Key Words: randomized controlled trial, bias, systematic review, meta-analysis, epidemiology
Abstract
Sequential analysis of clinical trials allows researchers a continuous monitoring of emerging data and greater security to avoid subjecting the trial participants to a less effective therapy before the inferiority is evident, while controlling the overall error rate. Although it has been widely used since its development, sequential analysis is not problem-free. Among them main issues to be mentioned are the balance between safety and efficacy, overestimation of the effect size of interventions and conditional bias. In this review, we develop different aspects of this methodology and the impact of including early-stopped clinical trials in systematic reviews with meta-analysis.
|
Main messages
|
Introduction
In human research, the clinical trial is the primary experimental methodological design. It is a controlled experiment used to evaluate the safety and efficacy of interventions for any health issue and is used to determine other effects of these interventions, including analyzing adverse events. Clinical trials are considered the paradigm of epidemiological research for establishing causal relationships between interventions and their effects[1],[2],[3]. They are the primary studies that nurture systematic reviews of interventions that will provide an even higher level of evidence. Therefore, the characteristics of the clinical trial will directly affect the result of a systematic review and, eventually, its meta-analysis.
Clinical trials usually include a pre-planned time frame, concluding once the expected events for the participants occur. Both the time frame and the minimum number of participants that must be included in the comparison groups to show significant differences in clinically relevant outcomes are defined a priori. However, due to ethical conditions, the study may have to be terminated early, which may be for efficacy reasons (for example, undoubted efficacy is observed in one intervention over another), safety reasons (for example, multiple adverse events in an intervention) or scientific reasons (for example, the emergence of new information that invalidates conducting the trial)[4]. An example could be what occurred with the use of human recombinant activated protein C in critically ill patients with sepsis: The original clinical trial, published in 2001, was stopped early after an interim analysis due to apparent differences in mortality[5], which led to the recommendation of its use in the Surviving Sepsis Campaign guidelines[6]. Some subsequent trials raised concern because of the increased risk of severe bleeding[7], questioning the reduction in mortality found in the original study until the drug was finally withdrawn from the market in 2011 and removed from clinical practice guidelines[6]. As observed, early discontinuation is not without contingencies and controversies, although it can be included during the protocol of some clinical trials: sequential clinical trials[8].
In this narrative review, we develop the main theoretical aspects and controversies regarding the execution of clinical trials with a sequential design that leads to early positive discontinuation, as well as their impact on systematic reviews with meta-analysis in order to clarify some concepts that have been scantly treated in the literature. There are different sequential analysis: classic or fixed, flexible, and by groups[9]. In this review, we will cover the latter, the most widely used in clinical trials.
What are sequential clinical trials?
Sequential clinical trials stipulate repeated intermediate analyses in their methodological design in order to investigate intergroup differences over time, which has various advantages. Intermediate analysis can result in:
- The continuity of the study: shows no differences, continues with the inclusion of participants, and carries out another intermediate analysis.
- Interruption due to futility or equivalence: reaches a maximum sample size defined in advance and accepts the null hypothesis of equal effects in case the analysis does not show statistically significant differences.
- Interruption due to superiority or inferiority: demonstrates differences and concludes the trial through an interim analysis[8],[10].
Characteristics and analysis of their results
Sequential clinical trials must have a large sample size since intermediate analyses involve fractionating and comparing blocks of the sample. Likewise, they must span at least two years, and the intermediate analyses must be carried out by an independent data monitoring committee to take care of masking[4]. Thus, if a statistical rule indicates the need to stop the study, an interim analysis can be performed with the data collected to assess the emerging evidence on the efficacy of treatment up to the moment of stoppage[11]. Due to their dynamic nature, clinical trials that follow this methodology must evaluate outcomes that may occur in the stipulated period, as well as guarantee the rapid availability of data for their interim analysis. Sequential analysis is a method that allows continuous monitoring of emerging data to conclude as soon as possible while controlling for the overall error rate[12]. Developed by Wald in the late 1940s and adapted for the medical sciences years later, sequential analysis has been widely used since then and has opened a new chapter in large-scale studies by creating and using statistical and data monitoring committees[12],[13]. The existence of these committees is justified based on some disadvantages of this type of study, which will be addressed later. It is important to note that the decision to stop a clinical trial is more complex and is not only subject to an interim analysis adjusted by a statistical method.
Sequential designs provide a hypothesis testing framework for making decisions regarding the early termination of a study, sometimes making it possible to save expenditures beyond the point where the evidence is convincing about the superiority of one therapy versus another. One effect is that the number of patients who would receive an inferior therapy will be reduced. Another is that this new information to patients will be more quickly disseminated to health personnel, providers, and decision-makers[11].
Intermediate analyses: periodic evaluation of the safety-efficacy balance
Intermediate analyses that seek to prove a benefit of the intervention raise the probability of a "false positive" or type I error. This error occurs when the researcher rejects the null hypothesis even while it is true in the population. It would be equivalent to concluding that there is a statistical association between both arms when, in fact, the association does not exist. Consequently, the more comparisons between interventions are made, the higher the probability of making a type I error, for which some statistical adjustments should be considered[14]. Existing methods of making these adjustments include sequential stopping rules such as the O'Brien and Fleming limits or Haybittle and Peto (rules also known as group sequential analysis methods with completion rule), as well as the α expense generalizations of Lan-DeMets[15],[16]. In these last rules, it is possible to carry out the intermediate analyses that are desired without them having been previously established since there is a decrease in the value of statistical significance by avoiding making an associated error to multiple comparisons[4]. These methods are difficult to implement and require advanced statistical knowledge[17]. Although there are recent methodologies in evaluating sequential designs[18], classical methods have been widely accepted and implemented in practice[19]. However, multiple problems can arise when researchers stop a trial earlier than planned, mainly when the decision to stop the trial is based on the finding of an apparently beneficial treatment effect[20]. The problem of balancing safety and efficacy information is possibly the most difficult to solve. It is common for safety-related outcomes to occur later, and more infrequently than efficacy-related outcomes. A trial stopped early for its efficacy could promote the adoption of therapy without fully understanding its consequences, because the trial was too small or the duration was too short to accumulate sufficient safety criteria, a potential problem even if the trial continues until the planned term[12]. This happens because the sequential analysis is based on the efficacy of the interventions, so it has less power to evaluate their safety, as illustrated by the example on the aforementioned human recombinant activated protein C.
Special considerations for interim analysis
A) Conditional bias
Another problem associated with sequential analysis is that when the trial is stopped due to a small or large effect size, there is a greater chance that the trial is at a "high" or "low" level regarding the magnitude of the effect estimation, a problem known as "conditional bias"[12]. Bias may arise due to large random fluctuations in the estimated treatment effect, particularly early in the progress of a trial. Figure 1 shows the variability in the effect's estimation according to time in three possible theoretical scenarios. The upper red line shows a trial whose effect fluctuates towards an exaggerated effect. The lower red line shows a trial whose effect fluctuates towards exaggerated harm. The blue line shows a trial whose effect oscillates around the true effect. The interruption limits in each scenario could determine very different effects regarding the true effect depending on whether traditional threshold or interruption rules are used.
Figure 1. The fluctuation of the effect measurement and possible interruption thresholds.
When researchers stop a trial based on an apparently beneficial treatment effect, its results may provide erroneous results[20]. It has been argued based on an empirical examination of studies stopped early that conditional bias is substantial and carries the potential to distort estimates of the risk-benefit balance and the meta-analytic effect. It has also been asserted that early discontinuation of a study is sometimes described with much hype, making it difficult to plan and carry out further studies on the same subject due to the unfounded belief that the effect is much more significant or safer than it is in actuality[12].
The probability of this conditional bias has been recognized for decades. Several methods of analysis have been proposed to control it after the completion of a sequential trial. However, such methods are less well known than methods for controlling type I error and are rarely used in practice when reporting the results of trials stopped early[19],[21].
B) Overestimation of treatment effect
Statistical models suggest that randomized clinical trials that are discontinued early because some benefit has been shown, overestimate the treatment effects systematically[20]. Similarly, by stopping early, the random error effect will be greater, since the number of observations will be fewer[8],[10]. By repeatedly analyzing the results of a trial, it will be the random fluctuations that exaggerate the effect that will lead to its termination: If a trial is interrupted after obtaining a low p-value, it is likely that, if the study was continued, the future p values would have been higher, which is associated with the regression to the mean phenomenon[14],[22].
Bassler and colleagues[20] compared the treatment effect of truncated randomized clinical trials with that of meta-analyses of randomized clinical trials that addressed the same research question but were not stopped early and explored factors associated with overestimation of the effect. They found substantial differences in treatment effect size between truncated and non-truncated randomized clinical trials (with a ratio of relative risks between truncated versus non-truncated of 0.71)—with truncated randomized clinical trials having less than 500 events regardless of the presence of a statistical interruption rule and the methodological quality of the studies, as assessed by concealment of allocation and blinding. Large overestimates were common when the total number of events was less than 200; smaller but significant overestimations occurred with 200 to 500 events, while trials with over 500 events showed small overestimates. This overestimation of the treatment effect arising from early discontinuation should be differentiated from any other type of overestimation due to selective reporting of research results; for example, when researchers report some of the multiple outcomes analyzed during research based on their nature and direction[23]. The tendency of truncated trials to overestimate treatment effects is particularly dangerous because it would allow for the introduction of publication bias. Their alleged convincing results are often published with no delay in featured journals and hastily disseminated in the media with decisions based on them, such as the incorporation into clinical practice guidelines, public policies, and quality assurance initiatives[24].
Implications for systematic reviews and meta-analyses
Given this rapid dissemination of truncated trials in mainstream journals[25],[26],[27], and their extensive incorporation into clinical practice, it is highly likely that, during searches of primary studies for systematic reviews, these studies are identified and included. Here, the aspects that should be adequately reported in the primary studies are the estimation of the sample size, the intermediate analysis that led to the interruption, and the rule that has been used to determine it. If systematic review authors do not notice truncation and do not consider early discontinuation of trials as a source of possible overestimation of treatment effects, meta-analyses may overestimate many of the treatment effects[28]. The inclusion of truncated randomized clinical trials may introduce artificial heterogeneity between studies, leading to an increase in the use of the random-effects meta-analytic technique, making it essential to examine the potential for bias in both study-fixed models and random-effects models[19].
In response to this risk, the exclusion of truncated studies in meta-analyses has been proposed. However, this exclusion generates an underestimation of the effect of the intervention that increases according to the number of interim analyses, which has been demonstrated through simulations[19]. This underestimation is fueled by the combination of two types of bias: estimation bias, related to how the effect measure is calculated, and information bias, related to the weighting mode of each study in the meta-analysis. When the proportion of sequential studies is low, the bias to exclude truncated studies is low. When at least half of the studies are subject to sequential analysis, the underestimation bias is in the order of five to 15%, regardless of whether the fixed or random effects approach was used. When all studies are subject to interim monitoring, the bias may be substantially higher than that range. Overall, these simulation results show that a strategy that excludes truncated studies from meta-analyses introduces a bias in the estimation of treatment effects[19].
On the other hand, although the studies stopped early would demonstrate an overestimated treatment benefit, it should not be too surprising that their inclusion in meta-analyses leads to a valid estimate. While it is true that, by including truncated trials in the meta-analysis, the difference observed in treatment overestimates the true effect, and that conditional to non-truncation, the difference observed in treatment underestimates the true effect, taken together, the effects of truncation and non-truncation would balance each other to allow an intermediate estimate. However, these simulations assume that the results of one trial do not influence how another trial is performed or whether that trial is directly ever performed[19]. This is unlikely to reflect reality since if a trial accidentally overestimates the effects of treatment and is therefore stopped early, it will be one of the first to be conducted and published and correction trials that would contribute to the combined estimation of a meta-analysis would never be carried out[24],[29].
Finally, the inclusion of trials concluded early could have implications for the certainty of the evidence. Since the number of events in these trials is usually fewer (precisely for stopping before reaching the required size), the confidence intervals of the estimator are usually wide, which leads to imprecision in the results of systematic reviews, making it highly likely that additional research has a significant impact on confidence in the effect estimates[30].
Conclusions
Sequential analysis of clinical trials can be a useful and interesting tool in terms of time and resources, but it is not without problems, such as conditional bias and overestimation of effect size. However, the combined effects obtained by meta-analyses that include truncated randomized clinical trials do not show a problem of bias, even if the truncated study prevents future experiments. Therefore, truncated randomized clinical trials should not be omitted in meta-analyses evaluating the effects of treatment. The superiority of one treatment over another demonstrated in an interim analysis of a randomized clinical trial designed with appropriate discontinuation rules, outlined in the study protocol and adequately executed, is probably a valid inference, even if the effect is slightly more significant than true, although the estimate derived from it may be imprecise.
Notes
Authors contribution
LG and JVAF conceptualized the original manuscript. All authors contributed to the writing, review, and editing of the final draft.
Funding
The authors declare that there were no external sources of funding.
Conflicts of interests
The authors declare that they have no conflicts of interest involving this work.
Ethics
This study did not require evaluation by an ethical scientific committee because it is based on secondary sources.
From the editors
The original version of this manuscript was submitted in Spanish. This English version was submitted by the authors and has been copyedited by the Journal.

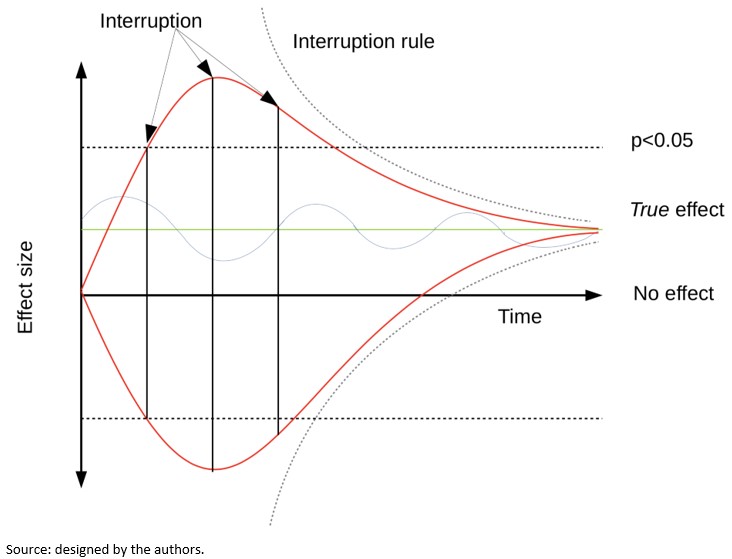 Figure 1. The fluctuation of the effect measurement and possible interruption thresholds.
Figure 1. The fluctuation of the effect measurement and possible interruption thresholds.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

El análisis secuencial de ensayos clínicos permite el monitoreo continuo de los datos emergentes para el investigador, así como una mayor seguridad para evitar someter a los participantes a una terapia inferior o fútil en términos de eficacia o seguridad, antes que esta sea evidente, mientras se controla la tasa de error general. Si bien ha sido extensamente empleado desde su desarrollo, no está exento de problemas. Entre ellos se puede mencionar el balance entre seguridad y eficacia, el sesgo condicional y la sobrestimación del tamaño del efecto de las intervenciones. En esta revisión se desarrollan distintos aspectos de esta metodología y el impacto que tiene la inclusión de ensayos clínicos precozmente interrumpidos en las revisiones sistemáticas con metanálisis.
 Authors:
Luis Ignacio Garegnani[1], Marcelo Arancibia[2], Eva Madrid[2], Juan Víctor Ariel Franco[1]
Authors:
Luis Ignacio Garegnani[1], Marcelo Arancibia[2], Eva Madrid[2], Juan Víctor Ariel Franco[1]
Affiliation:
[1] Departamento de Investigación, Centro Cochrane Asociado, Instituto Universitario Hospital Italiano, Buenos Aires, Argentina
[2] Centro Interdisciplinario de Estudios de Salud (CIESAL), Escuela de Medicina, Facultad de Medicina, Universidad de Valparaíso, Valparaíso, Chile
E-mail: marcelo.arancibiame@uv.cl
Author address:
[1] Angamos 655 Edificio R2, Oficina 1107 Reñaca, Viña del Mar, Chile

Citation: Garegnani LI, Arancibia M, Madrid E, Franco JVA. How to interpret clinical trials with sequential analysis that were stopped early. Medwave 2020;20(5):e7930 doi: 10.5867/medwave.2020.05.7930
Submission date: 29/11/2019
Acceptance date: 18/5/2020
Publication date: 5/6/2020
Origin: Not commissioned
Type of review: Externally peer-reviewed by three reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Höfler M. The Bradford Hill considerations on causality: a counterfactual perspective. Emerg Themes Epidemiol. 2005 Nov 3;2:11. | CrossRef | PubMed |
- Fedak KM, Bernal A, Capshaw ZA, Gross S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol. 2015 Sep 30;12:14. | CrossRef | PubMed |
- Lazcano-Ponce E, Salazar-Martínez E, Gutiérrez-Castrellón P, Angeles-Llerenas A, Hernández-Garduño A, Viramontes JL. Ensayos clínicos aleatorizados: variantes, métodos de aleatorización, análisis, consideraciones éticas y regulación [Randomized clinical trials: variants, randomization methods, analysis, ethical issues and regulations]. Salud Publica Mex. 2004 Nov-Dec;46(6):559-84. | CrossRef | PubMed |
- Monleón-Getino T, Barnadas-Molins A, Roset-Gamisans M. Diseños secuenciales y análisis intermedio en la investigación clínica: tamaño frente a dificultad [Sequential designs and intermediate analysis in clinical research: size vs difficulty]. Med Clin (Barc). 2009 Mar 28;132(11):437-42. | CrossRef | PubMed |
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001 Mar 8;344(10):699-709. | CrossRef | PubMed |
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013 Feb;39(2):165-228. | CrossRef | PubMed |
- Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005 Sep 29;353(13):1332-41. | CrossRef | PubMed |
- Molina-Arias M. Diseños secuenciales: interrupción precoz del ensayo clínico. Rev Pediatr Aten Primaria. 2017;19(73):87–90. [On line]. | Link |
- DeMets DL. Sequential designs in clinical trials. Card Electrophysiol Rev. 1998;2(1):57–60. | CrossRef |
- Muñoz Navarro SR, Bangdiwala SI. Análisis interino en ensayos clínicos: una guía metodológica [Interim analysis in clinical trials: a methodological guide]. Rev Med Chil. 2000 Aug;128(8):935-41. | PubMed |
- Wang H, Rosner GL, Goodman SN. Quantifying over-estimation in early stopped clinical trials and the "freezing effect" on subsequent research. Clin Trials. 2016 Dec;13(6):621-631. | CrossRef | PubMed |
- Goodman SN. Stopping trials for efficacy: an almost unbiased view. Clin Trials. 2009 Apr;6(2):133-5. | CrossRef | PubMed |
- Eckstein L. Assessing the legal duty to use or disclose interim data for ongoing clinical trials. J Law Biosci. 2019 Aug 13;6(1):51-84. | CrossRef | PubMed |
- Latour-Pérez J, Cabello-López JB. Interrupción precoz de los ensayos clínicos. Demasiado bueno para ser cierto? [Early interruption of clinical trials: too good to be true?]. Med Intensiva. 2007 Dec;31(9):518-20. | CrossRef | PubMed |
- Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659–63. | CrossRef |
- DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994 Jul 15-30;13(13-14):1341-52; discussion 1353-6. | CrossRef | PubMed |
- Hunt L. Sequential clinical trials—but not for beginners. Lancet. 1998 Mar 21;351(9106):919. | CrossRef |
- Chen DT, Schell MJ, Fulp WJ, Pettersson F, Kim S, Gray JE, et al. Application of Bayesian predictive probability for interim futility analysis in single-arm phase II trial. Transl Cancer Res. 2019 Jul;8(Suppl 4):S404-S420. | CrossRef | PubMed |
- Schou IM, Marschner IC. Meta-analysis of clinical trials with early stopping: an investigation of potential bias. Stat Med. 2013 Dec 10;32(28):4859-74. | CrossRef | PubMed |
- Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010 Mar 24;303(12):1180-7. | CrossRef | PubMed |
- Kumar A, Chakraborty BS. Interim analysis: A rational approach of decision making in clinical trial. J Adv Pharm Technol Res. 2016 Oct-Dec;7(4):118-122. | CrossRef | PubMed |
- Pocock SJ. When (not) to stop a clinical trial for benefit. JAMA. 2005 Nov 2;294(17):2228-30. | CrossRef | PubMed |
- Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions: version 6.0. Cochrane; 2019. [On line]. | Link |
- Briel M, Bassler D, Wang AT, Guyatt GH, Montori VM. The dangers of stopping a trial too early. J Bone Joint Surg Am. 2012 Jul 18;94 Suppl 1:56-60. | CrossRef | PubMed |
- Bothwell LE, Avorn J, Khan NF, Kesselheim AS. Adaptive design clinical trials: a review of the literature and ClinicalTrials.gov. BMJ Open. 2018 Feb 10;8(2):e018320. | CrossRef | PubMed |
- Cerqueira FP, Jesus AMC, Cotrim MD. Adaptive Design: A Review of the Technical, Statistical, and Regulatory Aspects of Implementation in a Clinical Trial. Ther Innov Regul Sci. 2020 Jan;54(1):246-258. | CrossRef | PubMed |
- Wayant C, Vassar M. A comparison of matched interim analysis publications and final analysis publications in oncology clinical trials. Ann Oncol. 2018 Dec 1;29(12):2384-2390. | CrossRef | PubMed |
- Bassler D, Ferreira-Gonzalez I, Briel M, Cook DJ, Devereaux PJ, Heels-Ansdell D, et al. Systematic reviewers neglect bias that results from trials stopped early for benefit. J Clin Epidemiol. 2007 Sep;60(9):869-73. | CrossRef | PubMed |
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ. 2012 Jun 15;344:e3863. | CrossRef | PubMed |
- Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. GRADE Work Gr. 2013. [On line]. | Link |
 Höfler M. The Bradford Hill considerations on causality: a counterfactual perspective. Emerg Themes Epidemiol. 2005 Nov 3;2:11. | CrossRef | PubMed |
Höfler M. The Bradford Hill considerations on causality: a counterfactual perspective. Emerg Themes Epidemiol. 2005 Nov 3;2:11. | CrossRef | PubMed | Fedak KM, Bernal A, Capshaw ZA, Gross S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol. 2015 Sep 30;12:14. | CrossRef | PubMed |
Fedak KM, Bernal A, Capshaw ZA, Gross S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol. 2015 Sep 30;12:14. | CrossRef | PubMed | Lazcano-Ponce E, Salazar-Martínez E, Gutiérrez-Castrellón P, Angeles-Llerenas A, Hernández-Garduño A, Viramontes JL. Ensayos clínicos aleatorizados: variantes, métodos de aleatorización, análisis, consideraciones éticas y regulación [Randomized clinical trials: variants, randomization methods, analysis, ethical issues and regulations]. Salud Publica Mex. 2004 Nov-Dec;46(6):559-84. | CrossRef | PubMed |
Lazcano-Ponce E, Salazar-Martínez E, Gutiérrez-Castrellón P, Angeles-Llerenas A, Hernández-Garduño A, Viramontes JL. Ensayos clínicos aleatorizados: variantes, métodos de aleatorización, análisis, consideraciones éticas y regulación [Randomized clinical trials: variants, randomization methods, analysis, ethical issues and regulations]. Salud Publica Mex. 2004 Nov-Dec;46(6):559-84. | CrossRef | PubMed | Monleón-Getino T, Barnadas-Molins A, Roset-Gamisans M. Diseños secuenciales y análisis intermedio en la investigación clínica: tamaño frente a dificultad [Sequential designs and intermediate analysis in clinical research: size vs difficulty]. Med Clin (Barc). 2009 Mar 28;132(11):437-42. | CrossRef | PubMed |
Monleón-Getino T, Barnadas-Molins A, Roset-Gamisans M. Diseños secuenciales y análisis intermedio en la investigación clínica: tamaño frente a dificultad [Sequential designs and intermediate analysis in clinical research: size vs difficulty]. Med Clin (Barc). 2009 Mar 28;132(11):437-42. | CrossRef | PubMed | Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001 Mar 8;344(10):699-709. | CrossRef | PubMed |
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001 Mar 8;344(10):699-709. | CrossRef | PubMed | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013 Feb;39(2):165-228. | CrossRef | PubMed |
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013 Feb;39(2):165-228. | CrossRef | PubMed | Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005 Sep 29;353(13):1332-41. | CrossRef | PubMed |
Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005 Sep 29;353(13):1332-41. | CrossRef | PubMed | Molina-Arias M. Diseños secuenciales: interrupción precoz del ensayo clínico. Rev Pediatr Aten Primaria. 2017;19(73):87–90. [On line]. | Link |
Molina-Arias M. Diseños secuenciales: interrupción precoz del ensayo clínico. Rev Pediatr Aten Primaria. 2017;19(73):87–90. [On line]. | Link | DeMets DL. Sequential designs in clinical trials. Card Electrophysiol Rev. 1998;2(1):57–60. | CrossRef |
DeMets DL. Sequential designs in clinical trials. Card Electrophysiol Rev. 1998;2(1):57–60. | CrossRef | Muñoz Navarro SR, Bangdiwala SI. Análisis interino en ensayos clínicos: una guía metodológica [Interim analysis in clinical trials: a methodological guide]. Rev Med Chil. 2000 Aug;128(8):935-41. | PubMed |
Muñoz Navarro SR, Bangdiwala SI. Análisis interino en ensayos clínicos: una guía metodológica [Interim analysis in clinical trials: a methodological guide]. Rev Med Chil. 2000 Aug;128(8):935-41. | PubMed | Wang H, Rosner GL, Goodman SN. Quantifying over-estimation in early stopped clinical trials and the "freezing effect" on subsequent research. Clin Trials. 2016 Dec;13(6):621-631. | CrossRef | PubMed |
Wang H, Rosner GL, Goodman SN. Quantifying over-estimation in early stopped clinical trials and the "freezing effect" on subsequent research. Clin Trials. 2016 Dec;13(6):621-631. | CrossRef | PubMed | Goodman SN. Stopping trials for efficacy: an almost unbiased view. Clin Trials. 2009 Apr;6(2):133-5. | CrossRef | PubMed |
Goodman SN. Stopping trials for efficacy: an almost unbiased view. Clin Trials. 2009 Apr;6(2):133-5. | CrossRef | PubMed | Eckstein L. Assessing the legal duty to use or disclose interim data for ongoing clinical trials. J Law Biosci. 2019 Aug 13;6(1):51-84. | CrossRef | PubMed |
Eckstein L. Assessing the legal duty to use or disclose interim data for ongoing clinical trials. J Law Biosci. 2019 Aug 13;6(1):51-84. | CrossRef | PubMed | Latour-Pérez J, Cabello-López JB. Interrupción precoz de los ensayos clínicos. Demasiado bueno para ser cierto? [Early interruption of clinical trials: too good to be true?]. Med Intensiva. 2007 Dec;31(9):518-20. | CrossRef | PubMed |
Latour-Pérez J, Cabello-López JB. Interrupción precoz de los ensayos clínicos. Demasiado bueno para ser cierto? [Early interruption of clinical trials: too good to be true?]. Med Intensiva. 2007 Dec;31(9):518-20. | CrossRef | PubMed | Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659–63. | CrossRef |
Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659–63. | CrossRef | DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994 Jul 15-30;13(13-14):1341-52; discussion 1353-6. | CrossRef | PubMed |
DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994 Jul 15-30;13(13-14):1341-52; discussion 1353-6. | CrossRef | PubMed | Hunt L. Sequential clinical trials—but not for beginners. Lancet. 1998 Mar 21;351(9106):919. | CrossRef |
Hunt L. Sequential clinical trials—but not for beginners. Lancet. 1998 Mar 21;351(9106):919. | CrossRef | Chen DT, Schell MJ, Fulp WJ, Pettersson F, Kim S, Gray JE, et al. Application of Bayesian predictive probability for interim futility analysis in single-arm phase II trial. Transl Cancer Res. 2019 Jul;8(Suppl 4):S404-S420. | CrossRef | PubMed |
Chen DT, Schell MJ, Fulp WJ, Pettersson F, Kim S, Gray JE, et al. Application of Bayesian predictive probability for interim futility analysis in single-arm phase II trial. Transl Cancer Res. 2019 Jul;8(Suppl 4):S404-S420. | CrossRef | PubMed | Schou IM, Marschner IC. Meta-analysis of clinical trials with early stopping: an investigation of potential bias. Stat Med. 2013 Dec 10;32(28):4859-74. | CrossRef | PubMed |
Schou IM, Marschner IC. Meta-analysis of clinical trials with early stopping: an investigation of potential bias. Stat Med. 2013 Dec 10;32(28):4859-74. | CrossRef | PubMed | Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010 Mar 24;303(12):1180-7. | CrossRef | PubMed |
Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010 Mar 24;303(12):1180-7. | CrossRef | PubMed | Kumar A, Chakraborty BS. Interim analysis: A rational approach of decision making in clinical trial. J Adv Pharm Technol Res. 2016 Oct-Dec;7(4):118-122. | CrossRef | PubMed |
Kumar A, Chakraborty BS. Interim analysis: A rational approach of decision making in clinical trial. J Adv Pharm Technol Res. 2016 Oct-Dec;7(4):118-122. | CrossRef | PubMed | Pocock SJ. When (not) to stop a clinical trial for benefit. JAMA. 2005 Nov 2;294(17):2228-30. | CrossRef | PubMed |
Pocock SJ. When (not) to stop a clinical trial for benefit. JAMA. 2005 Nov 2;294(17):2228-30. | CrossRef | PubMed | Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions: version 6.0. Cochrane; 2019. [On line]. | Link |
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions: version 6.0. Cochrane; 2019. [On line]. | Link | Briel M, Bassler D, Wang AT, Guyatt GH, Montori VM. The dangers of stopping a trial too early. J Bone Joint Surg Am. 2012 Jul 18;94 Suppl 1:56-60. | CrossRef | PubMed |
Briel M, Bassler D, Wang AT, Guyatt GH, Montori VM. The dangers of stopping a trial too early. J Bone Joint Surg Am. 2012 Jul 18;94 Suppl 1:56-60. | CrossRef | PubMed | Bothwell LE, Avorn J, Khan NF, Kesselheim AS. Adaptive design clinical trials: a review of the literature and ClinicalTrials.gov. BMJ Open. 2018 Feb 10;8(2):e018320. | CrossRef | PubMed |
Bothwell LE, Avorn J, Khan NF, Kesselheim AS. Adaptive design clinical trials: a review of the literature and ClinicalTrials.gov. BMJ Open. 2018 Feb 10;8(2):e018320. | CrossRef | PubMed | Cerqueira FP, Jesus AMC, Cotrim MD. Adaptive Design: A Review of the Technical, Statistical, and Regulatory Aspects of Implementation in a Clinical Trial. Ther Innov Regul Sci. 2020 Jan;54(1):246-258. | CrossRef | PubMed |
Cerqueira FP, Jesus AMC, Cotrim MD. Adaptive Design: A Review of the Technical, Statistical, and Regulatory Aspects of Implementation in a Clinical Trial. Ther Innov Regul Sci. 2020 Jan;54(1):246-258. | CrossRef | PubMed | Wayant C, Vassar M. A comparison of matched interim analysis publications and final analysis publications in oncology clinical trials. Ann Oncol. 2018 Dec 1;29(12):2384-2390. | CrossRef | PubMed |
Wayant C, Vassar M. A comparison of matched interim analysis publications and final analysis publications in oncology clinical trials. Ann Oncol. 2018 Dec 1;29(12):2384-2390. | CrossRef | PubMed | Bassler D, Ferreira-Gonzalez I, Briel M, Cook DJ, Devereaux PJ, Heels-Ansdell D, et al. Systematic reviewers neglect bias that results from trials stopped early for benefit. J Clin Epidemiol. 2007 Sep;60(9):869-73. | CrossRef | PubMed |
Bassler D, Ferreira-Gonzalez I, Briel M, Cook DJ, Devereaux PJ, Heels-Ansdell D, et al. Systematic reviewers neglect bias that results from trials stopped early for benefit. J Clin Epidemiol. 2007 Sep;60(9):869-73. | CrossRef | PubMed | Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ. 2012 Jun 15;344:e3863. | CrossRef | PubMed |
Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ. 2012 Jun 15;344:e3863. | CrossRef | PubMed | Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. GRADE Work Gr. 2013. [On line]. | Link |
Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. GRADE Work Gr. 2013. [On line]. | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








