Abstract
Patients with chronic kidney disease have higher cardiovascular risk than general population, a fact that has been linked to high homocysteine levels. Folic acid supplementation can reduce homocysteine levels, which would reduce cardiovascular events. However, there is controversy about the clinical effects of this measure. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified six systematic reviews comprising 13 trials addressing the question of this article. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded folic acid supplementation does not reduce the risk of myocardial infarction or stroke in patients with chronic kidney disease, and might have no effect on mortality.
Problem
It is clearly established patients with chronic kidney disease have a higher cardiovascular risk than the general population. However, despite control of classical risk factors (diabetes, hypertension, dyslipidemia, and smoking, among others) there is still a difference compared to population with these conditions without chronic kidney disease. In parallel, it has been found homocysteine levels are elevated in chronic kidney disease and there is an association with cardiovascular events and consequent mortality.
Considering folic acid can reduce homocysteine levels, it has been proposed as a potentially effective treatment to reduce cardiovascular diseases in patients with chronic kidney disease. However, there is controversy about the clinical effects of this measure.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1],[2],[3],[4],[5],[6] including 13 randomized controlled trials reported in 24 references [7],[8],[9],[10],[11],[12],[13],[14],[15],[16], [17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27], [28],[29],[30],[31],[32]. |
|
What types of patients were included |
General characteristics: The average age of patients in the trials was 58.7 years. The average percentage of men was 66%. Type and stage of kidney disease: Three trials [23],[30],[32] included patients on any stage of chronic kidney disease, four trials [7],[24],[27],[29] on pre-dialysis stage, five on dialysis [22],[25],[26], [28],[31] and one after transplantation [9]. Ten trials [9],[22],[23],[25],[26],[27],[28],[29],[31],[32] included any type of kidney disease, two trials [7],[30] included only diabetic nephropathy and one trial [24] included diabetic nephropathy or vascular nephropathy. Comorbidities: The average percentage of patients with a history of acute myocardial infarction was 40.9%, including one trial that restricted inclusion to post-infarction patients [27]. The average percentage of patients with diabetes was 40.9%, with two trials using this as an inclusion criterion [7],[30]. |
|
What types of interventions were included |
Five trials [25],[28],[30],[31],[32] evaluated folic acid as monotherapy, and eight trials [7],[9],[22],[23],[24],[26],[27],[29] in combination with vitamin B6 and/or B12. The average dose of folic acid was 8.8 mg/day, ranging from 2 to 40 mg/day. Eight trials [7],[23],[24],[27],[28],[29],[30],[32] compared against placebo, two trials [25], [26] compared against usual care, one trial [9] against vitamin B and two trials [22],[31] against a lower dose of folic acid with or without vitamin B supplement. |
|
What types of outcomes |
The different systematic reviews identified grouped the outcomes as follows:
|
Summary of findings
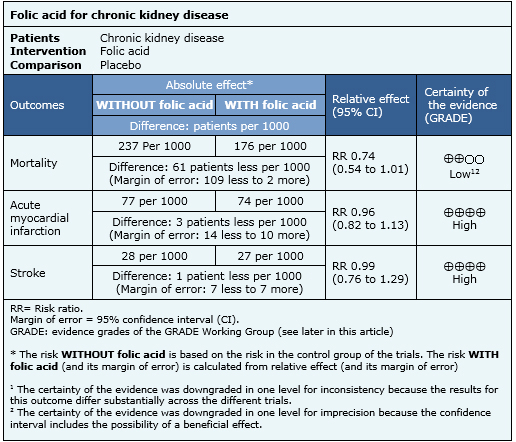
The information on the effects of folic acid in patients with chronic kidney disease is based on thirteen randomized trials involving 11,049 patients.
Nine trials (8,500 patients) measured the outcome mortality [7],[9],[22],[23],[24],[26],[28],[31],[32], seven trials (7,718 patients) measured myocardial infarction [7],[9],[22], [23],[26],[31],[32] and seven trials (8,536 patients) measured stroke [9],[22],[23],[24],[26],[31],[32].
The summary of findings is the following:
- Folic acid supplementation might have no effect on mortality in patients with chronic kidney disease, but the certainty of the evidence is low.
- Folic acid supplementation does not reduce the risk of acute myocardial infarction or stroke in patients with chronic kidney disease. The certainty of the evidence is high.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
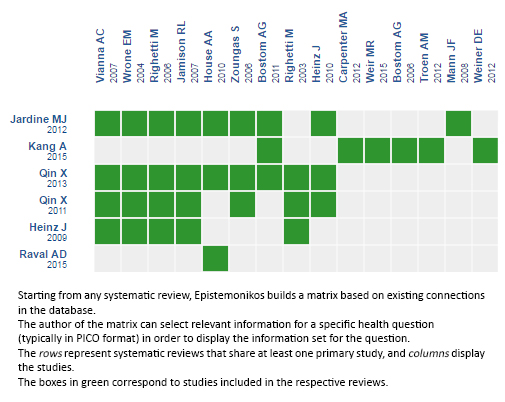
Follow the link to access the interactive version: Folic acid for chronic kidney disease.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Los pacientes con insuficiencia renal crónica poseen riesgo cardiovascular mayor que la población general, algo que ha sido relacionado con niveles altos de homocisteína. El uso de ácido fólico se ha propuesto como una medida que podría disminuir la hiperhomocisteinemia, y por esta vía disminuir los eventos cardiovasculares. Sin embargo, existe controversia sobre la verdadera utilidad de esta medida. Utilizando la base de datos Epistemonikos, la cual es mantenida mediante búsquedas en 30 bases de datos, identificamos seis revisiones sistemáticas que en conjunto incluyen 13 estudios aleatorizados que responden la pregunta de interés. Realizamos un metanálisis y tablas de resumen de los resultados utilizando el método GRADE. Concluimos que la suplementación con ácido fólico no disminuye el riesgo de infarto agudo al miocardio ni de accidente vascular encefálico en pacientes con insuficiencia renal crónica, y que podría no tener efecto sobre la mortalidad.
 Authors:
Gonzalo A. Bravo-Soto[1,2 ], Trinidad Madrid [2,3 ]
Authors:
Gonzalo A. Bravo-Soto[1,2 ], Trinidad Madrid [2,3 ]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Medicina Interna, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: madrid.trini@gmail.com
Author address:
[1] Facultad de Medicina
Pontificia Universidad Católica de Chile
Lira 63
Santiago Centro
Chile

Citation: Bravo-Soto GA, Madrid T . Is folic acid supplementation useful for chronic kidney disease?. Medwave 2016;16(Suppl 5):e6591 doi: 10.5867/medwave.2016.6591
Publication date: 7/11/2016

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Heinz J, Kropf S, Domröse U, Westphal S, Borucki K, Luley C, Neumann KH, et al. B vitamins and the risk of total mortality and cardiovascular disease in end-stage renal disease: results of a randomized controlled trial. Circulation. 2010 Mar 30;121(12):1432-8. | CrossRef | PubMed |
- Jardine MJ, Kang A, Zoungas S, Navaneethan SD, Ninomiya T, Nigwekar SU, et al. The effect of folic acid based homocysteine lowering on cardiovascular events in people with kidney disease: systematic review and meta-analysis. BMJ. 2012 Jun 13;344:e3533. | CrossRef | PubMed |
- Kang A, Nigwekar SU, Perkovic V, Kulshrestha S, Zoungas S, Navaneethan SD, et al. Interventions for lowering plasma homocysteine levels in kidney transplant recipients. Cochrane Database Syst Rev. 2015 May 4;(5):CD007910. | CrossRef | PubMed |
- Qin X, Huo Y, Langman CB, Hou F, Chen Y, Matossian D, et al. Folic acid therapy and cardiovascular disease in ESRD or advanced chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2011 Mar;6(3):482-8. | CrossRef | PubMed |
- Qin X, Huo Y, Xie D, Hou F, Xu X, Wang X. Homocysteine-lowering therapy with folic acid is effective in cardiovascular disease prevention in patients with kidney disease: a meta-analysis of randomized controlled trials. Clin Nutr. 2013 Oct;32(5):722-7. | CrossRef | PubMed |
- Raval AD, Thakker D, Rangoonwala AN, Gor D, Walia R. Vitamin B and its derivatives for diabetic kidney disease. Cochrane Database Syst Rev. 2015 Jan 12;1:CD009403. | CrossRef | PubMed |
- House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al. Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA. 2010 Apr 28;303(16):1603-9. | CrossRef | PubMed |
- House AA, Ehasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al. Vitamin therapy for homocysteine accelerates loss of renal function in diabetic kidney disease. Journal of the American Society of Nephrology. 2009;20:80A. | Link |
- Carpenter MA, John A, Weir MR, Smith SR, Hunsicker L, Kasiske BL, et al. BP, cardiovascular disease, and death in the Folic Acid for Vascular Outcome Reduction in Transplantation trial. J Am Soc Nephrol. 2014 Jul;25(7):1554-62. | CrossRef | PubMed |
- Bostom AG, Carpenter M, Kusek J. Baseline characteristics of the folic acid for vascular outcome reduction in transplantation (FAVOURIT) trial. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):883A. | Link |
- Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS. Baseline characteristics of participants in the folic acid for vascular outcome reduction in transplantation (FAVORIT) trial. American Journal of Transplantation 2009;9(Suppl 2):429. | Link |
- Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS, et al. Baseline characteristics of participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. Am J Kidney Dis. 2009 Jan;53(1):121-8. | CrossRef | PubMed |
- Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Jacques P, Levey AS, et al. Homocysteine-lowering in chronic stable renal transplant recipients: the FAVORIT Trial. American Society of Nephrology Renal Week; 2009 Oct 27-Nov 1; San Diego, CA. 2009. | Link |
- Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Pfeffer MA, Levey AS, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J. 2006 Sep;152(3):448.e1-7. | PubMed |
- Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, et al. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation. 2011 Apr 26;123(16):1763-70. | CrossRef | PubMed |
- Carpenter M, Bostom A, Hunsicker L, Ivanova A, Kasiske B, Kusek J, et al. Higher systolic blood pressure is associated with increased cardiovascular risk in patients with hypertension in the Folic Acid for Vascular Outcome Reduction in Transplantation trial fficacy of Folic Acid Therapy on the Progression of Chronic Kidney Disease. American Journal of Transplantation 2011;11(Suppl 2):375.
- Carpenter MA, Bostom A, Kusek J, Adey D, Cole E, House A, et al. Untreated CVD risk factors in chronic, stable kidney transplant recipients at baseline in the folic acid for vascular outcome reduction (FAVORIT) study. Journal of the American Society of Nephrology 2009;20:888A.
- Carpenter MA, Weir MR, Adey DB, House AA, Bostom AG, Kusek JW. Inadequacy of cardiovascular risk factor management in chronic kidney transplantation - evidence from the FAVORIT study. Clin Transplant. 2012 Jul-Aug;26(4):E438-46. | CrossRef | PubMed |
- Troen AM, Scott TM, D'Anci KE, Moorthy D, Dobson B, Rogers G, et al. Cognitive dysfunction and depression in adult kidney transplant recipients: baseline findings from the FAVORIT Ancillary Cognitive Trial (FACT). J Ren Nutr. 2012 Mar;22(2):268-76.e1-3. | CrossRef | PubMed |
- Weiner DE, Carpenter MA, Levey AS, Ivanova A, Cole EH, Hunsicker L, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. Am J Transplant. 2012 Sep;12(9):2437-45. | CrossRef | PubMed |
- Weir MR, Gravens-Muller L, Costa N, Ivanova A, Manitpisitkul W, Bostom AG, et al. Safety events in kidney transplant recipients: results from the folic Acid for vascular outcome reduction in transplant trial. Transplantation. 2015 May;99(5):1003-8. | CrossRef | PubMed |
- Heinz J, Kropf S, Luley C, Dierkes J. Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: a meta-analysis. Am J Kidney Dis. 2009 Sep;54(3):478-89. | CrossRef | PubMed |
- Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007 Sep 12;298(10):1163-70. | CrossRef | PubMed |
- Mann JF, Sheridan P, McQueen MJ, Held C, Arnold JM, Fodor G, et al. Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease--results of the renal Hope-2 study. Nephrol Dial Transplant. 2008 Feb;23(2):645-53. | PubMed |
- Righetti M, Ferrario GM, Milani S, Serbelloni P, La Rosa L, Uccellini M, et al. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit. 2003 Apr;9(4):PI19-24. | PubMed |
- Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif. 2006;24(4):379-86. | PubMed |
- Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010 Jun 23;303(24):2486-94. | CrossRef | PubMed |
- Vianna AC, Mocelin AJ, Matsuo T, Morais-Filho D, Largura A, Delfino VA, et al. Uremic hyperhomocysteinemia: a randomized trial of folate treatment for the prevention of cardiovascular events. Hemodial Int. 2007 Apr;11(2):210-6. | PubMed |
- VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010 Sep;9(9):855-65. | CrossRef | PubMed |
- Wotherspoon F, Laight DW, Turner C, Meeking DR, Allard SE, Munday LJ, et al. The effect of oral folic acid upon plasma homocysteine, endothelial function and oxidative stress in patients with type 1 diabetes and microalbuminuria. Int J Clin Pract. 2008 Apr;62(4):569-74. | CrossRef | PubMed |
- Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004 Feb;15(2):420-6. | PubMed |
- Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006 Mar 21;47(6):1108-16. | PubMed |
- Eknoyan G, Lameire N, Eckardt KU, Kasiske BL, Wheeler DC, Levin A, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013. 3:5-14.
- Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001 Jan;37(1 Suppl 2):S66-70. | PubMed |
- Levin A, Hemmelgarn B, Culleton B, Tobe S, McFarlane P, Ruzicka M, et al. Guidelines for the management of chronic kidney disease. CMAJ. 2008 Nov 18;179(11):1154-62. | CrossRef | PubMed |
- Xu X, Qin X, Li Y, Sun D, Wang J, Liang M, et al. Efficacy of Folic Acid Therapy on the Progression of Chronic Kidney Disease: The Renal Substudy of the China Stroke Primary Prevention Trial. JAMA Intern Med. 2016 Oct 1;176(10):1443-1450. | CrossRef | PubMed |
 Heinz J, Kropf S, Domröse U, Westphal S, Borucki K, Luley C, Neumann KH, et al. B vitamins and the risk of total mortality and cardiovascular disease in end-stage renal disease: results of a randomized controlled trial. Circulation. 2010 Mar 30;121(12):1432-8. | CrossRef | PubMed |
Heinz J, Kropf S, Domröse U, Westphal S, Borucki K, Luley C, Neumann KH, et al. B vitamins and the risk of total mortality and cardiovascular disease in end-stage renal disease: results of a randomized controlled trial. Circulation. 2010 Mar 30;121(12):1432-8. | CrossRef | PubMed | Jardine MJ, Kang A, Zoungas S, Navaneethan SD, Ninomiya T, Nigwekar SU, et al. The effect of folic acid based homocysteine lowering on cardiovascular events in people with kidney disease: systematic review and meta-analysis. BMJ. 2012 Jun 13;344:e3533. | CrossRef | PubMed |
Jardine MJ, Kang A, Zoungas S, Navaneethan SD, Ninomiya T, Nigwekar SU, et al. The effect of folic acid based homocysteine lowering on cardiovascular events in people with kidney disease: systematic review and meta-analysis. BMJ. 2012 Jun 13;344:e3533. | CrossRef | PubMed | Kang A, Nigwekar SU, Perkovic V, Kulshrestha S, Zoungas S, Navaneethan SD, et al. Interventions for lowering plasma homocysteine levels in kidney transplant recipients. Cochrane Database Syst Rev. 2015 May 4;(5):CD007910. | CrossRef | PubMed |
Kang A, Nigwekar SU, Perkovic V, Kulshrestha S, Zoungas S, Navaneethan SD, et al. Interventions for lowering plasma homocysteine levels in kidney transplant recipients. Cochrane Database Syst Rev. 2015 May 4;(5):CD007910. | CrossRef | PubMed | Qin X, Huo Y, Langman CB, Hou F, Chen Y, Matossian D, et al. Folic acid therapy and cardiovascular disease in ESRD or advanced chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2011 Mar;6(3):482-8. | CrossRef | PubMed |
Qin X, Huo Y, Langman CB, Hou F, Chen Y, Matossian D, et al. Folic acid therapy and cardiovascular disease in ESRD or advanced chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2011 Mar;6(3):482-8. | CrossRef | PubMed | Qin X, Huo Y, Xie D, Hou F, Xu X, Wang X. Homocysteine-lowering therapy with folic acid is effective in cardiovascular disease prevention in patients with kidney disease: a meta-analysis of randomized controlled trials. Clin Nutr. 2013 Oct;32(5):722-7. | CrossRef | PubMed |
Qin X, Huo Y, Xie D, Hou F, Xu X, Wang X. Homocysteine-lowering therapy with folic acid is effective in cardiovascular disease prevention in patients with kidney disease: a meta-analysis of randomized controlled trials. Clin Nutr. 2013 Oct;32(5):722-7. | CrossRef | PubMed | Raval AD, Thakker D, Rangoonwala AN, Gor D, Walia R. Vitamin B and its derivatives for diabetic kidney disease. Cochrane Database Syst Rev. 2015 Jan 12;1:CD009403. | CrossRef | PubMed |
Raval AD, Thakker D, Rangoonwala AN, Gor D, Walia R. Vitamin B and its derivatives for diabetic kidney disease. Cochrane Database Syst Rev. 2015 Jan 12;1:CD009403. | CrossRef | PubMed | House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al. Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA. 2010 Apr 28;303(16):1603-9. | CrossRef | PubMed |
House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al. Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA. 2010 Apr 28;303(16):1603-9. | CrossRef | PubMed | House AA, Ehasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al. Vitamin therapy for homocysteine accelerates loss of renal function in diabetic kidney disease. Journal of the American Society of Nephrology. 2009;20:80A. | Link |
House AA, Ehasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al. Vitamin therapy for homocysteine accelerates loss of renal function in diabetic kidney disease. Journal of the American Society of Nephrology. 2009;20:80A. | Link | Carpenter MA, John A, Weir MR, Smith SR, Hunsicker L, Kasiske BL, et al. BP, cardiovascular disease, and death in the Folic Acid for Vascular Outcome Reduction in Transplantation trial. J Am Soc Nephrol. 2014 Jul;25(7):1554-62. | CrossRef | PubMed |
Carpenter MA, John A, Weir MR, Smith SR, Hunsicker L, Kasiske BL, et al. BP, cardiovascular disease, and death in the Folic Acid for Vascular Outcome Reduction in Transplantation trial. J Am Soc Nephrol. 2014 Jul;25(7):1554-62. | CrossRef | PubMed | Bostom AG, Carpenter M, Kusek J. Baseline characteristics of the folic acid for vascular outcome reduction in transplantation (FAVOURIT) trial. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):883A. | Link |
Bostom AG, Carpenter M, Kusek J. Baseline characteristics of the folic acid for vascular outcome reduction in transplantation (FAVOURIT) trial. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):883A. | Link | Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS. Baseline characteristics of participants in the folic acid for vascular outcome reduction in transplantation (FAVORIT) trial. American Journal of Transplantation 2009;9(Suppl 2):429. | Link |
Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS. Baseline characteristics of participants in the folic acid for vascular outcome reduction in transplantation (FAVORIT) trial. American Journal of Transplantation 2009;9(Suppl 2):429. | Link | Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS, et al. Baseline characteristics of participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. Am J Kidney Dis. 2009 Jan;53(1):121-8. | CrossRef | PubMed |
Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS, et al. Baseline characteristics of participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. Am J Kidney Dis. 2009 Jan;53(1):121-8. | CrossRef | PubMed | Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Jacques P, Levey AS, et al. Homocysteine-lowering in chronic stable renal transplant recipients: the FAVORIT Trial. American Society of Nephrology Renal Week; 2009 Oct 27-Nov 1; San Diego, CA. 2009. | Link |
Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Jacques P, Levey AS, et al. Homocysteine-lowering in chronic stable renal transplant recipients: the FAVORIT Trial. American Society of Nephrology Renal Week; 2009 Oct 27-Nov 1; San Diego, CA. 2009. | Link | Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Pfeffer MA, Levey AS, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J. 2006 Sep;152(3):448.e1-7. | PubMed |
Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Pfeffer MA, Levey AS, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J. 2006 Sep;152(3):448.e1-7. | PubMed | Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, et al. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation. 2011 Apr 26;123(16):1763-70. | CrossRef | PubMed |
Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, et al. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation. 2011 Apr 26;123(16):1763-70. | CrossRef | PubMed | Carpenter M, Bostom A, Hunsicker L, Ivanova A, Kasiske B, Kusek J, et al. Higher systolic blood pressure is associated with increased cardiovascular risk in patients with hypertension in the Folic Acid for Vascular Outcome Reduction in Transplantation trial fficacy of Folic Acid Therapy on the Progression of Chronic Kidney Disease. American Journal of Transplantation 2011;11(Suppl 2):375.
Carpenter M, Bostom A, Hunsicker L, Ivanova A, Kasiske B, Kusek J, et al. Higher systolic blood pressure is associated with increased cardiovascular risk in patients with hypertension in the Folic Acid for Vascular Outcome Reduction in Transplantation trial fficacy of Folic Acid Therapy on the Progression of Chronic Kidney Disease. American Journal of Transplantation 2011;11(Suppl 2):375.  Carpenter MA, Bostom A, Kusek J, Adey D, Cole E, House A, et al. Untreated CVD risk factors in chronic, stable kidney transplant recipients at baseline in the folic acid for vascular outcome reduction (FAVORIT) study. Journal of the American Society of Nephrology 2009;20:888A.
Carpenter MA, Bostom A, Kusek J, Adey D, Cole E, House A, et al. Untreated CVD risk factors in chronic, stable kidney transplant recipients at baseline in the folic acid for vascular outcome reduction (FAVORIT) study. Journal of the American Society of Nephrology 2009;20:888A.  Carpenter MA, Weir MR, Adey DB, House AA, Bostom AG, Kusek JW. Inadequacy of cardiovascular risk factor management in chronic kidney transplantation - evidence from the FAVORIT study. Clin Transplant. 2012 Jul-Aug;26(4):E438-46. | CrossRef | PubMed |
Carpenter MA, Weir MR, Adey DB, House AA, Bostom AG, Kusek JW. Inadequacy of cardiovascular risk factor management in chronic kidney transplantation - evidence from the FAVORIT study. Clin Transplant. 2012 Jul-Aug;26(4):E438-46. | CrossRef | PubMed | Troen AM, Scott TM, D'Anci KE, Moorthy D, Dobson B, Rogers G, et al. Cognitive dysfunction and depression in adult kidney transplant recipients: baseline findings from the FAVORIT Ancillary Cognitive Trial (FACT). J Ren Nutr. 2012 Mar;22(2):268-76.e1-3. | CrossRef | PubMed |
Troen AM, Scott TM, D'Anci KE, Moorthy D, Dobson B, Rogers G, et al. Cognitive dysfunction and depression in adult kidney transplant recipients: baseline findings from the FAVORIT Ancillary Cognitive Trial (FACT). J Ren Nutr. 2012 Mar;22(2):268-76.e1-3. | CrossRef | PubMed | Weiner DE, Carpenter MA, Levey AS, Ivanova A, Cole EH, Hunsicker L, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. Am J Transplant. 2012 Sep;12(9):2437-45. | CrossRef | PubMed |
Weiner DE, Carpenter MA, Levey AS, Ivanova A, Cole EH, Hunsicker L, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. Am J Transplant. 2012 Sep;12(9):2437-45. | CrossRef | PubMed | Weir MR, Gravens-Muller L, Costa N, Ivanova A, Manitpisitkul W, Bostom AG, et al. Safety events in kidney transplant recipients: results from the folic Acid for vascular outcome reduction in transplant trial. Transplantation. 2015 May;99(5):1003-8. | CrossRef | PubMed |
Weir MR, Gravens-Muller L, Costa N, Ivanova A, Manitpisitkul W, Bostom AG, et al. Safety events in kidney transplant recipients: results from the folic Acid for vascular outcome reduction in transplant trial. Transplantation. 2015 May;99(5):1003-8. | CrossRef | PubMed | Heinz J, Kropf S, Luley C, Dierkes J. Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: a meta-analysis. Am J Kidney Dis. 2009 Sep;54(3):478-89. | CrossRef | PubMed |
Heinz J, Kropf S, Luley C, Dierkes J. Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: a meta-analysis. Am J Kidney Dis. 2009 Sep;54(3):478-89. | CrossRef | PubMed | Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007 Sep 12;298(10):1163-70. | CrossRef | PubMed |
Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007 Sep 12;298(10):1163-70. | CrossRef | PubMed | Mann JF, Sheridan P, McQueen MJ, Held C, Arnold JM, Fodor G, et al. Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease--results of the renal Hope-2 study. Nephrol Dial Transplant. 2008 Feb;23(2):645-53. | PubMed |
Mann JF, Sheridan P, McQueen MJ, Held C, Arnold JM, Fodor G, et al. Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease--results of the renal Hope-2 study. Nephrol Dial Transplant. 2008 Feb;23(2):645-53. | PubMed | Righetti M, Ferrario GM, Milani S, Serbelloni P, La Rosa L, Uccellini M, et al. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit. 2003 Apr;9(4):PI19-24. | PubMed |
Righetti M, Ferrario GM, Milani S, Serbelloni P, La Rosa L, Uccellini M, et al. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit. 2003 Apr;9(4):PI19-24. | PubMed | Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif. 2006;24(4):379-86. | PubMed |
Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif. 2006;24(4):379-86. | PubMed | Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010 Jun 23;303(24):2486-94. | CrossRef | PubMed |
Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010 Jun 23;303(24):2486-94. | CrossRef | PubMed | Vianna AC, Mocelin AJ, Matsuo T, Morais-Filho D, Largura A, Delfino VA, et al. Uremic hyperhomocysteinemia: a randomized trial of folate treatment for the prevention of cardiovascular events. Hemodial Int. 2007 Apr;11(2):210-6. | PubMed |
Vianna AC, Mocelin AJ, Matsuo T, Morais-Filho D, Largura A, Delfino VA, et al. Uremic hyperhomocysteinemia: a randomized trial of folate treatment for the prevention of cardiovascular events. Hemodial Int. 2007 Apr;11(2):210-6. | PubMed | VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010 Sep;9(9):855-65. | CrossRef | PubMed |
VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010 Sep;9(9):855-65. | CrossRef | PubMed | Wotherspoon F, Laight DW, Turner C, Meeking DR, Allard SE, Munday LJ, et al. The effect of oral folic acid upon plasma homocysteine, endothelial function and oxidative stress in patients with type 1 diabetes and microalbuminuria. Int J Clin Pract. 2008 Apr;62(4):569-74. | CrossRef | PubMed |
Wotherspoon F, Laight DW, Turner C, Meeking DR, Allard SE, Munday LJ, et al. The effect of oral folic acid upon plasma homocysteine, endothelial function and oxidative stress in patients with type 1 diabetes and microalbuminuria. Int J Clin Pract. 2008 Apr;62(4):569-74. | CrossRef | PubMed | Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004 Feb;15(2):420-6. | PubMed |
Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004 Feb;15(2):420-6. | PubMed | Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006 Mar 21;47(6):1108-16. | PubMed |
Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006 Mar 21;47(6):1108-16. | PubMed | Eknoyan G, Lameire N, Eckardt KU, Kasiske BL, Wheeler DC, Levin A, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013. 3:5-14.
Eknoyan G, Lameire N, Eckardt KU, Kasiske BL, Wheeler DC, Levin A, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013. 3:5-14.  Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001 Jan;37(1 Suppl 2):S66-70. | PubMed |
Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001 Jan;37(1 Suppl 2):S66-70. | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








