Key Words: Work of Breathing, Mechanical Ventilation, Ventilator Weaning, Intensive Care Units, COVID- 19
Abstract
Technological advances in mechanical ventilation have been essential to increasing the survival rate in intensive care units. Usually, patients needing mechanical ventilation use controlled ventilation to override the patient’s respiratory muscles and favor lung protection. Weaning from mechanical ventilation implies a transition towards spontaneous breathing, mainly using assisted mechanical ventilation. In this transition, the challenge for clinicians is to avoid under and over assistance and minimize excessive respiratory effort and iatrogenic diaphragmatic and lung damage. Esophageal balloon monitoring allows objective measurements of respiratory muscle activity in real time, but there are still limitations to its routine application in intensive care unit patients using mechanical ventilation. Like the esophageal balloon, respiratory muscle electromyography and diaphragmatic ultrasound are minimally invasive tools requiring specific training that monitor respiratory muscle activity. Particularly during the coronavirus disease pandemic, non invasive tools available on mechanical ventilators to monitor respiratory drive, inspiratory effort, and work of breathing have been extended to individualize mechanical ventilation based on patient’s needs. This review aims to identify the conceptual definitions of respiratory drive, inspiratory effort, and work of breathing and to identify non invasive maneuvers available on intensive care ventilators to measure these parameters. The literature highlights that although respiratory drive, inspiratory effort, and work of breathing are intuitive concepts, even distinguished authors disagree on their definitions.
|
Main messages
|
Introduction
Patients hospitalized in intensive care units requiring mechanical ventilation are increasing worldwide, especially during the coronavirus pandemic (COVID-19) [1],[2]. Technological and procedural advances in mechanical ventilation are essential in increasing survival in the intensive care unit, mainly those related to monitoring and maintaining synchrony between ventilator support and respiratory muscle activity [3]. Controlled ventilation is most commonly used, temporarily attenuating or overriding respiratory muscles and favoring lung protection [4].
Weaning from mechanical ventilation requires a transition period towards spontaneous breathing [5]. During this period, clinicians should avoid under and over-assistance of the respiratory muscles, minimizing excessive inspiratory effort and ventilator-induced lung and diaphragmatic injury [6]. These threats reinforce the need to personalize mechanical ventilation and identify precise ventilatory parameters based on specific physiological variables and individualized goals [7]. Individualization of ventilatory settings becomes even more challenging during assisted ventilation [8], where a delicate balance between respiratory muscle load and ventilator support must be maintained. Following clinicians' knowledge, assisted ventilation is mainly modulated through tidal volume as an indicator of over-assistance [9] rather than considering specific inspiratory effort parameters [8],[10].
Esophageal pressure (Peso) measurement by esophageal balloon is useful for:
- Characterizing the mechanical properties of the respiratory system (primarily during controlled mechanical ventilation).
- Understanding the patient-ventilator interaction.
- Monitoring continuously real-time respiratory muscle activity during assisted mechanical ventilation [11],[12].
Notably, the esophageal balloon allows breath-by-breath monitoring of the patient’s work of breathing (WOBp) through the Campbell diagram [13]. Additionally, it allows measuring more specific components of inspiratory effort through the pressure time product (PTP) and the respiratory muscle pressure (Pmus) [11,12]. An average respiratory effort haves a pressure-time product between 50 and 150 centimeters of water per second per minute or a muscle pressure between 5 and 10 centimeters of water. Therefore, the esophageal balloon permits adjusting respiratory support based on each patient’s performance, thus customizing mechanical ventilation programming.
Although esophageal balloon monitoring provides objective measurements of respiratory effort and work of breathing, it requires time, specialized equipment, and correct physiological interpretation of signals, limiting its routine application in ventilated patients [11],[12]. The respiratory effort can also be estimated through the diaphragmatic electrical activity, either using diaphragmatic surface electromyography [14] or neurally adjusted ventilatory assist [15], although it requires electrodes or a nasogastric tube to operate. On the other hand, measuring the thickening fraction by diaphragmatic ultrasound approximates inspiratory effort and correlates well with esophageal balloon variables. However, this measurement also needs equipment and training, and it is not continuous or synchronized with ventilator waveforms [16]. Therefore, clinicians require simple, feasible, non-invasive techniques to assess patient ventilator interaction and respiratory effort.
During the last pandemic years, an emphasis has been placed on studying respiratory drive and inspiratory effort due to its correlation with hypoxemia and dyspnea in COVID-19 patients with acute respiratory distress syndrome [17]. Moreover, noninvasive maneuvers available on intensive care ventilators to monitor respiratory drive, inspiratory effort, and work of breathing – especially during pressure support ventilation [17],[18],[19],[20] – have become popular to promote mechanical ventilation tailored to the patient’s needs. This review aims to identify the conceptual definitions of respiratory drive, inspiratory effort, and work of breathing and to identify non-invasive maneuvers available on intensive care ventilators to measure these parameters.
Methods
References from a previously published scoping review related to this topic were used to select articles [21]. First, a search update was performed in PubMed/MEDLINE, combining keywords such as: "respiratory drive", "respiratory effort", "work of breathing", "assisted ventilation", "intensive care unit", and "mechanical ventilation". All studies that responded to the objective were non-systematically selected, without limiting by year of publication or type of study. Articles related to COVID-19 were also included. Second, the operating manuals of intensive care ventilators used in Chile were reviewed. Finally, a field review was made of the most commonly used ventilators in public and private hospitals of Chile (in person or remotely by video call), searching for non-invasive maneuvers to measure or estimate respiratory drive, inspiratory effort, or work of breathing. Hospitals and companies were visited until the brands and models of the most used mechanical ventilators in Chile during the pandemic were included. All ventilators designed for invasive ventilation with valve operation were included, except ventilators for hospital transport.
This review presents physiological concepts and definitions of respiratory drive, inspiratory effort, and work of breathing and describes and collects the maneuvers available in intensive care ventilators. For this purpose, we relied on selected literature and the on-site review of ventilators, especially for non-invasive maneuvers.
Results and discussion
Definitions
The literature highlights that although respiratory drive, inspiratory effort, and work of breathing are intuitive concepts, even distinguished authors disagree on their definitions [22] (Table 1). Under healthy conditions, respiratory drive, inspiratory effort, and work of breathing are closely related, but this may be untrue in pathological states [23]. This background justifies analyzing definitions of the parameters addressed in this review (Figure 1).
Definition of respiratory drive
The primary objective of respiration is to maintain balanced gas exchange. In other words, a balance between oxygen intake and carbon dioxide (CO2) production. This goal is achieved by tight control of respiration through regulation of the respiratory center. The intensity and speed of the response depend on feedback from four input sources: biochemical, mechanical, emotional, and inflammatory [23].
The first and foremost input source is biochemical, which corresponds to reflex feedback mediated by input originating from central and peripheral chemoreceptors [24]. This control mechanism minimizes arterial carbon dioxide levels and pH fluctuations and avoids hypoxemia [25]. Peripheral chemoreceptors in the carotid bodies also regulate the respiratory drive by modifying central chemoreceptors' sensitivity and excitatory threshold [26].
The second input corresponds to information from mechanoreceptors at the lungs, costal grid, airway, and respiratory muscles. Muscle spindle use and slowly adapting stretch receptors are examples of this, as they provide information on stretch and lung volume through their vagal inputs. These receptors are responsible for the Hering-Breuer reflex, which terminates inspiration and facilitates expiration at high tidal volumes [27].
The third input corresponds to suprapontine feedback. Fear, anxiety, pain, and delirium transmit sensory feedback to the respiratory centers through the cerebral cortex and hypothalamus modulations.
The fourth input is mediated by inflammatory responses [23] from different pathological states, including sepsis and acute respiratory failure. Inflammatory mediators (histamine, bradykinins, and prostaglandins) elicit a vagal stimulus that increases respiratory rate and decreases tidal volume [25], triggering a rapid and shallow respiratory pattern.
Once the information from all four inputs is integrated, a response is elaborated by the respiratory center, known as the respiratory drive. Thus, the respiratory drive is defined as the intensity of the respiratory efference generated in the central nervous system [18],[23],[28]. Several authors agree that the drive does not necessarily reflect changes in the inspiratory effort since it is an electrical signal and not directly a mechanical force. The respiratory drive is originated from an interneuron group (respiratory center) at the brainstem [27]. The amplitude of the signal emerging from this neuronal group determines the mechanical response of the respiratory muscles.
In patients with mechanical ventilation, a diminished respira-tory drive – either due to over- assistance or sedation – will con-tribute to diaphragmatic weakness [29]. A weak drive will also be responsible for the loss of synchrony between the patient and the ventilator. This asynchrony may cause a high drive that damages the diaphragm due to overload [30]. Likewise, increased respiratory drive leads to a rapid and shallow respiratory pattern, with the recruitment of accessory musculature, increased dyspnea, and, consequently, failure of spontaneous breathing trials [31]. Another effect of an excessive respiratory drive is patient- ventilator asynchrony. During assisted ventila-tion, parameter adjustement that is not following the ventila-tory demands can lead to flow starvation, premature cycling, or ineffective triggering [32],[33]. Therefore, the drive (and its inputs) is an antecedent to respiratory effort and work. Its intensity and magnitude determine muscle activity and rhythm.
Definition of inspiratory effort
The definition of inspiratory effort seems intuitive; however, it has been little explored in the literature and could be confused with work of breathing [22],[34]. Moreover, in certain situations, such as air trapping (PEEPi) or respiratory muscle weakness, its conceptualization can be confusing whenever effort and work are assumed to be the same. We should keep in mind that respiratory drive controls inspiratory effort to maintain a balance with ventilatory demands. Thus, inspiratory effort depends on the intensity of the efference from the higher centers and the viscoelastic properties of the respiratory system [19],[34].
The respiratory muscle pump is responsible for maintaining a balance according to metabolic demands and comprises different skeletal muscles that act in a coordinated manner to support alveolar ventilation [22]. The diaphragm carries out the inspiratory muscle pump function [35]. However, when the respiratory load increases and the diaphragm alone cannot respond to the demand, accessory muscles are recruited to contribute to inspiration. Sternocleidomastoid, parasternal, and external intercostal muscles are the main contributing muscles [36]. Therefore, during spontaneous breathing, the fall in the pleural pressure depends on the contraction of the inspiratory muscles (muscle pressure) and the pressure gradient generated over the chest wall [22],[37].
According to the literature, the inspiratory effort is defined as any consumption of energy by the respiratory muscles to conduct respiration [22], which considers the time and type of muscle contraction. However, isometric contractions consume energy and constitute an effort but do not translate into a volume change. This aspect is crucial because it differentiates inspiratory effort from the work of breathing. Not every contraction of the respiratory muscles results in a change of volume, but it always translates into energy consumption. In healthy subjects, inspiration begins at the equilibrium point of the respiratory system. That is, where the collapsing tendency of the lungs is equal to the tendency toward rib cage expansion [38]. At this point, the action of the inspiratory muscles (muscle pressure) causes a fall in pleural pressure and, consequently, generates a volume change.
Definition of work of breathing
In physics, work refers to the force applied to an object and the displacement generated by it [34]; in other words, it corre-sponds to force times displacement (W= F × d). In the respiratory system, work of breathing (WOB) is calculated as the product of the integral of pressure and volume:
WOB = ∫ pressure × volume
I.e., the area in a pressure- volume diagram [13]. Conceptually, it is defined as the work required to cope with the load imposed by the respiratory system. This work can be carried out by the respiratory muscles when breathing is spontaneous, exclusively by the mechanical ventilator if breathing is artificially controlled, or by both in the case of assisted ventilation [39]. To understand the components of the work of breathing, we need to mention the equation of motion. This equation shows that the airway pressure has an elastic component (necessary to overcome the elastance of the parenchyma), a resistive component (the circulation of a flow is constantly opposed by a resis-tive load), and an inertial component (negligible for respiratory frequencies lower than one Hertz) [40]:
Paw = Ers × V + Raw × Q + PEEPtot
Where, Paw: airway pressure, Ers: elastance of the respiratory system, V: volume, Raw: airway resistance, Q: gas flow, and PEEPtot: positive end-expiratory pressure.
From this equation, it can be deduced that the work of breath-ing must overcome resistive and elastic components and the air trapped at the end of expiration (positive end- expiratory pres-sure) [13].
The concept of work of breathing can be confusing because the classical definition of work states there must always be a change in volume following a change in pressure. However, the consumption of energy from the isometric component of respiratory muscles in healthy and diseased individuals is not included in the classical concept [34].
Non-invasive maneuvers of drive, effort, and work of breathing available in intensive care ventilators
Within the maneuvers available in ventilators, we identified the airway occlusion pressure of the first 100 milliseconds (P0,1), the expiratory occlusion pressure difference (ΔPocc), and the pressure muscle index (PMI). In addition, variables calculable with ventilator information such as muscle pressure, pressure- time product, and patient work of breathing are also considered (Table 2).
Airway occlusion pressure
Occlusion pressure at the first 100 milliseconds is a non-invasive measurement for drive monitoring, defined by the airway pressure drop during the first 100 milliseconds (0.1 seconds) against an end-expiratory occlusion. It was initially described by Whitelaw et al. in 1975 in healthy individuals [41], later began to be used in critically ill patients [42],[43], and is still a subject of study [44],[45],[46]. It is also a tool used to predict weaning from mechanical ventilation and to adjust respiratory support, even in patients on extracorporeal life support [47]. Occlusion pres-sure in the first 100 milliseconds ranges from 0.5 to 1.5 centimeters of water in healthy individuals and 1.3 to 3.5 centimeters of water in critically ill patients [18]. An occlusion pressure at the first 100 milliseconds greater than 3.5 centimeters of water has a sensitivity of 92% and a specificity of 89% for detecting excessive inspiratory effort [48]. Modern ventilators continuously display occlusion pressure at the first 100 milliseconds by estimating it according to the airway pressure drop during the activation phase or by a brief occlusion maneuver at the end of expiration [49]. Occlusion pressure at the first 100 milliseconds increases proportionally to alveolar carbon dioxide pressure during assisted ventilation and is unaffected by respiratory muscle weakness [47],[50]. As the breath-to-breath variability of occlusion pressure at the first 100 milliseconds is significant, it is recommended to consider an average of three to four measurements [18].
Expiratory occlusion maneuver
With an expiratory occlusion maneuver in assisted ventilation, we can obtain a difference in the expiratory occlusion pressure, i.e., the difference between the baseline pressure (commonly the positive end-expiratory pressure) and the most negative value during the pressure drop after a mechanical occlusion at the end of expiration (usually a five- second expiratory pause) [51]. In simple terms, it is the continuation of the occlusion pressure maneuver to the first 100 milliseconds considering the most negative value.
The expiratory occlusion pressure difference maneuver was first described by Bertoni et al. in 2019 [51] as a non-invasive method to estimate inspiratory effort during assisted ventilation. Expiratory occlusion is commonly used to measure posi-tive intrinsic end-expiratory pressure in controlled ventilation or peak inspiratory pressure (PIP) during maximal inspiratory efforts. Inspiratory effort can also be assessed by airway pressure oscillation during a brief randomly applied end-expiratory occlusion maneuver without maximal voluntary effort. Indeed, the airway pressure oscillation during occlusion correlates with the pleural pressure oscillation [37], as long as this single, brief, unexpected occlusion does not majorly modify the respiratory drive [52].
Considering the differences in esophageal pressure oscillation between occluded and dynamic conditions, it has been pro-posed to multiply the result of the expiratory occlusion pressure difference by 0.75 to obtain a predicted muscle pressure [20]. That is:
Pmus = −0.75 × ΔPocc
This corresponds to the global force generated by the inspira-tory muscles (https://rtmaven.com). The expected muscle pressure for a patient with adequate inspiratory effort is between 5 and 10 centimeters of water [53]. For expiratory occlusion, pressure differences are between 7 and 15 centime-ters of water [20]. Alternatively, using the expiratory occlusion maneuver, the predicted dynamic transpulmonary driving pres-sure (ΔPL) can be calculated as:
ΔPL = (peak Paw − PEEP) −0.66 × ΔPocc
This equation indicates overdistention with values over 16 to 17 centimeters of the water. The expiratory occlusion maneuver can detect potential lung damage and excessive or absent respi-ratory effort, and it can also be used to differentiate various forms of patient- ventilator asynchronies. This maneuver can unmask ineffective efforts, identify auto triggers, and differentiate double triggers from reverse triggers (in which case there will be apnea during the expiratory occlusion) [54]. Suppose the ventilator can measure the start and end of inspiratory effort during the expiratory pause. In that case, one could estimate the patient’s neural inspiratory time and observe the morphology of the pressure drop given by the slope between the occlusion pressure at the first 100 milliseconds and the difference of the expiratory occlusion pressure. A notable limitation of the expiratory occlusion maneuver is that dynamic hyperinflation and intrinsic positive end-expiratory pressure can underestimate the effort, especially if the intrinsic positive end-expiratory pressure has not equilibrated with the occluded airway [54].
Inspiratory occlusion maneuver
A brief end- inspiratory occlusion maneuver is widely used to measure plateau pressure (Pplat) and driving pressure (ΔP) in controlled mechanical ventilation. Bellani et al. suggested that a brief inspiratory occlusion maneuver allows reliable plateau pressure measurements even in assisted mechanical ventilation [55]. During an inspiratory occlusion in mechanically assisted ventilation, patients relax the inspiratory muscles, increasing airway distending pressure, which is detectable on the ventilator waveform.
The inspiratory occlusion maneuver in assisted ventilation is not new. Foti et al. first described it in 1997 as a method to assess the pressure developed by the inspiratory muscles in patients with acute lung injury during pressure support ventilation [56]. When the patient is over-assisted, and the respiratory effort is low, the airway pressure falls below the plateau pressure during inspiratory occlusion in assisted ventilation. On the contrary, when the airway pressure rises above the plateau pressure value, it could indicate a high respiratory effort or under-assistance, indicated by the magnitude of the relaxation of the inspiratory muscles. The difference between airway and plateau pressure during an inspiratory occlusion is known as PMI, Pmusc index, or pressure muscle index [56]. During pressure support ventilation, the muscle pressure index is calculated as:
PMI = Pplat − (PEEP + PS)
Where the Pplat is the pressure reached during inspiratory occlusion. Values of pressure muscle index greater than three centimeters of water indicate an elevated inspiratory effort [56]. Part of the reasoning behind the pressure muscle index was described by Prinianakis et al. in 2008 while studying the cause of the increased ventilator pressure above the end- inspiratory pressure support during pressure support ventila-tion [57]. Since inspiratory muscle relaxation precedes expiratory muscle contraction, the increase in ventilator pres-sure using pressure support ventilation may imply the end of neural inspiration. However, the technique used to measure pressure muscle index is not without limitations. Because the pressure is obtained under quasi- static conditions, this mea-surement may be affected by Pendelluft mechanisms, espe-cially in patients with acute respiratory distress syndrome [58]. Moreover, the airway pressure tracing may be unstable during occlusion, which could confound inspiratory muscle relaxation with abdominal muscle activity that may rapidly increase air-way pressure in the onset of neural expiration during inspira-tory occlusion [59].
Calculations derived from ventilator information
Current ventilators provide estimated values of inspiratory effort and work of breathing variables without needing a probe or additional equipment, including the pressure-time product and the patient’s work of breathing parameters. The methods available in ventilators to approximate these parameters – derived initially from esophageal balloons [60] – are not entirely accurate. For this reason, they should be taken with caution, as each ventilator has different pressurization characteristics [61]. Some ventilators deliver the pressure- time product or patient’s work of breathing based on estimates of the area of the pressure-time curve.
Particularly, proportional assist ventilation with adjustable load was initially described by Magdy Jones in 1991 as a ventilatory mode that delivered support according to patient demand, considering the mechanical properties of the respiratory system [62]. Since 2007, proportional assist ventilation with load- adjustable gain factors (PAV+) delivers ventilator and patient’s elastic and resistive work of breathing values from a brief 300- millisecond inspiratory occlusion. From this analysis, we can calculate the plateau pressure and, consequently, the main components of the equation of motion – i.e., compliance and resistance [63],[64]. When using proportional assist ventilation with load- adjustable gain factors, the expected values for a good work of breathing range from 0.3 to 0.7 Joules per liter. Since 2013, proportional assist ventilation with load- adjustable gain factors permits calculating muscle pressure and pressure- time product using Carteaux et al. formulas [53] without an esophageal balloon. Therefore, muscle pressure can be calculated as:
Pmus = (Ppeak – PEEP) × (% support ÷ % patient)
Where % support is the percentage of assistance set in the ventilator and % patient is the percentage of work performed by the patient (https://bit.ly/3kj6oTz). Consequently, by obtain-ing the muscle pressure, it is possible to calculate:
PTP = [(Pmus x inspiratory time) × respiratory frequency] ÷ 2.
Estimation of the pressure-time product (PTP) was only possi-ble with proportional assist ventilation with load- adjustable gain factors until the expiratory occlusion pressure difference was described using the expiratory occlusion maneuver, from which muscle pressure is obtained. However, – although it has not yet been studied – it is now possible to estimate the pressure- time product during ventilation with pressure support using the muscle pressure calculation with the expiratory occlusion maneuver.
Waveforms and asynchronies
The presence of asynchronies in pressure- time and flow- time curves can alert high respiratory drive and high respiratory effort, particularly in flow starvation, premature cycling, and double triggering [18]. In general, the interpretation of the ventilatory waveform may help identify a mismatch between neural and mechanical inspiratory time, which is common under pressure support ventilation [65]. Just as the slopes of pressure and flow versus time waveforms can help measure the ’stress index' and ineffective efforts, they can also help estimate the level of inspiratory effort.
Recently, a new method based on the concavity of inspiratory flow waveform called 'flow index' was described [66]. Although this method still requires more studies on clinical applicability without additional equipment, it allows approaching the inspi-ratory effort concept using the analysis and interpretation of the ventilatory waveform to adjust pressure support ventilation according to the patient’s effort level and tidal volume [63].
Future directions
It is often assumed that deep sedation minimizes or abolishes spontaneous inspiratory effort ("respirolysis") [67]. Future management of mechanical ventilation aims to reduce deep sedation as routine clinical practice by including other alterna-tives for inspiratory effort control [68]. First, by developing innovative ventilator features that improve patient- ventilator synchrony, such as neurally adjusted ventilatory assist. Second, by using extracorporeal support in patients with severe acute respiratory distress syndrome to eliminate excess carbon diox-ide as an undesirable stimulus to the respiratory center [67],[68]. Finally, by approaching current mechanical ventilation settings based on respiratory muscle demand to mitigate asynchronies without excessive sedation.
Conclusions
The differences between respiratory drive, inspiratory effort, and work of breathing concepts are essential to understanding the interaction between the patient’s respiratory muscle pump and the ventilatory support.
These three concepts are co-dependent and can be monitored noninvasively using maneuvers or tools from intensive care mechanical ventilators.
Nevertheless, not all ventilators can measure respiratory drive, inspiratory effort, or work of breathing variables using pressure support ventilation. The most commonly available maneuvers are the occlusion pressure in the first 100 milliseconds and expiratory occlusion pressure. These maneuvers have growing sci-entific evidence and could be used to monitor drive and inspiratory effort in more than 60% of ventilators used in Chile. Moreover, the accessibility of these maneuvers is even more relevant in pandemic times due to the large volume of critically ill patients.
Future studies should explore the accuracy and relevance of the non- invasive maneuvers identified in this review.
Notes
Contributor roles
FR- C: conceptualization, methodology, research, data curation, preparation of the original manuscript, review and editing of the article, visualization, and project management. FG- S: conceptualization, methodology, research, data curation, original manuscript preparation, manuscript review and editing, visualization, and project management. JM: conceptualization, manuscript review and editing, and supervision.
Acknowledgments
We thank the following institutions and collaborators during the field review of the intensive care ventilators: Hospital Metropolitano, Santiago (Nicolás Aguirre and Javier Leiva), Clínica INDISA, Santiago (Felipe Castillo Merino and Juan Enrique Lee Goic), Hospital del Salvador, Santiago (Antonieta Rosales and Andrea Fuentes), Hospital Eduardo Pereira, Valparaíso (Sebastián Castro), Clínica Bicentenario, Santiago (Nicolás Piñeiro), Hospital San Pablo, Coquimbo (Eduardo González), Critical Patient Department, Clínica Alemana, Santiago (Agustín Camus- Molina) and DTS - Neyün, Santiago (Roger Burgos, Patricia Espinosa, Michael Espinoza).
Competing interests
The authors declare no competing interests with this work.
Funding
The authors declare that this work was not financed.
Ethics
This article is exempt from approval by an accredited ethics committee because it is a narrative review article.
Provenance and peer review
Not commissioned. Externally peer- reviewed by three reviewers, double- blind.
Language of submission
Spanish

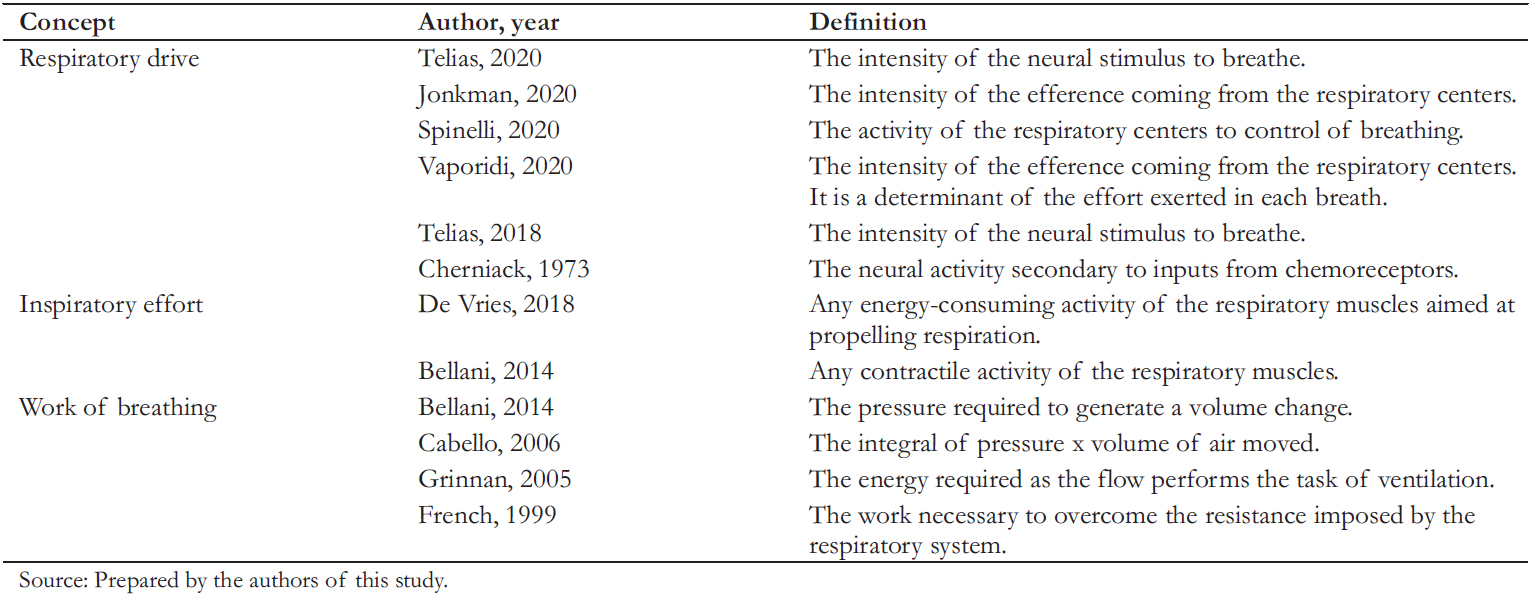 Table 1. Definitions of respiratory drive, inspiratory effort, and work of breathing identified in the literature.
Table 1. Definitions of respiratory drive, inspiratory effort, and work of breathing identified in the literature.

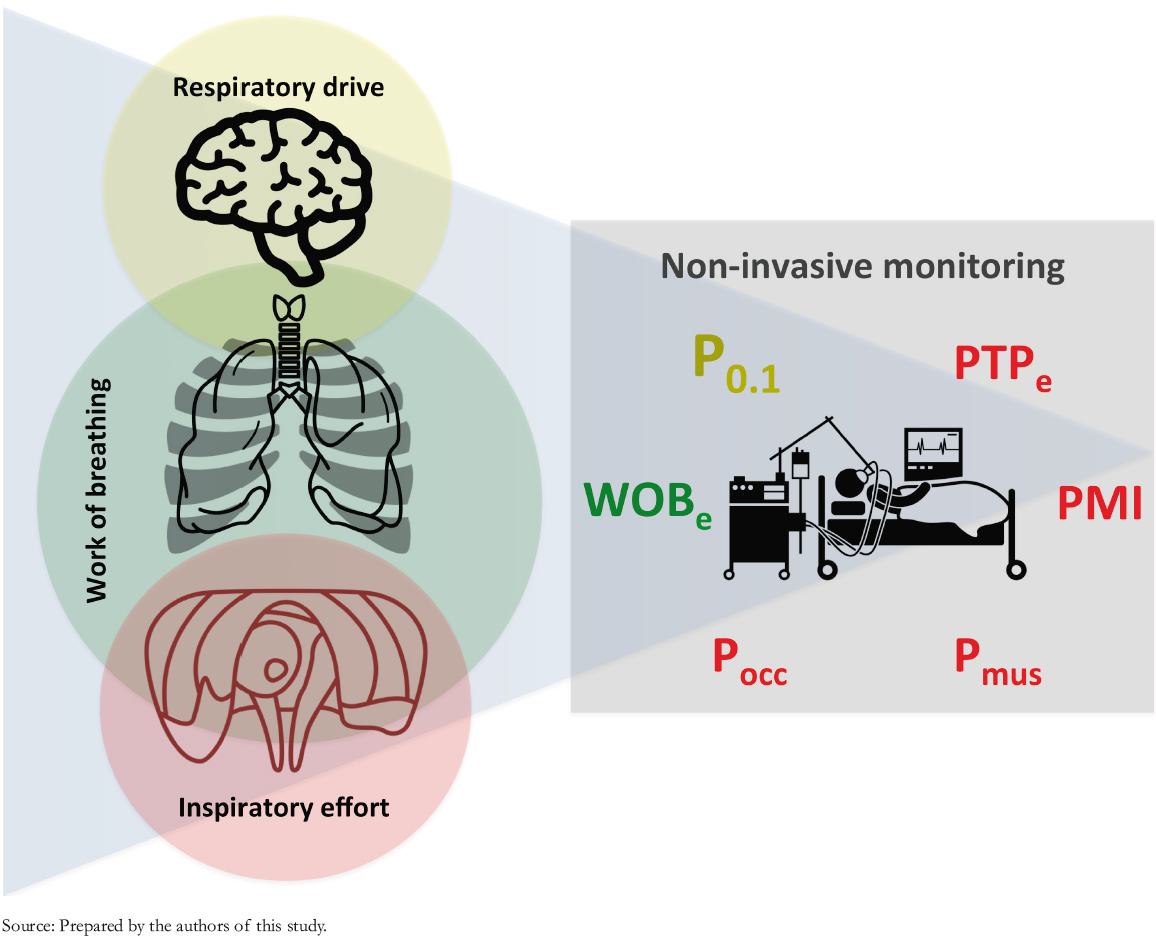 Figure 1. Graphical definition and alternatives for monitoring respiratory drive, inspiratory effort, and work of breathing in intensive care ventilators.
Figure 1. Graphical definition and alternatives for monitoring respiratory drive, inspiratory effort, and work of breathing in intensive care ventilators.

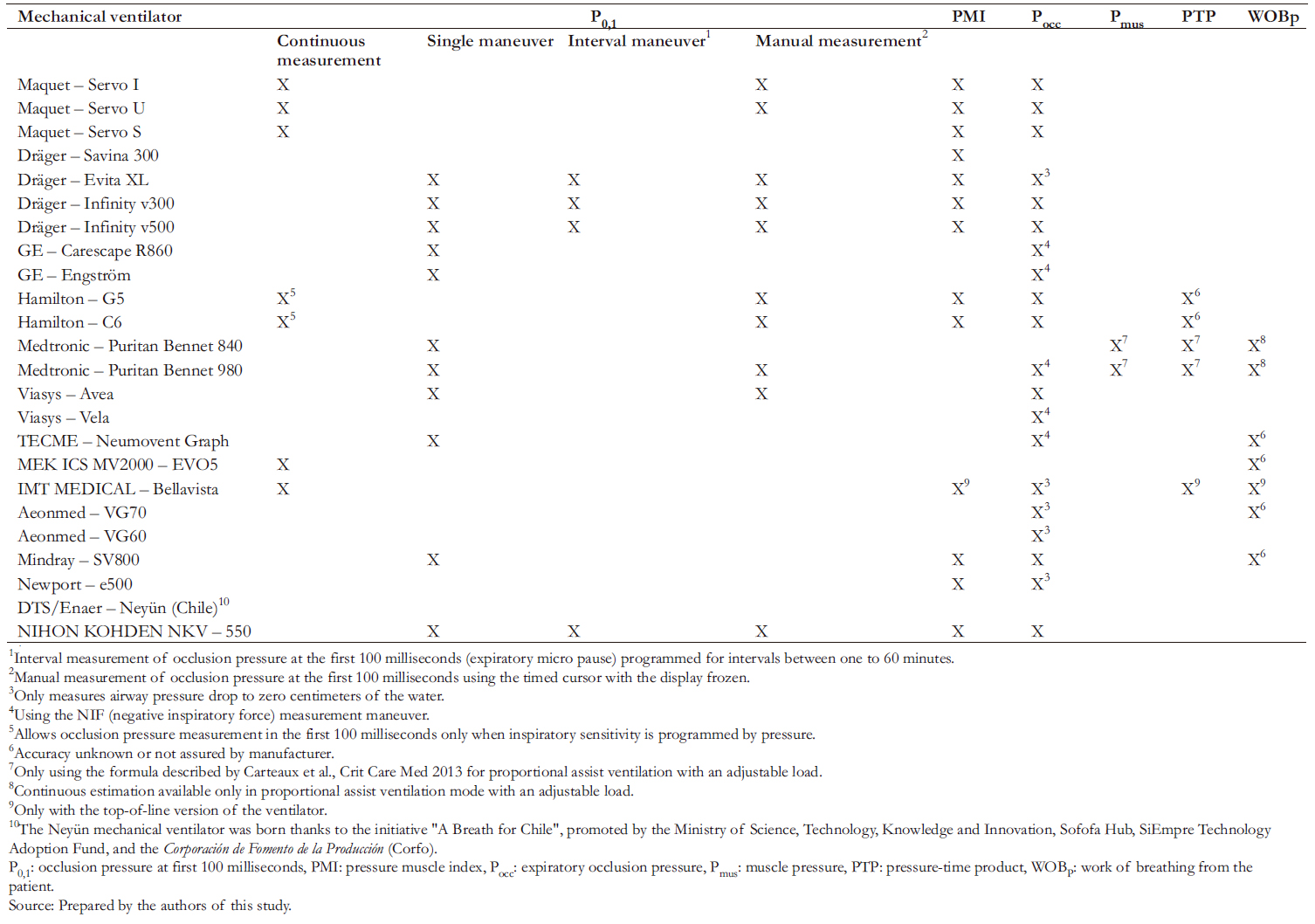 Table 2. Non-invasive maneuvers to monitor respiratory drive, inspiratory effort and/or work of breathing during pressure support ventilation available in intensive care ventilators used in Chile.
Table 2. Non-invasive maneuvers to monitor respiratory drive, inspiratory effort and/or work of breathing during pressure support ventilation available in intensive care ventilators used in Chile.

Los avances tecnológicos de la ventilación mecánica han sido parte esencial del aumento de la sobrevida en las unidades de cuidados intensivos. Desde la conexión a la ventilación mecánica, comúnmente se utiliza ventilación controlada sin la consecuente participación de los músculos respiratorios del paciente, con el fin de favorecer la protección pulmonar. El retiro de la ventilación mecánica implica un periodo de transición hacia la respiración espontánea, utilizando principalmente ventilación mecánica asistida. En esta transición, el desafío de los clínicos es evitar la sub y sobre asistencia ventilatoria, minimizando el esfuerzo respiratorio excesivo, daño diafragmático y pulmonar inducidos por la ventilación mecánica. La monitorización con balón esofágico permite mediciones objetivas de la actividad muscular respiratoria en tiempo real, pero aún hay limitaciones para su aplicación rutinaria en pacientes ventilados mecánicamente en la unidad de cuidados intensivos. Al igual que el balón esofágico, la electromiografía de los músculos respiratorios y la ecografía diafragmática son herramientas que permiten monitorizar la actividad muscular de la respiración, siendo mínimamente invasivas y con requerimiento de entrenamiento específico. Particularmente, durante la actual pandemia de enfermedad por coronavirus se ha extendido el uso de herramientas no invasivas disponibles en los ventiladores mecánicos para monitorizar el impulso (drive), esfuerzo y trabajo respiratorio, para promover una ventilación mecánica ajustada a las necesidades del paciente. Consecuentemente, el objetivo de esta revisión es identificar las definiciones conceptuales de impulso, esfuerzo y trabajo respiratorio utilizadas en el contexto de la unidad de cuidados intensivos, e identificar las maniobras de medición no invasivas disponibles en los ventiladores de cuidados intensivos para monitorizar impulso, esfuerzo y trabajo respiratorio. La literatura destaca que, aunque los conceptos de impulso, esfuerzo y trabajo respiratorio se perciben intuitivos, no existe una definición clara. Asimismo, destacados autores los definen como conceptos diferentes.
 Authors:
Francisco Ríos-Castro[1,2], Felipe González-Seguel[1,2], Jorge Molina[1]
Authors:
Francisco Ríos-Castro[1,2], Felipe González-Seguel[1,2], Jorge Molina[1]
Affiliation:
[1] Carrera de Kinesiología, Facultad de Medicina, Clínica Alemana Universidad del Desarrollo, Santiago, Chile
[2] Servicio de Medicina Física y Rehabilitación, Facultad de Medicina, Clínica Alemana Universidad del Desarrollo, Santiago, Chile
E-mail: feligonzalezs@udd.cl

Citation: Ríos-Castro F, González-Seguel F, Molina J. Respiratory drive, inspiratory effort, and work of breathing: review of definitions and non-invasive monitoring tools for intensive care ventilators during pandemic times. Medwave 2022; 22(3):e002550 doi: 10.5867/medwave.2022.03.002550
Submission date: 30/3/2022
Acceptance date: 28/4/2022
Publication date: 29/4/2022
Origin: Not commissioned
Type of review: Externally peer-reviewed by three reviewers, double-blind.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Wunsch H. Mechanical Ventilation in COVID- 19: Interpreting the Current Epidemiology. Am J Respir Crit Care Med. 2020;202: 1–4. | CrossRef |
- Carson SS, Cox CE, Holmes GM, Howard A, Carey TS. The changing epidemiology of mechanical ventilation: A population- based study. J Intensive Care Med. 2006;21: 173–82. | CrossRef |
- Slutsky AS. History of Mechanical Ventilation. From Vesalius to Ventilator- induced Lung Injury. Am J Respir Crit Care Med. 2015;191: 1106–15. | CrossRef |
- Rittayamai N, Katsios CM, Beloncle F, Friedrich JO, Mancebo J, Brochard L. Pressure- Controlled vs Volume- Controlled Ventilation in Acute Respiratory Failure. Chest. 2015;148: 340–355. | CrossRef |
- Girard TD, Alhazzani W, Kress JP, Ouellette DR, Schmidt GA, Truwit JD, et al. An Official American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: Liberation from Mechanical Ventilation in Critically Ill Adults. Rehabilitation Protocols, Ventilator Liberation Protocols, and Cuff Leak Tests. Am J Respir Crit Care Med. 2017;195: 120–133. | CrossRef |
- Kacmarek RM, Pirrone M, Berra L. Assisted mechanical ventilation: the future is now! BMC Anesthesiol. 2015;15: 1–3. | CrossRef |
- Pelosi P, Ball L, Barbas CSV, Bellomo R, Burns KEA, Einav S, et al. Personalized mechanical ventilation in acute respiratory distress syndrome. Crit Care. 2021;25: 250. | CrossRef |
- Ruiz Ferrón F, Serrano Simón JM. La monitorización convencional no es suficiente para valorar el esfuerzo respiratorio durante la ventilación asistida. Medicina Intensiva. 2019;43: 197–206. | CrossRef |
- Pérez J, Dorado JH, Papazian AC, Berastegui M, Gilgado DI, Cardoso GP, et al. Titration and characteristics of pressure- support ventilation use in Argentina: an online cross- sectional survey study. Rev Bras Ter Intensiva. 2020;32: 81–91. | CrossRef |
- Brochard L, Telias I. Bedside Detection of Overassistance During Pressure Support Ventilation. Crit Care Med. 2018;46: 488–490. | CrossRef |
- Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189: 520–31. | CrossRef |
- Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42: 1360–73. | CrossRef |
- Cabello B, Mancebo J. Work of breathing. Intensive Care Med. 2006;32: 1311–4. | CrossRef |
- Bellani G, Mauri T, Coppadoro A, Grasselli G, Patroniti N, Spadaro S, et al. Estimation of patient’s inspiratory effort from the electrical activity of the diaphragm. Crit Care Med. 2013;41: 1483–91. | CrossRef |
- Yuan X, Lu X, Chao Y, Beck J, Sinderby C, Xie J, et al. Neurally adjusted ventilatory assist as a weaning mode for adults with invasive mechanical ventilation: a systematic review and meta- analysis. Crit Care. 2021;25: 222. | CrossRef |
- Vivier E, Mekontso Dessap A, Dimassi S, Vargas F, Lyazidi A, Thille AW, et al. Diaphragm ultrasonography to estimate the work of breathing during non- invasive ventilation. Intensive Care Med. 2012;38: 796–803. | CrossRef |
- Esnault P, Cardinale M, Hraiech S, Goutorbe P, Baumstrack K, Prud’homme E, et al. High Respiratory Drive and Excessive Respiratory Efforts Predict Relapse of Respiratory Failure in Critically Ill Patients with COVID- 19. Am J Respir Crit Care Med. 2020;202: 1173–1178. | CrossRef |
- Telias I, Spadaro S. Techniques to monitor respiratory drive and inspiratory effort. Curr Opin Crit Care. 2020;26: 3–10. | CrossRef |
- Cruces P, Retamal J, Hurtado DE, Erranz B, Iturrieta P, González C, et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS- CoV- 2 infection. Crit Care. 2020;24: 494. | CrossRef |
- Roesthuis L, van den Berg M, van der Hoeven H. Non- invasive method to detect high respiratory effort and transpulmonary driving pressures in COVID- 19 patients during mechanical ventilation. Ann Intensive Care. 2021;11. | CrossRef |
- González- Seguel F, Camus- Molina A, Jasmén A, Molina J, Pérez- Araos R, Graf J. Respiratory Support Adjustments and Monitoring of Mechanically Ventilated Patients Performing Early Mobilization: A Scoping Review. Crit Care Explor. 2021;3: e0407. | CrossRef |
- de Vries H, Jonkman A, Shi Z- H, Spoelstra- de Man A, Heunks L. Assessing breathing effort in mechanical ventilation: physiology and clinical implications. Ann Transl Med. 2018;6: 387. | CrossRef |
- Jonkman AH, de Vries HJ, Heunks LMA. Physiology of the Respiratory Drive in ICU Patients: Implications for Diagnosis and Treatment. Crit Care. 2020;24: 104. | CrossRef |
- Telias I, Brochard L, Goligher EC. Is my patient’s respiratory drive (too) high? Intensive Care Med. 2018;44: 1936–1939. | CrossRef |
- Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46: 606–618. | CrossRef |
- Biscoe TJ, Purves MJ, Sampson SR. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970;208: 121–31. | CrossRef |
- Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci. 2018;19: 351–367. | CrossRef |
- Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory Drive in Critically Ill Patients. Pathophysiology and Clinical Implications Am J Respir Crit Care Med. 2020;201: 20–32. | CrossRef |
- Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes ARJ, Spoelstra- de Man AME, et al. Diaphragm muscle fiber weakness and ubiquitin- proteasome activation in critically ill patients. Am J Respir Crit Care Med. 2015;191: 1126–38. | CrossRef |
- Ebihara S, Hussain SNA, Danialou G, Cho WK, Gottfried SB, Petrof BJ. Mechanical ventilation protects against diaphragm injury in sepsis: interaction of oxidative and mechanical stresses. Am J Respir Crit Care Med. 2002;165: 221–8. | CrossRef |
- Schmidt M, Kindler F, Gottfried SB, Raux M, Hug F, Similowski T, et al. Dyspnea and surface inspiratory electromyograms in mechanically ventilated patients. Intensive Care Med. 2013;39: 1368–1376. | CrossRef |
- Schmidt M, Demoule A, Polito A, Porchet R, Aboab J, Siami S, et al. Dyspnea in mechanically ventilated critically ill patients. Crit Care Med. 2011;39: 2059–65. | CrossRef |
- Schmidt M, Banzett RB, Raux M, Morélot- Panzini C, Dangers L, Similowski T, et al. Unrecognized suffering in the ICU: addressing dyspnea in mechanically ventilated patients. Intensive Care Med. 2014;40: 1–10. | CrossRef |
- Bellani G, Pesenti A. Assessing effort and work of breathing. Curr Opin Crit Care. 2014;20: 352–8. | CrossRef |
- Troyer AD, Wilson TA. Action of the diaphragm on the rib cage. J Appl Physiol (1985). 2016;121: 391–400. | CrossRef |
- De Troyer A, Boriek AM. Mechanics of the respiratory muscles. Compr Physiol. 2011;1: 1273–300. | CrossRef |
- Bertoni M, Spadaro S, Goligher EC. Monitoring Patient Respiratory Effort During Mechanical Ventilation: Lung and Diaphragm- Protective Ventilation. Crit Care. 2020;24: 106. | CrossRef |
- Vassilakopoulos T. Understanding wasted/ineffective efforts in mechanically ventilated COPD patients using the Campbell diagram. Intensive Care Med. 2008;34: 1336–9. | CrossRef |
- French CJ. Work of breathing measurement in the critically ill patient. Anaesth Intensive Care. 1999;27: 561–73. | CrossRef |
- García- Prieto E, Amado-R odríguez L, Albaiceta GM. Monitorization of respiratory mechanics in the ventilated patient. Medicina Intensiva (English Edition). 2014;38: 49–55. | CrossRef |
- Whitelaw WA, Derenne J- P, Milic- Emili J. Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol. 1975;23: 181–99. | CrossRef |
- Herrera M, Blasco J, Venegas J, Barba R, Doblas A, Marquez E. Mouth occlusion pressure (P0.1) in acute respiratory failure. Intensive Care Med. 1985;11: 134–9. | CrossRef |
- Aubier M, Murciano D, Fournier M, Milic- Emili J, Pariente R, Derenne JP. Central respiratory drive in acute respiratory failure of patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1980;122: 191–9. | CrossRef |
- Telias I, Junhasavasdikul D, Rittayamai N, Piquilloud L, Chen L, Ferguson ND, et al. Airway Occlusion Pressure As an Estimate of Respiratory Drive and Inspiratory Effort during Assisted Ventilation. Am J Respir Crit Care Med. 2020;201: 1086–1098. | CrossRef |
- Sato R, Hasegawa D, Hamahata NT, Narala S, Nishida K, Takahashi K, et al. The predictive value of airway occlusion pressure at 100 msec (P0.1) on successful weaning from mechanical ventilation: A systematic review and meta- analysis. J Crit Care. 2021;63: 124–132. | CrossRef |
- elias I, Damiani F, Brochard L. The airway occlusion pressure P0.1) to monitor respiratory drive during mechanical ventilation: increasing awareness of a not- so- new problem. Intensive Care Med. 2018;44: 1532–1535. | CrossRef |
- Mauri T, Grasselli G, Suriano G, Eronia N, Spadaro S, Turrini C, et al. Control of Respiratory Drive and Effort in Extracorporeal Membrane Oxygenation Patients Recovering from Severe Acute Respiratory Distress Syndrome. Anesthesiology. 2016;125: 159–67. | CrossRef |
- Rittayamai N, Beloncle F, Goligher EC, Chen L, Mancebo J, Richard J- CM, et al. Effect of inspiratory synchronization during pressure- controlled ventilation on lung distension and inspiratory effort. Ann Intensive Care. 2017;7. | CrossRef |
- Beloncle F, Piquilloud L, Olivier P- Y, Vuillermoz A, Yvin E, Mercat A, et al. Accuracy of P0.1 measurements performed by ICU ventilators: a bench study. Ann Intensive Care. 2019;9. | CrossRef |
- Holle RH, Schoene RB, Pavlin EJ. Effect of respiratory muscle weakness on P0.1 induced by partial curarization. J Appl Physiol Respir Environ Exerc Physiol. 1984;57: 1150–7. | CrossRef |
- Bertoni M, Telias I, Urner M, Long M, Del Sorbo L, Fan E, et al. A novel non- invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care. 2019;23: 346. | CrossRef |
- Grassino A, Goldman MD, Mead J, Sears TA. Mechanics of the human diaphragm during voluntary contraction: statics. J Appl Physiol Respir Environ Exerc Physiol. 1978;44: 829–39. | CrossRef |
- Carteaux G, Mancebo J, Mercat A, Dellamonica J, Richard J- CM, Aguirre- Bermeo H, et al. Bedside adjustment of proportional assist ventilation to target a predefined range of respiratory effort. Crit Care Med. 2013;41: 2125–32. | CrossRef |
- Dianti J, Bertoni M, Goligher EC. Monitoring patient- ventilator interaction by an end- expiratory occlusion maneuver. Intensive Care Med. 2020;46: 2338–2341. | CrossRef |
- Bellani G, Grassi A, Sosio S, Foti G. Plateau and driving pressure in the presence of spontaneous breathing. Intensive Care Med. 2019;45: 97–98. | CrossRef |
- Foti G, Cereda M, Banfi G, Pelosi P, Fumagalli R, Pesenti A. End- inspiratory airway occlusion: a method to assess the pressure developed by inspiratory muscles in patients with acute lung injury undergoing pressure support. Am J Respir Crit Care Med. 1997;156: 1210–6. | CrossRef |
- Prinianakis G, Plataki M, Kondili E, Klimathianaki M, Vaporidi K, Georgopoulos D. Effects of relaxation of inspiratory muscles on ventilator pressure during pressure support. Intensive Care Med. 2008;34: 70–4. | CrossRef |
- Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, Costa ELV, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med. 2013;188: 1420–7. | CrossRef |
- Natalini G, Buizza B, Granato A, Aniballi E, Pisani L, Ciabatti G, et al. Non- invasive assessment of respiratory muscle activity during pressure support ventilation: accuracy of end- inspiration occlusion and least square fitting methods. J Clin Monit Comput. 2021;35: 913–921. | CrossRef |
- Calzia E, Lindner KH, Witt S, Schirmer U, Lange H, Stenz R, et al. Pressure- time product and work of breathing during biphasic continuous positive airway pressure and assisted spontaneous breathing. Am J Respir Crit Care Med. 1994;150: 904–10. | CrossRef |
- Thille AW, Lyazidi A, Richard J- C, Galia F, Brochard L. A bench study of intensive- care- unit ventilators: new versus old and turbine- based versus compressed gas- based ventilators. Intensive Care Med. 2009;35: 1368–76. | CrossRef |
- Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis. 1992;145: 114–20. | CrossRef |
- Younes M, Kun J, Masiowski B, Webster K, Roberts D. A method for noninvasive determination of inspiratory resistance during proportional assist ventilation. Am J Respir Crit Care Med. 2001;163: 829–39. | CrossRef |
- Younes M, Webster K, Kun J, Roberts D, Masiowski B. A method for measuring passive elastance during proportional assist ventilation. Am J Respir Crit Care Med. 2001;164: 50–60. | CrossRef |
- Spahija J, de Marchie M, Albert M, Bellemare P, Delisle S, Beck J, et al. Patient- ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. 2010;38: 518–26. | CrossRef |
- Albani F, Pisani L, Ciabatti G, Fusina F, Buizza B, Granato A, et al. Flow Index: a novel, non- invasive, continuous, quantitative method to evaluate patient inspiratory effort during pressure support ventilation. Crit Care. 2021;25: 196. | CrossRef |
- Dzierba AL, Khalil AM, Derry KL, Madahar P, Beitler JR. Discordance Between Respiratory Drive and Sedation Depth in Critically Ill Patients Receiving Mechanical Ventilation. Crit Care Med. 2021;49: 2090–2101. | CrossRef |
- MacIntyre N. Managing Patient- Ventilator Dyssynchrony. Crit Care Med. 2021;49: 2149–2151. | CrossRef |
 Wunsch H. Mechanical Ventilation in COVID- 19: Interpreting the Current Epidemiology. Am J Respir Crit Care Med. 2020;202: 1–4. | CrossRef |
Wunsch H. Mechanical Ventilation in COVID- 19: Interpreting the Current Epidemiology. Am J Respir Crit Care Med. 2020;202: 1–4. | CrossRef | Carson SS, Cox CE, Holmes GM, Howard A, Carey TS. The changing epidemiology of mechanical ventilation: A population- based study. J Intensive Care Med. 2006;21: 173–82. | CrossRef |
Carson SS, Cox CE, Holmes GM, Howard A, Carey TS. The changing epidemiology of mechanical ventilation: A population- based study. J Intensive Care Med. 2006;21: 173–82. | CrossRef | Slutsky AS. History of Mechanical Ventilation. From Vesalius to Ventilator- induced Lung Injury. Am J Respir Crit Care Med. 2015;191: 1106–15. | CrossRef |
Slutsky AS. History of Mechanical Ventilation. From Vesalius to Ventilator- induced Lung Injury. Am J Respir Crit Care Med. 2015;191: 1106–15. | CrossRef | Rittayamai N, Katsios CM, Beloncle F, Friedrich JO, Mancebo J, Brochard L. Pressure- Controlled vs Volume- Controlled Ventilation in Acute Respiratory Failure. Chest. 2015;148: 340–355. | CrossRef |
Rittayamai N, Katsios CM, Beloncle F, Friedrich JO, Mancebo J, Brochard L. Pressure- Controlled vs Volume- Controlled Ventilation in Acute Respiratory Failure. Chest. 2015;148: 340–355. | CrossRef | Girard TD, Alhazzani W, Kress JP, Ouellette DR, Schmidt GA, Truwit JD, et al. An Official American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: Liberation from Mechanical Ventilation in Critically Ill Adults. Rehabilitation Protocols, Ventilator Liberation Protocols, and Cuff Leak Tests. Am J Respir Crit Care Med. 2017;195: 120–133. | CrossRef |
Girard TD, Alhazzani W, Kress JP, Ouellette DR, Schmidt GA, Truwit JD, et al. An Official American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: Liberation from Mechanical Ventilation in Critically Ill Adults. Rehabilitation Protocols, Ventilator Liberation Protocols, and Cuff Leak Tests. Am J Respir Crit Care Med. 2017;195: 120–133. | CrossRef | Kacmarek RM, Pirrone M, Berra L. Assisted mechanical ventilation: the future is now! BMC Anesthesiol. 2015;15: 1–3. | CrossRef |
Kacmarek RM, Pirrone M, Berra L. Assisted mechanical ventilation: the future is now! BMC Anesthesiol. 2015;15: 1–3. | CrossRef | Pelosi P, Ball L, Barbas CSV, Bellomo R, Burns KEA, Einav S, et al. Personalized mechanical ventilation in acute respiratory distress syndrome. Crit Care. 2021;25: 250. | CrossRef |
Pelosi P, Ball L, Barbas CSV, Bellomo R, Burns KEA, Einav S, et al. Personalized mechanical ventilation in acute respiratory distress syndrome. Crit Care. 2021;25: 250. | CrossRef | Ruiz Ferrón F, Serrano Simón JM. La monitorización convencional no es suficiente para valorar el esfuerzo respiratorio durante la ventilación asistida. Medicina Intensiva. 2019;43: 197–206. | CrossRef |
Ruiz Ferrón F, Serrano Simón JM. La monitorización convencional no es suficiente para valorar el esfuerzo respiratorio durante la ventilación asistida. Medicina Intensiva. 2019;43: 197–206. | CrossRef | Pérez J, Dorado JH, Papazian AC, Berastegui M, Gilgado DI, Cardoso GP, et al. Titration and characteristics of pressure- support ventilation use in Argentina: an online cross- sectional survey study. Rev Bras Ter Intensiva. 2020;32: 81–91. | CrossRef |
Pérez J, Dorado JH, Papazian AC, Berastegui M, Gilgado DI, Cardoso GP, et al. Titration and characteristics of pressure- support ventilation use in Argentina: an online cross- sectional survey study. Rev Bras Ter Intensiva. 2020;32: 81–91. | CrossRef | Brochard L, Telias I. Bedside Detection of Overassistance During Pressure Support Ventilation. Crit Care Med. 2018;46: 488–490. | CrossRef |
Brochard L, Telias I. Bedside Detection of Overassistance During Pressure Support Ventilation. Crit Care Med. 2018;46: 488–490. | CrossRef | Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189: 520–31. | CrossRef |
Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189: 520–31. | CrossRef | Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42: 1360–73. | CrossRef |
Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42: 1360–73. | CrossRef | Bellani G, Mauri T, Coppadoro A, Grasselli G, Patroniti N, Spadaro S, et al. Estimation of patient’s inspiratory effort from the electrical activity of the diaphragm. Crit Care Med. 2013;41: 1483–91. | CrossRef |
Bellani G, Mauri T, Coppadoro A, Grasselli G, Patroniti N, Spadaro S, et al. Estimation of patient’s inspiratory effort from the electrical activity of the diaphragm. Crit Care Med. 2013;41: 1483–91. | CrossRef | Yuan X, Lu X, Chao Y, Beck J, Sinderby C, Xie J, et al. Neurally adjusted ventilatory assist as a weaning mode for adults with invasive mechanical ventilation: a systematic review and meta- analysis. Crit Care. 2021;25: 222. | CrossRef |
Yuan X, Lu X, Chao Y, Beck J, Sinderby C, Xie J, et al. Neurally adjusted ventilatory assist as a weaning mode for adults with invasive mechanical ventilation: a systematic review and meta- analysis. Crit Care. 2021;25: 222. | CrossRef | Vivier E, Mekontso Dessap A, Dimassi S, Vargas F, Lyazidi A, Thille AW, et al. Diaphragm ultrasonography to estimate the work of breathing during non- invasive ventilation. Intensive Care Med. 2012;38: 796–803. | CrossRef |
Vivier E, Mekontso Dessap A, Dimassi S, Vargas F, Lyazidi A, Thille AW, et al. Diaphragm ultrasonography to estimate the work of breathing during non- invasive ventilation. Intensive Care Med. 2012;38: 796–803. | CrossRef | Esnault P, Cardinale M, Hraiech S, Goutorbe P, Baumstrack K, Prud’homme E, et al. High Respiratory Drive and Excessive Respiratory Efforts Predict Relapse of Respiratory Failure in Critically Ill Patients with COVID- 19. Am J Respir Crit Care Med. 2020;202: 1173–1178. | CrossRef |
Esnault P, Cardinale M, Hraiech S, Goutorbe P, Baumstrack K, Prud’homme E, et al. High Respiratory Drive and Excessive Respiratory Efforts Predict Relapse of Respiratory Failure in Critically Ill Patients with COVID- 19. Am J Respir Crit Care Med. 2020;202: 1173–1178. | CrossRef | Telias I, Spadaro S. Techniques to monitor respiratory drive and inspiratory effort. Curr Opin Crit Care. 2020;26: 3–10. | CrossRef |
Telias I, Spadaro S. Techniques to monitor respiratory drive and inspiratory effort. Curr Opin Crit Care. 2020;26: 3–10. | CrossRef | Cruces P, Retamal J, Hurtado DE, Erranz B, Iturrieta P, González C, et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS- CoV- 2 infection. Crit Care. 2020;24: 494. | CrossRef |
Cruces P, Retamal J, Hurtado DE, Erranz B, Iturrieta P, González C, et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS- CoV- 2 infection. Crit Care. 2020;24: 494. | CrossRef | Roesthuis L, van den Berg M, van der Hoeven H. Non- invasive method to detect high respiratory effort and transpulmonary driving pressures in COVID- 19 patients during mechanical ventilation. Ann Intensive Care. 2021;11. | CrossRef |
Roesthuis L, van den Berg M, van der Hoeven H. Non- invasive method to detect high respiratory effort and transpulmonary driving pressures in COVID- 19 patients during mechanical ventilation. Ann Intensive Care. 2021;11. | CrossRef | González- Seguel F, Camus- Molina A, Jasmén A, Molina J, Pérez- Araos R, Graf J. Respiratory Support Adjustments and Monitoring of Mechanically Ventilated Patients Performing Early Mobilization: A Scoping Review. Crit Care Explor. 2021;3: e0407. | CrossRef |
González- Seguel F, Camus- Molina A, Jasmén A, Molina J, Pérez- Araos R, Graf J. Respiratory Support Adjustments and Monitoring of Mechanically Ventilated Patients Performing Early Mobilization: A Scoping Review. Crit Care Explor. 2021;3: e0407. | CrossRef | de Vries H, Jonkman A, Shi Z- H, Spoelstra- de Man A, Heunks L. Assessing breathing effort in mechanical ventilation: physiology and clinical implications. Ann Transl Med. 2018;6: 387. | CrossRef |
de Vries H, Jonkman A, Shi Z- H, Spoelstra- de Man A, Heunks L. Assessing breathing effort in mechanical ventilation: physiology and clinical implications. Ann Transl Med. 2018;6: 387. | CrossRef | Jonkman AH, de Vries HJ, Heunks LMA. Physiology of the Respiratory Drive in ICU Patients: Implications for Diagnosis and Treatment. Crit Care. 2020;24: 104. | CrossRef |
Jonkman AH, de Vries HJ, Heunks LMA. Physiology of the Respiratory Drive in ICU Patients: Implications for Diagnosis and Treatment. Crit Care. 2020;24: 104. | CrossRef | Telias I, Brochard L, Goligher EC. Is my patient’s respiratory drive (too) high? Intensive Care Med. 2018;44: 1936–1939. | CrossRef |
Telias I, Brochard L, Goligher EC. Is my patient’s respiratory drive (too) high? Intensive Care Med. 2018;44: 1936–1939. | CrossRef | Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46: 606–618. | CrossRef |
Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46: 606–618. | CrossRef | Biscoe TJ, Purves MJ, Sampson SR. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970;208: 121–31. | CrossRef |
Biscoe TJ, Purves MJ, Sampson SR. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970;208: 121–31. | CrossRef | Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci. 2018;19: 351–367. | CrossRef |
Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci. 2018;19: 351–367. | CrossRef | Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory Drive in Critically Ill Patients. Pathophysiology and Clinical Implications Am J Respir Crit Care Med. 2020;201: 20–32. | CrossRef |
Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory Drive in Critically Ill Patients. Pathophysiology and Clinical Implications Am J Respir Crit Care Med. 2020;201: 20–32. | CrossRef | Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes ARJ, Spoelstra- de Man AME, et al. Diaphragm muscle fiber weakness and ubiquitin- proteasome activation in critically ill patients. Am J Respir Crit Care Med. 2015;191: 1126–38. | CrossRef |
Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes ARJ, Spoelstra- de Man AME, et al. Diaphragm muscle fiber weakness and ubiquitin- proteasome activation in critically ill patients. Am J Respir Crit Care Med. 2015;191: 1126–38. | CrossRef | Ebihara S, Hussain SNA, Danialou G, Cho WK, Gottfried SB, Petrof BJ. Mechanical ventilation protects against diaphragm injury in sepsis: interaction of oxidative and mechanical stresses. Am J Respir Crit Care Med. 2002;165: 221–8. | CrossRef |
Ebihara S, Hussain SNA, Danialou G, Cho WK, Gottfried SB, Petrof BJ. Mechanical ventilation protects against diaphragm injury in sepsis: interaction of oxidative and mechanical stresses. Am J Respir Crit Care Med. 2002;165: 221–8. | CrossRef | Schmidt M, Kindler F, Gottfried SB, Raux M, Hug F, Similowski T, et al. Dyspnea and surface inspiratory electromyograms in mechanically ventilated patients. Intensive Care Med. 2013;39: 1368–1376. | CrossRef |
Schmidt M, Kindler F, Gottfried SB, Raux M, Hug F, Similowski T, et al. Dyspnea and surface inspiratory electromyograms in mechanically ventilated patients. Intensive Care Med. 2013;39: 1368–1376. | CrossRef | Schmidt M, Demoule A, Polito A, Porchet R, Aboab J, Siami S, et al. Dyspnea in mechanically ventilated critically ill patients. Crit Care Med. 2011;39: 2059–65. | CrossRef |
Schmidt M, Demoule A, Polito A, Porchet R, Aboab J, Siami S, et al. Dyspnea in mechanically ventilated critically ill patients. Crit Care Med. 2011;39: 2059–65. | CrossRef | Schmidt M, Banzett RB, Raux M, Morélot- Panzini C, Dangers L, Similowski T, et al. Unrecognized suffering in the ICU: addressing dyspnea in mechanically ventilated patients. Intensive Care Med. 2014;40: 1–10. | CrossRef |
Schmidt M, Banzett RB, Raux M, Morélot- Panzini C, Dangers L, Similowski T, et al. Unrecognized suffering in the ICU: addressing dyspnea in mechanically ventilated patients. Intensive Care Med. 2014;40: 1–10. | CrossRef | Bellani G, Pesenti A. Assessing effort and work of breathing. Curr Opin Crit Care. 2014;20: 352–8. | CrossRef |
Bellani G, Pesenti A. Assessing effort and work of breathing. Curr Opin Crit Care. 2014;20: 352–8. | CrossRef | Troyer AD, Wilson TA. Action of the diaphragm on the rib cage. J Appl Physiol (1985). 2016;121: 391–400. | CrossRef |
Troyer AD, Wilson TA. Action of the diaphragm on the rib cage. J Appl Physiol (1985). 2016;121: 391–400. | CrossRef | De Troyer A, Boriek AM. Mechanics of the respiratory muscles. Compr Physiol. 2011;1: 1273–300. | CrossRef |
De Troyer A, Boriek AM. Mechanics of the respiratory muscles. Compr Physiol. 2011;1: 1273–300. | CrossRef | Bertoni M, Spadaro S, Goligher EC. Monitoring Patient Respiratory Effort During Mechanical Ventilation: Lung and Diaphragm- Protective Ventilation. Crit Care. 2020;24: 106. | CrossRef |
Bertoni M, Spadaro S, Goligher EC. Monitoring Patient Respiratory Effort During Mechanical Ventilation: Lung and Diaphragm- Protective Ventilation. Crit Care. 2020;24: 106. | CrossRef | Vassilakopoulos T. Understanding wasted/ineffective efforts in mechanically ventilated COPD patients using the Campbell diagram. Intensive Care Med. 2008;34: 1336–9. | CrossRef |
Vassilakopoulos T. Understanding wasted/ineffective efforts in mechanically ventilated COPD patients using the Campbell diagram. Intensive Care Med. 2008;34: 1336–9. | CrossRef | French CJ. Work of breathing measurement in the critically ill patient. Anaesth Intensive Care. 1999;27: 561–73. | CrossRef |
French CJ. Work of breathing measurement in the critically ill patient. Anaesth Intensive Care. 1999;27: 561–73. | CrossRef | García- Prieto E, Amado-R odríguez L, Albaiceta GM. Monitorization of respiratory mechanics in the ventilated patient. Medicina Intensiva (English Edition). 2014;38: 49–55. | CrossRef |
García- Prieto E, Amado-R odríguez L, Albaiceta GM. Monitorization of respiratory mechanics in the ventilated patient. Medicina Intensiva (English Edition). 2014;38: 49–55. | CrossRef | Whitelaw WA, Derenne J- P, Milic- Emili J. Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol. 1975;23: 181–99. | CrossRef |
Whitelaw WA, Derenne J- P, Milic- Emili J. Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol. 1975;23: 181–99. | CrossRef | Herrera M, Blasco J, Venegas J, Barba R, Doblas A, Marquez E. Mouth occlusion pressure (P0.1) in acute respiratory failure. Intensive Care Med. 1985;11: 134–9. | CrossRef |
Herrera M, Blasco J, Venegas J, Barba R, Doblas A, Marquez E. Mouth occlusion pressure (P0.1) in acute respiratory failure. Intensive Care Med. 1985;11: 134–9. | CrossRef | Aubier M, Murciano D, Fournier M, Milic- Emili J, Pariente R, Derenne JP. Central respiratory drive in acute respiratory failure of patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1980;122: 191–9. | CrossRef |
Aubier M, Murciano D, Fournier M, Milic- Emili J, Pariente R, Derenne JP. Central respiratory drive in acute respiratory failure of patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1980;122: 191–9. | CrossRef | Telias I, Junhasavasdikul D, Rittayamai N, Piquilloud L, Chen L, Ferguson ND, et al. Airway Occlusion Pressure As an Estimate of Respiratory Drive and Inspiratory Effort during Assisted Ventilation. Am J Respir Crit Care Med. 2020;201: 1086–1098. | CrossRef |
Telias I, Junhasavasdikul D, Rittayamai N, Piquilloud L, Chen L, Ferguson ND, et al. Airway Occlusion Pressure As an Estimate of Respiratory Drive and Inspiratory Effort during Assisted Ventilation. Am J Respir Crit Care Med. 2020;201: 1086–1098. | CrossRef | Sato R, Hasegawa D, Hamahata NT, Narala S, Nishida K, Takahashi K, et al. The predictive value of airway occlusion pressure at 100 msec (P0.1) on successful weaning from mechanical ventilation: A systematic review and meta- analysis. J Crit Care. 2021;63: 124–132. | CrossRef |
Sato R, Hasegawa D, Hamahata NT, Narala S, Nishida K, Takahashi K, et al. The predictive value of airway occlusion pressure at 100 msec (P0.1) on successful weaning from mechanical ventilation: A systematic review and meta- analysis. J Crit Care. 2021;63: 124–132. | CrossRef | elias I, Damiani F, Brochard L. The airway occlusion pressure P0.1) to monitor respiratory drive during mechanical ventilation: increasing awareness of a not- so- new problem. Intensive Care Med. 2018;44: 1532–1535. | CrossRef |
elias I, Damiani F, Brochard L. The airway occlusion pressure P0.1) to monitor respiratory drive during mechanical ventilation: increasing awareness of a not- so- new problem. Intensive Care Med. 2018;44: 1532–1535. | CrossRef | Mauri T, Grasselli G, Suriano G, Eronia N, Spadaro S, Turrini C, et al. Control of Respiratory Drive and Effort in Extracorporeal Membrane Oxygenation Patients Recovering from Severe Acute Respiratory Distress Syndrome. Anesthesiology. 2016;125: 159–67. | CrossRef |
Mauri T, Grasselli G, Suriano G, Eronia N, Spadaro S, Turrini C, et al. Control of Respiratory Drive and Effort in Extracorporeal Membrane Oxygenation Patients Recovering from Severe Acute Respiratory Distress Syndrome. Anesthesiology. 2016;125: 159–67. | CrossRef | Rittayamai N, Beloncle F, Goligher EC, Chen L, Mancebo J, Richard J- CM, et al. Effect of inspiratory synchronization during pressure- controlled ventilation on lung distension and inspiratory effort. Ann Intensive Care. 2017;7. | CrossRef |
Rittayamai N, Beloncle F, Goligher EC, Chen L, Mancebo J, Richard J- CM, et al. Effect of inspiratory synchronization during pressure- controlled ventilation on lung distension and inspiratory effort. Ann Intensive Care. 2017;7. | CrossRef | Beloncle F, Piquilloud L, Olivier P- Y, Vuillermoz A, Yvin E, Mercat A, et al. Accuracy of P0.1 measurements performed by ICU ventilators: a bench study. Ann Intensive Care. 2019;9. | CrossRef |
Beloncle F, Piquilloud L, Olivier P- Y, Vuillermoz A, Yvin E, Mercat A, et al. Accuracy of P0.1 measurements performed by ICU ventilators: a bench study. Ann Intensive Care. 2019;9. | CrossRef | Holle RH, Schoene RB, Pavlin EJ. Effect of respiratory muscle weakness on P0.1 induced by partial curarization. J Appl Physiol Respir Environ Exerc Physiol. 1984;57: 1150–7. | CrossRef |
Holle RH, Schoene RB, Pavlin EJ. Effect of respiratory muscle weakness on P0.1 induced by partial curarization. J Appl Physiol Respir Environ Exerc Physiol. 1984;57: 1150–7. | CrossRef | Bertoni M, Telias I, Urner M, Long M, Del Sorbo L, Fan E, et al. A novel non- invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care. 2019;23: 346. | CrossRef |
Bertoni M, Telias I, Urner M, Long M, Del Sorbo L, Fan E, et al. A novel non- invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care. 2019;23: 346. | CrossRef | Grassino A, Goldman MD, Mead J, Sears TA. Mechanics of the human diaphragm during voluntary contraction: statics. J Appl Physiol Respir Environ Exerc Physiol. 1978;44: 829–39. | CrossRef |
Grassino A, Goldman MD, Mead J, Sears TA. Mechanics of the human diaphragm during voluntary contraction: statics. J Appl Physiol Respir Environ Exerc Physiol. 1978;44: 829–39. | CrossRef | Carteaux G, Mancebo J, Mercat A, Dellamonica J, Richard J- CM, Aguirre- Bermeo H, et al. Bedside adjustment of proportional assist ventilation to target a predefined range of respiratory effort. Crit Care Med. 2013;41: 2125–32. | CrossRef |
Carteaux G, Mancebo J, Mercat A, Dellamonica J, Richard J- CM, Aguirre- Bermeo H, et al. Bedside adjustment of proportional assist ventilation to target a predefined range of respiratory effort. Crit Care Med. 2013;41: 2125–32. | CrossRef | Dianti J, Bertoni M, Goligher EC. Monitoring patient- ventilator interaction by an end- expiratory occlusion maneuver. Intensive Care Med. 2020;46: 2338–2341. | CrossRef |
Dianti J, Bertoni M, Goligher EC. Monitoring patient- ventilator interaction by an end- expiratory occlusion maneuver. Intensive Care Med. 2020;46: 2338–2341. | CrossRef | Bellani G, Grassi A, Sosio S, Foti G. Plateau and driving pressure in the presence of spontaneous breathing. Intensive Care Med. 2019;45: 97–98. | CrossRef |
Bellani G, Grassi A, Sosio S, Foti G. Plateau and driving pressure in the presence of spontaneous breathing. Intensive Care Med. 2019;45: 97–98. | CrossRef | Foti G, Cereda M, Banfi G, Pelosi P, Fumagalli R, Pesenti A. End- inspiratory airway occlusion: a method to assess the pressure developed by inspiratory muscles in patients with acute lung injury undergoing pressure support. Am J Respir Crit Care Med. 1997;156: 1210–6. | CrossRef |
Foti G, Cereda M, Banfi G, Pelosi P, Fumagalli R, Pesenti A. End- inspiratory airway occlusion: a method to assess the pressure developed by inspiratory muscles in patients with acute lung injury undergoing pressure support. Am J Respir Crit Care Med. 1997;156: 1210–6. | CrossRef | Prinianakis G, Plataki M, Kondili E, Klimathianaki M, Vaporidi K, Georgopoulos D. Effects of relaxation of inspiratory muscles on ventilator pressure during pressure support. Intensive Care Med. 2008;34: 70–4. | CrossRef |
Prinianakis G, Plataki M, Kondili E, Klimathianaki M, Vaporidi K, Georgopoulos D. Effects of relaxation of inspiratory muscles on ventilator pressure during pressure support. Intensive Care Med. 2008;34: 70–4. | CrossRef | Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, Costa ELV, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med. 2013;188: 1420–7. | CrossRef |
Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, Costa ELV, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med. 2013;188: 1420–7. | CrossRef | Natalini G, Buizza B, Granato A, Aniballi E, Pisani L, Ciabatti G, et al. Non- invasive assessment of respiratory muscle activity during pressure support ventilation: accuracy of end- inspiration occlusion and least square fitting methods. J Clin Monit Comput. 2021;35: 913–921. | CrossRef |
Natalini G, Buizza B, Granato A, Aniballi E, Pisani L, Ciabatti G, et al. Non- invasive assessment of respiratory muscle activity during pressure support ventilation: accuracy of end- inspiration occlusion and least square fitting methods. J Clin Monit Comput. 2021;35: 913–921. | CrossRef | Calzia E, Lindner KH, Witt S, Schirmer U, Lange H, Stenz R, et al. Pressure- time product and work of breathing during biphasic continuous positive airway pressure and assisted spontaneous breathing. Am J Respir Crit Care Med. 1994;150: 904–10. | CrossRef |
Calzia E, Lindner KH, Witt S, Schirmer U, Lange H, Stenz R, et al. Pressure- time product and work of breathing during biphasic continuous positive airway pressure and assisted spontaneous breathing. Am J Respir Crit Care Med. 1994;150: 904–10. | CrossRef | Thille AW, Lyazidi A, Richard J- C, Galia F, Brochard L. A bench study of intensive- care- unit ventilators: new versus old and turbine- based versus compressed gas- based ventilators. Intensive Care Med. 2009;35: 1368–76. | CrossRef |
Thille AW, Lyazidi A, Richard J- C, Galia F, Brochard L. A bench study of intensive- care- unit ventilators: new versus old and turbine- based versus compressed gas- based ventilators. Intensive Care Med. 2009;35: 1368–76. | CrossRef | Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis. 1992;145: 114–20. | CrossRef |
Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis. 1992;145: 114–20. | CrossRef | Younes M, Kun J, Masiowski B, Webster K, Roberts D. A method for noninvasive determination of inspiratory resistance during proportional assist ventilation. Am J Respir Crit Care Med. 2001;163: 829–39. | CrossRef |
Younes M, Kun J, Masiowski B, Webster K, Roberts D. A method for noninvasive determination of inspiratory resistance during proportional assist ventilation. Am J Respir Crit Care Med. 2001;163: 829–39. | CrossRef | Younes M, Webster K, Kun J, Roberts D, Masiowski B. A method for measuring passive elastance during proportional assist ventilation. Am J Respir Crit Care Med. 2001;164: 50–60. | CrossRef |
Younes M, Webster K, Kun J, Roberts D, Masiowski B. A method for measuring passive elastance during proportional assist ventilation. Am J Respir Crit Care Med. 2001;164: 50–60. | CrossRef | Spahija J, de Marchie M, Albert M, Bellemare P, Delisle S, Beck J, et al. Patient- ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. 2010;38: 518–26. | CrossRef |
Spahija J, de Marchie M, Albert M, Bellemare P, Delisle S, Beck J, et al. Patient- ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. 2010;38: 518–26. | CrossRef | Albani F, Pisani L, Ciabatti G, Fusina F, Buizza B, Granato A, et al. Flow Index: a novel, non- invasive, continuous, quantitative method to evaluate patient inspiratory effort during pressure support ventilation. Crit Care. 2021;25: 196. | CrossRef |
Albani F, Pisani L, Ciabatti G, Fusina F, Buizza B, Granato A, et al. Flow Index: a novel, non- invasive, continuous, quantitative method to evaluate patient inspiratory effort during pressure support ventilation. Crit Care. 2021;25: 196. | CrossRef | Dzierba AL, Khalil AM, Derry KL, Madahar P, Beitler JR. Discordance Between Respiratory Drive and Sedation Depth in Critically Ill Patients Receiving Mechanical Ventilation. Crit Care Med. 2021;49: 2090–2101. | CrossRef |
Dzierba AL, Khalil AM, Derry KL, Madahar P, Beitler JR. Discordance Between Respiratory Drive and Sedation Depth in Critically Ill Patients Receiving Mechanical Ventilation. Crit Care Med. 2021;49: 2090–2101. | CrossRef | MacIntyre N. Managing Patient- Ventilator Dyssynchrony. Crit Care Med. 2021;49: 2149–2151. | CrossRef |
MacIntyre N. Managing Patient- Ventilator Dyssynchrony. Crit Care Med. 2021;49: 2149–2151. | CrossRef |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis









