Abstract
Intravenous antibiotic prophylaxis is routinely administered to prevent surgical site infection in spinal surgery. Adding intrawound vancomycin powder before surgical closure might further decrease infection risk. However, its use is controversial. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified six systematic reviews that considered 16 studies, including one randomized controlled trial. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded vancomycin probably does not decrease the risk of infection in low risk surgery, but there is uncertainty about its effects in populations or surgeries with a higher risk because the certainty of the evidence is very low.
Problem
The infection rate after spinal surgery ranges from 0.5 to 12 %. For decades, efforts have been made in order to implement different measures to reduce this risk and then to improve surgical outcomes. Adding intrawound vancomycin powder could decrease the risk of infection and associated complications.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1-6] that consider 16 primary studies[7-22], including only one randomized controlled trial [21]. |
|
What types of patients were included |
The 16 studies included adults; three studies included posterior cervical surgery [7],[15],[19], six studies (including the only randomized trial) cervical and posterior thoracolumbar surgery [8],[11],[14],[17],[20],[21], four posterior thoracolumbar surgery [12],[13],[18],[22], one posterior lumbar [16] and two studies did not specify the type of surgery [9],[10]. Three studies (including the only randomized trial) analyzed separately instrumented and non-instrumented surgery [10],[16],[21]. |
|
What types of interventions were included |
The intervention was vancomycin powder. Nine studies (including the randomized) administered one gram of vancomycin powder [7-9],[14],[16],[19-22], three studies used two grams [12],[13],[18], two studies 0.5 to two grams [10,11], one study one to two grams [17] and one study 500 mg [15]. All studies compared against standard treatment which corresponds to intravenous cefazolin. |
|
What types of outcomes were measured |
Risk of infection, Staphylococcus aureus infection, pseudarthrosis. |
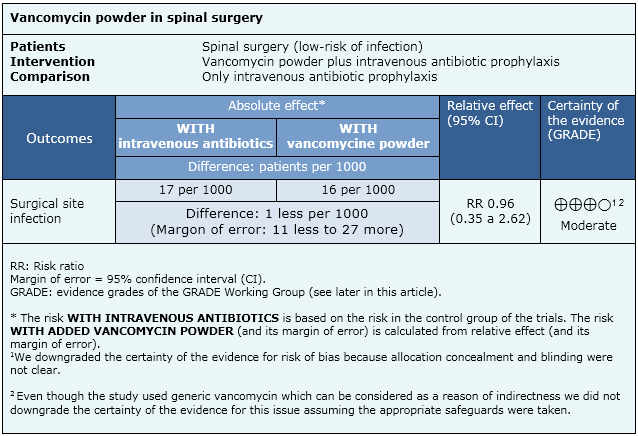
Summary of findings
The information on the effects of vancomycin powder on the surgical site is based on one randomised controlled trial including 907 patients. We conducted an evaluation of the certainty of the evidence coming from 15 non-randomised studies, which produced lower certainty tan the only randomised trial. So, we considered it for the formulation of key messages and considerations for decision-making, but not for the summary of findings table.
- Vancomycin powder probably does not decrease surgical site infection in low-risk spinal surgery. The certainty of the evidence is moderate.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
Follow the link to access the interactive version Vancomycin powder vs endovenous antibiotic prophylaxis to avoid surgical site infection in patients with spine surgery
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

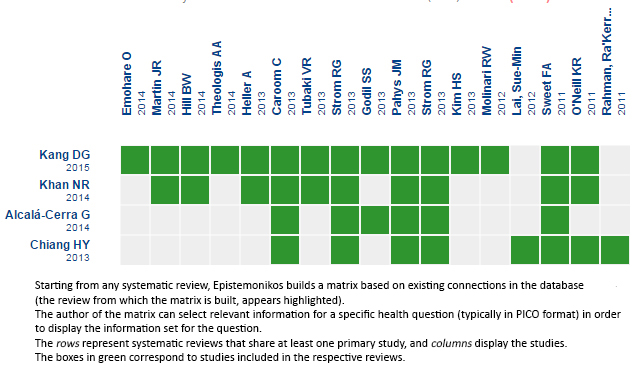 Matrix of evidence
Matrix of evidence
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

La profilaxis antibiótica endovenosa es de uso rutinario para la prevención de infección postoperatoria en cirugía de columna. Se ha planteado que la aplicación de vancomicina en polvo en el campo quirúrgico previo al cierre por planos podría tener un efecto adicional, sin embargo, su uso es controvertido. Utilizando la base de datos Epistemonikos, la cual es mantenida mediante búsquedas en 30 bases de datos, identificamos seis revisiones sistemáticas que en conjunto consideran 16 estudios pertinentes, incluyendo un estudio controlado aleatorizado. Realizamos un metanálisis y tablas de resumen de los resultados utilizando el método GRADE. Se concluye que el uso de vancomicina probablemente no disminuye el riesgo de infección en cirugías de bajo riesgo, pero existe incertidumbre sobre el efecto en poblaciones o cirugías con un riesgo mayor porque la certeza de la evidencia es muy baja.
 Authors:
Mario López[1,3], Marcelo Molina[2,3]
Authors:
Mario López[1,3], Marcelo Molina[2,3]
Affiliation:
[1] Pontificia Universidad Católica de Chile
[2] Departamento de traumatología y ortopedia, Facultad de Medicina, Pontificia Universidad Católica de Chile
[3] Proyecto Epistemonikos
E-mail: mmolinas@med.puc.cl

Citation: López M, Molina M. Should we add vancomycin antibiotic powder to prevent post operative infection in spine surgery?. Medwave 2015 Jun;15(Suppl 1):e6160 doi: 10.5867/medwave.2015.6160
Publication date: 12/6/2015

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Khan NR, Thompson CJ, DeCuypere M, Angotti JM, Kalobwe E, Muhlbauer MS, et al. A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine. 2014 Dec;21(6):974-83. | CrossRef | PubMed |
- Kang DG, Holekamp TF, Wagner SC, Lehman RA Jr. Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: a systematic literature review. Spine J. 2015 Apr 1;15(4):762-70. | CrossRef | PubMed |
- Alcalá-Cerra G, Paternina-Caicedo AJ, Moscote-Salazar LR, Gutiérrez-Paternina JJ, Niño-Hernández LM. [Application of vancomycin powder into the wound during spine surgery: systematic review and meta-analysis]. Rev Esp Cir Ortop Traumatol.2014 May-Jun;58(3):182-91. | CrossRef | PubMed |
- Chiang HY, Herwaldt LA, Blevins AE, Cho E, Schweizer ML. Effectiveness of local vancomycin powder to decrease surgical site infections: a meta-analysis. Spine J. 2014 Mar 1;14(3):397-407. | CrossRef | PubMed |
- Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA. The Use of Vancomycin Powder In Modern Spine Surgery: Systematic Review and Meta-Analysis of the Clinical Evidence. World Neurosurg. 2015 May;83(5):816-823. | CrossRef | PubMed |
- Kanj WW, Flynn JM, Spiegel DA, Dormans JP, Baldwin KD. Vancomycin prophylaxis of surgical site infection in clean orthopedic surgery. Orthopedics. 2013 Feb;36(2):138-46. | CrossRef | PubMed |
- Caroom C, Tullar JM, Benton EG Jr, Jones JR, Chaput CD. Intrawound vancomycin powder reduces surgical site infections in posterior cervical fusion. Spine (Phila Pa 1976). 2013 Jun 15;38(14):1183-7. | CrossRef | PubMed |
- Godil SS, Parker SL, O'Neill KR, Devin CJ, McGirt MJ. Comparative effectiveness and cost-benefit analysis of local application of vancomycin powder in posterior spinal fusion for spine trauma: clinical article. J Neurosurg Spine. 2013 Sep;19(3):331-5. | CrossRef | PubMed |
- Rahman RKK, Lenke LG, Bridwell KH, Buchowski J, Dickson DD, Aleem A, et al. Intrawound vancomycin powder lowers the acute deep wound infection rate in adult spinal deformity patients. Spine. 2011;(suppl 1):73. | Link |
- Heller A. The effect of intrawound vancomycin powder on surgical site infections in posterior instrumented spinal arthrodesis. [tesis] University of Kansas, 2012. | Link |
- Heller A, McIff TE, Lai SM, Burton DC. Intrawound vancomycin powder decreases staphylococcal surgical site infections following posterior instrumented spinal arthrodesis. J Spinal Disord Tech. 2013 Oct 30. | PubMed |
- Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976). 2011 Nov 15;36(24):2084-8. | CrossRef | PubMed |
- Theologis AA, Demirkiran G, Callahan M, Pekmezci M, Ames C, Deviren V. Local intrawound vancomycin powder decreases the risk of surgical site infections in complex adult deformity reconstruction: a cost analysis. Spine (Phila Pa 1976).2014 Oct 15;39(22):1875-80. | CrossRef | PubMed |
- Kim HS, Lee SG, Kim WK, Park CW, Son S. Prophylactic intrawound application of vancomycin powder in instrumented spinal fusion surgery. Korean J Spine. 2013 Sep;10(3):121-5. | CrossRef | PubMed |
- Pahys JM, Pahys JR, Cho SK, Kang MM, Zebala LP, Hawasli AH, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am. 2013 Mar 20;95(6):549-54. | CrossRef | PubMed |
- Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Lumbar laminectomy and fusion with routine local application of vancomycin powder: decreased infection rate in instrumented and non-instrumented cases. Clin Neurol Neurosurg. 2013 Sep;115(9):1766-9. | CrossRef | PubMed |
- Hill BW, Emohare O, Song B, Davis R, Kang MM. The use of vancomycin powder reduces surgical reoperation in posterior instrumented and noninstrumented spinal surgery. Acta Neurochir (Wien). 2014 Apr;156(4):749-54. | CrossRef | PubMed |
- Martin JR, Adogwa O, Brown CR, Bagley CA, Richardson WJ, Lad SP, et al. Experience with intrawound vancomycin powder for spinal deformity surgery. Spine (Phila Pa 1976). 2014 Jan 15;39(2):177-84. | CrossRef | PubMed |
- Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Decreased risk of wound infection after posterior cervical fusion with routine local application of vancomycin powder. Spine (Phila Pa 1976). 2013 May 20;38(12):991-4. | CrossRef | PubMed |
- O'Neill KR, Smith JG, Abtahi AM, Archer KR, Spengler DM, McGirt MJ, et al. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J. 2011 Jul;11(7):641-6. | CrossRef | PubMed |
- Tubaki VR, Rajasekaran S, Shetty AP. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine (Phila Pa 1976). 2013 Dec 1;38(25):2149-55. | CrossRef | PubMed |
- Emohare O, Ledonio CG, Hill BW, Davis RA, Polly DW Jr, Kang MM. Cost savings analysis of intrawound vancomycin powder in posterior spinal surgery. Spine J. 2014 Nov 1;14(11):2710-5. | CrossRef | PubMed |
- Shaffer WO, Baisden JL, Fernand R, Matz PG; North American Spine Society. An evidence-based clinical guideline for antibiotic prophylaxis in spine surgery. Spine J. 2013 Oct;13(10):1387-92. | CrossRef | PubMed |
 Khan NR, Thompson CJ, DeCuypere M, Angotti JM, Kalobwe E, Muhlbauer MS, et al. A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine. 2014 Dec;21(6):974-83. | CrossRef | PubMed |
Khan NR, Thompson CJ, DeCuypere M, Angotti JM, Kalobwe E, Muhlbauer MS, et al. A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine. 2014 Dec;21(6):974-83. | CrossRef | PubMed | Kang DG, Holekamp TF, Wagner SC, Lehman RA Jr. Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: a systematic literature review. Spine J. 2015 Apr 1;15(4):762-70. | CrossRef | PubMed |
Kang DG, Holekamp TF, Wagner SC, Lehman RA Jr. Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: a systematic literature review. Spine J. 2015 Apr 1;15(4):762-70. | CrossRef | PubMed | Alcalá-Cerra G, Paternina-Caicedo AJ, Moscote-Salazar LR, Gutiérrez-Paternina JJ, Niño-Hernández LM. [Application of vancomycin powder into the wound during spine surgery: systematic review and meta-analysis]. Rev Esp Cir Ortop Traumatol.2014 May-Jun;58(3):182-91. | CrossRef | PubMed |
Alcalá-Cerra G, Paternina-Caicedo AJ, Moscote-Salazar LR, Gutiérrez-Paternina JJ, Niño-Hernández LM. [Application of vancomycin powder into the wound during spine surgery: systematic review and meta-analysis]. Rev Esp Cir Ortop Traumatol.2014 May-Jun;58(3):182-91. | CrossRef | PubMed | Chiang HY, Herwaldt LA, Blevins AE, Cho E, Schweizer ML. Effectiveness of local vancomycin powder to decrease surgical site infections: a meta-analysis. Spine J. 2014 Mar 1;14(3):397-407. | CrossRef | PubMed |
Chiang HY, Herwaldt LA, Blevins AE, Cho E, Schweizer ML. Effectiveness of local vancomycin powder to decrease surgical site infections: a meta-analysis. Spine J. 2014 Mar 1;14(3):397-407. | CrossRef | PubMed | Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA. The Use of Vancomycin Powder In Modern Spine Surgery: Systematic Review and Meta-Analysis of the Clinical Evidence. World Neurosurg. 2015 May;83(5):816-823. | CrossRef | PubMed |
Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA. The Use of Vancomycin Powder In Modern Spine Surgery: Systematic Review and Meta-Analysis of the Clinical Evidence. World Neurosurg. 2015 May;83(5):816-823. | CrossRef | PubMed | Kanj WW, Flynn JM, Spiegel DA, Dormans JP, Baldwin KD. Vancomycin prophylaxis of surgical site infection in clean orthopedic surgery. Orthopedics. 2013 Feb;36(2):138-46. | CrossRef | PubMed |
Kanj WW, Flynn JM, Spiegel DA, Dormans JP, Baldwin KD. Vancomycin prophylaxis of surgical site infection in clean orthopedic surgery. Orthopedics. 2013 Feb;36(2):138-46. | CrossRef | PubMed | Caroom C, Tullar JM, Benton EG Jr, Jones JR, Chaput CD. Intrawound vancomycin powder reduces surgical site infections in posterior cervical fusion. Spine (Phila Pa 1976). 2013 Jun 15;38(14):1183-7. | CrossRef | PubMed |
Caroom C, Tullar JM, Benton EG Jr, Jones JR, Chaput CD. Intrawound vancomycin powder reduces surgical site infections in posterior cervical fusion. Spine (Phila Pa 1976). 2013 Jun 15;38(14):1183-7. | CrossRef | PubMed | Godil SS, Parker SL, O'Neill KR, Devin CJ, McGirt MJ. Comparative effectiveness and cost-benefit analysis of local application of vancomycin powder in posterior spinal fusion for spine trauma: clinical article. J Neurosurg Spine. 2013 Sep;19(3):331-5. | CrossRef | PubMed |
Godil SS, Parker SL, O'Neill KR, Devin CJ, McGirt MJ. Comparative effectiveness and cost-benefit analysis of local application of vancomycin powder in posterior spinal fusion for spine trauma: clinical article. J Neurosurg Spine. 2013 Sep;19(3):331-5. | CrossRef | PubMed | Rahman RKK, Lenke LG, Bridwell KH, Buchowski J, Dickson DD, Aleem A, et al. Intrawound vancomycin powder lowers the acute deep wound infection rate in adult spinal deformity patients. Spine. 2011;(suppl 1):73. | Link |
Rahman RKK, Lenke LG, Bridwell KH, Buchowski J, Dickson DD, Aleem A, et al. Intrawound vancomycin powder lowers the acute deep wound infection rate in adult spinal deformity patients. Spine. 2011;(suppl 1):73. | Link | Heller A. The effect of intrawound vancomycin powder on surgical site infections in posterior instrumented spinal arthrodesis. [tesis] University of Kansas, 2012. | Link |
Heller A. The effect of intrawound vancomycin powder on surgical site infections in posterior instrumented spinal arthrodesis. [tesis] University of Kansas, 2012. | Link | Heller A, McIff TE, Lai SM, Burton DC. Intrawound vancomycin powder decreases staphylococcal surgical site infections following posterior instrumented spinal arthrodesis. J Spinal Disord Tech. 2013 Oct 30. | PubMed |
Heller A, McIff TE, Lai SM, Burton DC. Intrawound vancomycin powder decreases staphylococcal surgical site infections following posterior instrumented spinal arthrodesis. J Spinal Disord Tech. 2013 Oct 30. | PubMed | Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976). 2011 Nov 15;36(24):2084-8. | CrossRef | PubMed |
Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976). 2011 Nov 15;36(24):2084-8. | CrossRef | PubMed | Theologis AA, Demirkiran G, Callahan M, Pekmezci M, Ames C, Deviren V. Local intrawound vancomycin powder decreases the risk of surgical site infections in complex adult deformity reconstruction: a cost analysis. Spine (Phila Pa 1976).2014 Oct 15;39(22):1875-80. | CrossRef | PubMed |
Theologis AA, Demirkiran G, Callahan M, Pekmezci M, Ames C, Deviren V. Local intrawound vancomycin powder decreases the risk of surgical site infections in complex adult deformity reconstruction: a cost analysis. Spine (Phila Pa 1976).2014 Oct 15;39(22):1875-80. | CrossRef | PubMed | Kim HS, Lee SG, Kim WK, Park CW, Son S. Prophylactic intrawound application of vancomycin powder in instrumented spinal fusion surgery. Korean J Spine. 2013 Sep;10(3):121-5. | CrossRef | PubMed |
Kim HS, Lee SG, Kim WK, Park CW, Son S. Prophylactic intrawound application of vancomycin powder in instrumented spinal fusion surgery. Korean J Spine. 2013 Sep;10(3):121-5. | CrossRef | PubMed | Pahys JM, Pahys JR, Cho SK, Kang MM, Zebala LP, Hawasli AH, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am. 2013 Mar 20;95(6):549-54. | CrossRef | PubMed |
Pahys JM, Pahys JR, Cho SK, Kang MM, Zebala LP, Hawasli AH, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am. 2013 Mar 20;95(6):549-54. | CrossRef | PubMed | Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Lumbar laminectomy and fusion with routine local application of vancomycin powder: decreased infection rate in instrumented and non-instrumented cases. Clin Neurol Neurosurg. 2013 Sep;115(9):1766-9.
| CrossRef | PubMed |
Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Lumbar laminectomy and fusion with routine local application of vancomycin powder: decreased infection rate in instrumented and non-instrumented cases. Clin Neurol Neurosurg. 2013 Sep;115(9):1766-9.
| CrossRef | PubMed | Hill BW, Emohare O, Song B, Davis R, Kang MM. The use of vancomycin powder reduces surgical reoperation in posterior instrumented and noninstrumented spinal surgery. Acta Neurochir (Wien). 2014 Apr;156(4):749-54.
| CrossRef | PubMed |
Hill BW, Emohare O, Song B, Davis R, Kang MM. The use of vancomycin powder reduces surgical reoperation in posterior instrumented and noninstrumented spinal surgery. Acta Neurochir (Wien). 2014 Apr;156(4):749-54.
| CrossRef | PubMed | Martin JR, Adogwa O, Brown CR, Bagley CA, Richardson WJ, Lad SP, et al. Experience with intrawound vancomycin powder for spinal deformity surgery. Spine (Phila Pa 1976). 2014 Jan 15;39(2):177-84. | CrossRef | PubMed |
Martin JR, Adogwa O, Brown CR, Bagley CA, Richardson WJ, Lad SP, et al. Experience with intrawound vancomycin powder for spinal deformity surgery. Spine (Phila Pa 1976). 2014 Jan 15;39(2):177-84. | CrossRef | PubMed | Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Decreased risk of wound infection after posterior cervical fusion with routine local application of vancomycin powder. Spine (Phila Pa 1976). 2013 May 20;38(12):991-4. | CrossRef | PubMed |
Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Decreased risk of wound infection after posterior cervical fusion with routine local application of vancomycin powder. Spine (Phila Pa 1976). 2013 May 20;38(12):991-4. | CrossRef | PubMed | O'Neill KR, Smith JG, Abtahi AM, Archer KR, Spengler DM, McGirt MJ, et al. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J. 2011 Jul;11(7):641-6. | CrossRef | PubMed |
O'Neill KR, Smith JG, Abtahi AM, Archer KR, Spengler DM, McGirt MJ, et al. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J. 2011 Jul;11(7):641-6. | CrossRef | PubMed | Tubaki VR, Rajasekaran S, Shetty AP. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine (Phila Pa 1976). 2013 Dec 1;38(25):2149-55. | CrossRef | PubMed |
Tubaki VR, Rajasekaran S, Shetty AP. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine (Phila Pa 1976). 2013 Dec 1;38(25):2149-55. | CrossRef | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








