13],[14], and nebulized in one [19]. The dose ranged from 0.3 mg/kg/day to 1.7 mg/kg/day. In five trials the treatment lasted one day [7],[8],[11], [13],[14], in two trials [12],[17] it lasted two days, and we were not able to obtain this information for one trial [19].
Three trials [7],[8],[11] compared against prednisolone and five trials [12],[13],[14],[17],[19] against prednisone. The comparison was administered orally in all of the trials. The dose varied between 1-2 mg/kg/day. The duration of treatment was 3 to 5 days, except for one trial [14] that lasted two days. We could not obtain this information for another trial [19]. |
|
What types of outcomes |
The outcomes were pooled by the systematic reviews as follows:
|
Summary of findings
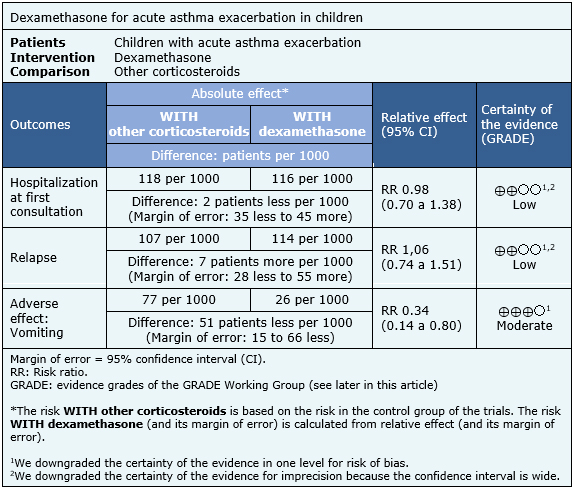
The information about the effects of dexamethasone compared to others corticosteroids is based on eight randomized trials [7],[8],[11],[12],[13],[14],[17],[19] including 1280 patients.
Three trials [7],[8],[17] reported hospitalization at first consultation (1007 patients), eight trials [7],[8],[11],[12],[13],[14],[17],[19] reported relapse (1280 patients) and five trials [7],[8],[11],[12], [17] (1112 patients) reported adverse effects (vomiting).
The summary of findings is the following:
- Dexamethasone may be equally effective as other corticosteroids in reducing hospitalizations in children with acute asthma exacerbation, but the certainty of the evidence is low.
- Dexamethasone may be equally effective as other corticosteroids in reducing hospitalizations, and revisit to emergency services or unscheduled medical consultations in children with acute asthma exacerbations, but the certainty of the evidence is low.
- Dexamethasone may be equally effective as other corticosteroids in reducing revisit to emergency service or non-scheduled medical consultation in children with acute asthma exacerbation, but the certainty of the evidence is low.
- Dexamethasone, compared to other corticosteroids, probably has less adverse effects (vomiting) in children with acute asthma exacerbation. The certainty of the evidence is moderate.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
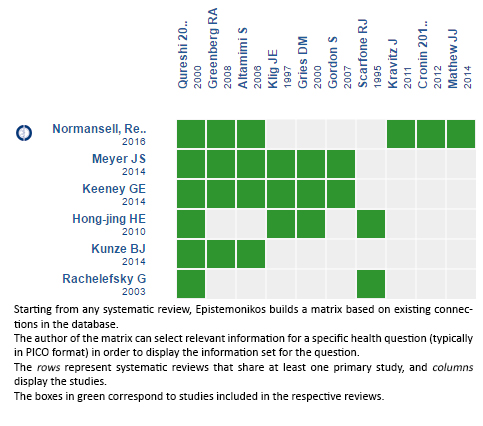
Follow the link to access the interactive version: Dexamethasone versus others corticoids for acute asthma exacerbation
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

La dexametasona se ha planteado como una opción en el tratamiento de exacerbación asmática en niños, acortando el tratamiento, lo que además de ventajas prácticas, podría reducir costos y potenciales efectos adversos. No está claro, sin embargo, si su eficacia es similar al esquema esteroidal tradicional. Para responder esta interrogante utilizamos la base de datos Epistemonikos, la cual es mantenida mediante búsquedas en múltiples fuentes de información. Identificamos seis revisiones sistemáticas que en conjunto incluyen diez ensayos controlados aleatorizados. Extrajimos los datos, realizamos un metanálisis y preparamos una tabla de resumen de los resultados utilizando el método GRADE. Concluimos que la dexametasona, en comparación con otros corticoides, probablemente tiene menos efectos adversos, y podría ser igual de efectiva en reducir hospitalizaciones y reconsulta.
 Authors:
Gonzalo A. Bravo-Soto[1,2], Constanza Harismendy[2,3], Pamela Rojas[2,3], Rodrigo Silva[2,3], Pamela von Borries[2,3]
Authors:
Gonzalo A. Bravo-Soto[1,2], Constanza Harismendy[2,3], Pamela Rojas[2,3], Rodrigo Silva[2,3], Pamela von Borries[2,3]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Medicina Familiar, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: rsilva@medfamiliar.cl
Author address:
[1] Facultad de Medicina Pontificia Universidad Católica de Chile Diagonal Paraguay 362 Santiago Centro Chile.

Citation: Bravo-Soto GA, Harismendy C, Rojas P, Silva R, von Borries P. Is dexamethasone as effective as other corticosteroids for acute asthma exacerbation in children?. Medwave2017;17(Suppl2):e6931 doi: 10.5867/medwave.2017.6931
Submission date: 23/2/2017
Acceptance date: 28/2/2017
Publication date: 20/4/2017

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Hong-jing HE, Hui-min W, Jia C, Jiang H, Gui-li X. System Review on Curative Effect and Safety of Dexamethasone for Severe Asthma and Severe Sepsis [J]. China Pharmacy. 2010;22:025 | Link |
- Keeney GE, Gray MP, Morrison AK, Levas MN, Kessler EA, Hill GD, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014 Mar;133(3):493-9 | CrossRef | PubMed |
- Kunze, Benhamin J. Is Oral Dexamethasone Safe and Effective For Treating Asthma Exacerbations In Pediatric Patients?. PCOM Physician Assistant Studies Student Scholarship. 2014. Paper 168 | Link |
- Meyer JS, Riese J, Biondi E. Is dexamethasone an effective alternative to oral prednisone in the treatment of pediatric asthma exacerbations? Hosp Pediatr. 2014 May;4(3):172-80 | CrossRef | PubMed |
- Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016 May 13;(5):CD011801 | CrossRef | PubMed |
- Rachelefsky G. Treating exacerbations of asthma in children: the role of systemic corticosteroids. Pediatrics. 2003 Aug;112(2):382-97 | PubMed |
- Altamimi S, Robertson G, Jastaniah W, Davey A, Dehghani N, Chen R, et al. Single-dose oral dexamethasone in the emergency management of children with exacerbations of mild to moderate asthma. PediatrEmerg Care. 2006 Dec;22(12):786-93 | PubMed |
- Cronin J, Kennedy U, McCoy S, An Fhailí SN, Crispino-O'Connell G, Hayden J, et al. Single dose oral dexamethasone versus multi-dose prednisolone in the treatment of acute exacerbations of asthma in children who attend the emergency department: study protocol for a randomized controlled trial. Trials. 2012 Aug 21;13:141 | CrossRef | PubMed |
- Cronin JJ, McCoy S, Kennedy U, An Fhailí SN, Wakai A, Hayden J, et al. A Randomized Trial of Single-Dose Oral Dexamethasone Versus Multidose Prednisolone for Acute Exacerbations of Asthma in Children Who Attend the Emergency Department. Ann Emerg Med. 2016 May;67(5):593-601.e3 | CrossRef | PubMed |
- Cronin J, McCoy S, Nally S, Kennedy U, Crispino-O’Connell G, Walsh S, O’Sullivan R. 370 A Randomised Trial of Dexamethasone Versus Prednisolone in the Treatment of Acute Paediatric Asthma Exacerbations. Archives of Disease in Childhood. 2012;97(Suppl 2):A109-A109 | Link |
- Gordon S, Tompkins T, Dayan PS. Randomized trial of single-dose intramuscular dexamethasone compared with prednisolone for children with acute asthma. PediatrEmerg Care. 2007 Aug;23(8):521-7 | PubMed |
- Greenberg RA, Kerby G, Roosevelt GE. A comparison of oral dexamethasone with oral prednisone in pediatric asthma exacerbations treated in the emergency department. ClinPediatr (Phila). 2008 Oct;47(8):817-23 | CrossRef | PubMed |
- Gries DM, Moffitt DR, Pulos E, Carter ER. A single dose of intramuscularly administered dexamethasone acetate is as effective as oral prednisone to treat asthma exacerbations in young children. J Pediatr. 2000 Mar;136(3):298-303. | PubMed |
- Klig JE, Hodge D 3rd, Rutherford MW. Symptomatic improvement following emergency department management of asthma: a pilot study of intramuscular dexamethasone versus oral prednisone. J Asthma. 1997;34(5):419-25 | PubMed |
- Kravitz J, Dominici P, Ufberg J, Fisher J, Giraldo P. Two days of dexamethasone versus 5 days of prednisone in the treatment of acute asthma: a randomized controlled trial. Ann Emerg Med. 2011 Aug;58(2):200-4 | CrossRef | PubMed |
- Mathew JJ, Manickavel S, Riss A, McNamee J, Debari V, Mahmood N, et al. Dexamethasone Versus Prednisone In The Treatment Of Acute Asthma In Adults: Can An Easier Regimen Provide The Same Results?. American Journal of Respiratory and Critical Care Medicine. 2014;189:A1360. | Link |
- Oureshi F, Poirier MP, Zaritsky A. Oral dexamethasone versus oral prednisone: Effect on relapse in acute asthma. Pediatric Research. 2000;47(4):116A-116A. | Link |
- Qureshi F, Zaritsky A, Poirier MP. Comparative efficacy of oral dexamethasone versus oral prednisone in acute pediatric asthma. J Pediatr. 2001 Jul;139(1):20-6 | PubMed |
- Scarfone RJ, Loiselle JM, Wiley JF 2nd, Decker JM, Henretig FM, Joffe MD. Nebulized dexamethasone versus oral prednisone in the emergency treatment of asthmatic children. Ann Emerg Med. 1995 Oct;26(4):480-6 | PubMed |
- Identifying and overcoming challenges in asthma care and self-care. Oct 2014. A workshop supported by the National Institute for Health Research, UKCochrane Centre and the Cochrane Airways Group | Link |
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2016. | Link |
- GEMA4.1. Guía española para el manejo del asma, 2016 | Link |
- British guideline on the management of asthma. A national clinical guideline, Sept 2016. British Thoracic Society, Scottish Intercollegiate Guidelines Network. | Link |
- MatoqA. Oral Dexamethasone Versus Oral Prednisone in Children Hospitalized With Asthma: A Randomized Control Study. NCT02780479 | Link |
- Benito F. Pilot randomized controlled trial using dexamethasone versus prednisolone/prednisone in children with acute asthma in pediatric emergency ward. EUCTR2013-003145-42-ES | Link |
- O'Sullivan R. A randomised trial of single dose oral dexamethasone versus multi-dose prednisolone in the treatment of acute exacerbations of asthma in children who attend the Emergency Department. ISRCTN26944158 | Link |
- SonnenbergL. Dexamethasone Tolerability in the Treatment of Acute Asthma in Children. NCT00973687 | Link |
 Hong-jing HE, Hui-min W, Jia C, Jiang H, Gui-li X. System Review on Curative Effect and Safety of Dexamethasone for Severe Asthma and Severe Sepsis [J]. China Pharmacy. 2010;22:025 | Link |
Hong-jing HE, Hui-min W, Jia C, Jiang H, Gui-li X. System Review on Curative Effect and Safety of Dexamethasone for Severe Asthma and Severe Sepsis [J]. China Pharmacy. 2010;22:025 | Link | Keeney GE, Gray MP, Morrison AK, Levas MN, Kessler EA, Hill GD, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014 Mar;133(3):493-9 | CrossRef | PubMed |
Keeney GE, Gray MP, Morrison AK, Levas MN, Kessler EA, Hill GD, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014 Mar;133(3):493-9 | CrossRef | PubMed | Kunze, Benhamin J. Is Oral Dexamethasone Safe and Effective For Treating Asthma Exacerbations In Pediatric Patients?. PCOM Physician Assistant Studies Student Scholarship. 2014. Paper 168 | Link |
Kunze, Benhamin J. Is Oral Dexamethasone Safe and Effective For Treating Asthma Exacerbations In Pediatric Patients?. PCOM Physician Assistant Studies Student Scholarship. 2014. Paper 168 | Link | Meyer JS, Riese J, Biondi E. Is dexamethasone an effective alternative to oral prednisone in the treatment of pediatric asthma exacerbations? Hosp Pediatr. 2014 May;4(3):172-80 | CrossRef | PubMed |
Meyer JS, Riese J, Biondi E. Is dexamethasone an effective alternative to oral prednisone in the treatment of pediatric asthma exacerbations? Hosp Pediatr. 2014 May;4(3):172-80 | CrossRef | PubMed | Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016 May 13;(5):CD011801 | CrossRef | PubMed |
Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016 May 13;(5):CD011801 | CrossRef | PubMed | Rachelefsky G. Treating exacerbations of asthma in children: the role of systemic corticosteroids. Pediatrics. 2003 Aug;112(2):382-97 | PubMed |
Rachelefsky G. Treating exacerbations of asthma in children: the role of systemic corticosteroids. Pediatrics. 2003 Aug;112(2):382-97 | PubMed | Altamimi S, Robertson G, Jastaniah W, Davey A, Dehghani N, Chen R, et al. Single-dose oral dexamethasone in the emergency management of children with exacerbations of mild to moderate asthma. PediatrEmerg Care. 2006 Dec;22(12):786-93 | PubMed |
Altamimi S, Robertson G, Jastaniah W, Davey A, Dehghani N, Chen R, et al. Single-dose oral dexamethasone in the emergency management of children with exacerbations of mild to moderate asthma. PediatrEmerg Care. 2006 Dec;22(12):786-93 | PubMed | Cronin J, Kennedy U, McCoy S, An Fhailí SN, Crispino-O'Connell G, Hayden J, et al. Single dose oral dexamethasone versus multi-dose prednisolone in the treatment of acute exacerbations of asthma in children who attend the emergency department: study protocol for a randomized controlled trial. Trials. 2012 Aug 21;13:141 | CrossRef | PubMed |
Cronin J, Kennedy U, McCoy S, An Fhailí SN, Crispino-O'Connell G, Hayden J, et al. Single dose oral dexamethasone versus multi-dose prednisolone in the treatment of acute exacerbations of asthma in children who attend the emergency department: study protocol for a randomized controlled trial. Trials. 2012 Aug 21;13:141 | CrossRef | PubMed | Cronin JJ, McCoy S, Kennedy U, An Fhailí SN, Wakai A, Hayden J, et al. A Randomized Trial of Single-Dose Oral Dexamethasone Versus Multidose Prednisolone for Acute Exacerbations of Asthma in Children Who Attend the Emergency Department. Ann Emerg Med. 2016 May;67(5):593-601.e3 | CrossRef | PubMed |
Cronin JJ, McCoy S, Kennedy U, An Fhailí SN, Wakai A, Hayden J, et al. A Randomized Trial of Single-Dose Oral Dexamethasone Versus Multidose Prednisolone for Acute Exacerbations of Asthma in Children Who Attend the Emergency Department. Ann Emerg Med. 2016 May;67(5):593-601.e3 | CrossRef | PubMed | Cronin J, McCoy S, Nally S, Kennedy U, Crispino-O’Connell G, Walsh S, O’Sullivan R. 370 A Randomised Trial of Dexamethasone Versus Prednisolone in the Treatment of Acute Paediatric Asthma Exacerbations. Archives of Disease in Childhood. 2012;97(Suppl 2):A109-A109 | Link |
Cronin J, McCoy S, Nally S, Kennedy U, Crispino-O’Connell G, Walsh S, O’Sullivan R. 370 A Randomised Trial of Dexamethasone Versus Prednisolone in the Treatment of Acute Paediatric Asthma Exacerbations. Archives of Disease in Childhood. 2012;97(Suppl 2):A109-A109 | Link | Gordon S, Tompkins T, Dayan PS. Randomized trial of single-dose intramuscular dexamethasone compared with prednisolone for children with acute asthma. PediatrEmerg Care. 2007 Aug;23(8):521-7 | PubMed |
Gordon S, Tompkins T, Dayan PS. Randomized trial of single-dose intramuscular dexamethasone compared with prednisolone for children with acute asthma. PediatrEmerg Care. 2007 Aug;23(8):521-7 | PubMed | Greenberg RA, Kerby G, Roosevelt GE. A comparison of oral dexamethasone with oral prednisone in pediatric asthma exacerbations treated in the emergency department. ClinPediatr (Phila). 2008 Oct;47(8):817-23 | CrossRef | PubMed |
Greenberg RA, Kerby G, Roosevelt GE. A comparison of oral dexamethasone with oral prednisone in pediatric asthma exacerbations treated in the emergency department. ClinPediatr (Phila). 2008 Oct;47(8):817-23 | CrossRef | PubMed | Gries DM, Moffitt DR, Pulos E, Carter ER. A single dose of intramuscularly administered dexamethasone acetate is as effective as oral prednisone to treat asthma exacerbations in young children. J Pediatr. 2000 Mar;136(3):298-303. | PubMed |
Gries DM, Moffitt DR, Pulos E, Carter ER. A single dose of intramuscularly administered dexamethasone acetate is as effective as oral prednisone to treat asthma exacerbations in young children. J Pediatr. 2000 Mar;136(3):298-303. | PubMed | Klig JE, Hodge D 3rd, Rutherford MW. Symptomatic improvement following emergency department management of asthma: a pilot study of intramuscular dexamethasone versus oral prednisone. J Asthma. 1997;34(5):419-25 | PubMed |
Klig JE, Hodge D 3rd, Rutherford MW. Symptomatic improvement following emergency department management of asthma: a pilot study of intramuscular dexamethasone versus oral prednisone. J Asthma. 1997;34(5):419-25 | PubMed | Kravitz J, Dominici P, Ufberg J, Fisher J, Giraldo P. Two days of dexamethasone versus 5 days of prednisone in the treatment of acute asthma: a randomized controlled trial. Ann Emerg Med. 2011 Aug;58(2):200-4 | CrossRef | PubMed |
Kravitz J, Dominici P, Ufberg J, Fisher J, Giraldo P. Two days of dexamethasone versus 5 days of prednisone in the treatment of acute asthma: a randomized controlled trial. Ann Emerg Med. 2011 Aug;58(2):200-4 | CrossRef | PubMed | Mathew JJ, Manickavel S, Riss A, McNamee J, Debari V, Mahmood N, et al. Dexamethasone Versus Prednisone In The Treatment Of Acute Asthma In Adults: Can An Easier Regimen Provide The Same Results?. American Journal of Respiratory and Critical Care Medicine. 2014;189:A1360. | Link |
Mathew JJ, Manickavel S, Riss A, McNamee J, Debari V, Mahmood N, et al. Dexamethasone Versus Prednisone In The Treatment Of Acute Asthma In Adults: Can An Easier Regimen Provide The Same Results?. American Journal of Respiratory and Critical Care Medicine. 2014;189:A1360. | Link | Oureshi F, Poirier MP, Zaritsky A. Oral dexamethasone versus oral prednisone: Effect on relapse in acute asthma. Pediatric Research. 2000;47(4):116A-116A. | Link |
Oureshi F, Poirier MP, Zaritsky A. Oral dexamethasone versus oral prednisone: Effect on relapse in acute asthma. Pediatric Research. 2000;47(4):116A-116A. | Link | Qureshi F, Zaritsky A, Poirier MP. Comparative efficacy of oral dexamethasone versus oral prednisone in acute pediatric asthma. J Pediatr. 2001 Jul;139(1):20-6 | PubMed |
Qureshi F, Zaritsky A, Poirier MP. Comparative efficacy of oral dexamethasone versus oral prednisone in acute pediatric asthma. J Pediatr. 2001 Jul;139(1):20-6 | PubMed | Scarfone RJ, Loiselle JM, Wiley JF 2nd, Decker JM, Henretig FM, Joffe MD. Nebulized dexamethasone versus oral prednisone in the emergency treatment of asthmatic children. Ann Emerg Med. 1995 Oct;26(4):480-6 | PubMed |
Scarfone RJ, Loiselle JM, Wiley JF 2nd, Decker JM, Henretig FM, Joffe MD. Nebulized dexamethasone versus oral prednisone in the emergency treatment of asthmatic children. Ann Emerg Med. 1995 Oct;26(4):480-6 | PubMed | Identifying and overcoming challenges in asthma care and self-care. Oct 2014. A workshop supported by the National Institute for Health Research, UKCochrane Centre and the Cochrane Airways Group | Link |
Identifying and overcoming challenges in asthma care and self-care. Oct 2014. A workshop supported by the National Institute for Health Research, UKCochrane Centre and the Cochrane Airways Group | Link | British guideline on the management of asthma. A national clinical guideline, Sept 2016. British Thoracic Society, Scottish Intercollegiate Guidelines Network. | Link |
British guideline on the management of asthma. A national clinical guideline, Sept 2016. British Thoracic Society, Scottish Intercollegiate Guidelines Network. | Link | MatoqA. Oral Dexamethasone Versus Oral Prednisone in Children Hospitalized With Asthma: A Randomized Control Study. NCT02780479 | Link |
MatoqA. Oral Dexamethasone Versus Oral Prednisone in Children Hospitalized With Asthma: A Randomized Control Study. NCT02780479 | Link | Benito F. Pilot randomized controlled trial using dexamethasone versus prednisolone/prednisone in children with acute asthma in pediatric emergency ward. EUCTR2013-003145-42-ES | Link |
Benito F. Pilot randomized controlled trial using dexamethasone versus prednisolone/prednisone in children with acute asthma in pediatric emergency ward. EUCTR2013-003145-42-ES | Link | O'Sullivan R. A randomised trial of single dose oral dexamethasone versus multi-dose prednisolone in the treatment of acute exacerbations of asthma in children who attend the Emergency Department. ISRCTN26944158 | Link |
O'Sullivan R. A randomised trial of single dose oral dexamethasone versus multi-dose prednisolone in the treatment of acute exacerbations of asthma in children who attend the Emergency Department. ISRCTN26944158 | Link | SonnenbergL. Dexamethasone Tolerability in the Treatment of Acute Asthma in Children. NCT00973687 | Link |
SonnenbergL. Dexamethasone Tolerability in the Treatment of Acute Asthma in Children. NCT00973687 | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








