Key Words: antimalarials, retina, toxicity, retinopathy, chloroquine, hydroxychloroquine
Abstract
Antimalarial drugs are widely used in several countries for control of rheumatologic diseases such as systemic lupus erythematosus and rheumatoid arthritis. They are still used in Mexico because of their low cost and few secondary effects, most of which are mild and reversible. Even so, at an ophthalmological level, they could produce irreversible visual damage, which is why it is important to have ophthalmological evaluation and proper follow up. We present a clinical case as an example of characteristic ophthalmological findings as well as risk factors for retinal toxicity. We then discuss guidelines for diagnosis and follow up of patients who use antimalarial drugs for the treatment of rheumatologic illnesses.
Introduction
The anti-malarial drugs, chloroquine and hydroxychloroquine, are widely known and used since their introduction in 1943 and 1955 respectively [1]. They are frequently used as disease-modifying antirheumatic drugs, for autoimmune disorders like rheumatoid arthritis [2] and systemic lupus erythematosus [3].
Antimalarial drugs are used in Mexico and other developing countries due to their low cost and safety profile compared with second-line drugs [4], or in combination with other drugs [5]. Although hydroxychloroquine is less toxic than chloroquine [6], the latter is used more frequently in Mexico because of their lower cost.
The main reason to stop antimalarial drugs is the profile of adverse effects associated with their use. Multiple adverse effects have been described, the most frequent are gastrointestinal (anorexia, weight loss, abdominal pain, heartburn, nausea and vomiting) (10-20%) and cutaneous (alopecia, pigmentation changes, dryness, pruritus, exanthema: exfoliative, maculopapular, urticaria, lichenoid) (10%) [1], most of them being mild and reversible. Damage to the retina is the most serious adverse effect of these drugs, with the risk of causing irreversible visual loss. In addition to retinal toxicity, antimalarial drugs may have other manifestations of toxicity at eye level like epithelial keratopathy with spiral pattern, subcapsular cataracts, optic nerve atrophy, and extraocular muscle paralysis with accommodation reflex palsy [7],[8].
Although retinal toxic effects have been described even with a few months of treatment [9],[10], in the vast majority of cases the risk increases over time, 1% after 5 to 7 years of chloroquine use [11]. The deposit of antimalarial drugs into the retina has been described for many years; the original description of chloroquine toxicity dates back to 1959 by Hobbs et al. [12], while hydroxychloroquine toxicity was first described in 1967 by Scheareret et al. [13]. Retinal toxicity incidence varies, depending on the drug, diagnostic criteria, and screening strategies used. Beinsten reported an incidence of chloroquine initial toxicity of 10% and only 0.5% of advanced retinopathy [8], while the incidence of toxicity from hydroxychloroquine has been reported from 0.08% [14] to 3.4% [15].
The exact mechanism by which antimalarial drugs cause retinal toxicity is not well elucidated, however, since chloroquine is a lysosomotropic agent (accumulates inside lysosomes increasing intralysosomal pH and inducing an osmotic edema with lysosomal hydrolase release) [16], studies in animal models suggest that the damage in lysosomal function produces an accumulation of lipofuscin [17],[18], which is toxic to photoreceptors and retinal pigment epithelium cells (RPE). This causes degeneration of the retina similar to that described in age-related macular degeneration [19] or some retinal dystrophies associated with ABCA4 gene mutation [20]. Other changes found in electron microscopy are: presence of membranous cytoplasmic bodies in ganglion, amacrine, horizontal and bipolar cells as well as autophagocytic granules accumulation in photoreceptors, primarily in the cones [17], with late involvement of the photoreceptors and retinal pigment epithelium [21]. In vivo studies with optical coherence tomography (OCT) in patients who used hydroxychloroquine suggest that, in contrast to those with chloroquine use, in these patients there is primary involvement of photoreceptors their binding to the retinal pigment epithelium melanin which would prolong the toxic effects of the drug [11]. In addition, the ganglion cells layer thickness is preserved unchanged during all the time of use, even in cases that develop retinal toxicity [22].
The clinical manifestations of retinal toxicity by antimalarial drugs have been well described; in early stages, changes are little evident but it is possible to observe diminished foveolar brightness or subtle pigmentary changes in both eyes. Usually during this stage, the patient remains asymptomatic or presents only minor reading difficulties; as the disease progresses, scotomas and more difficulties to read may appear since this function is done by macular cells. In the ophthalmologic examination the characteristic "bulls-eye" image can be seen, which is caused by depigmentation of the retinal pigment epithelium in the macula with a small central island spared. If exposure to the drug continues, there would be retinal pigment epithelium atrophy initially in the macular region that can progress outwards to cause retinal atrophy. This will produce a decrease in visual acuity, as well as in color and night vision. In advanced stages, funduscopic changes can resemble to those of retinitis pigmentosa with diffuse retinal changes and vascular thinning [23]. Recently described, there is a different presentation in Asian patients in whom initial damage occurs in the extramacular region close to the vascular arcades [24],[25].
Diagnosis, detection and follow up
Because of the severity of ocular damage associated with the use of antimalarial drugs, various associations have developed guidelines for the follow-up of these patients. One of the first and more used were the ones made by the American Academy of Ophthalmology (AAO) in 2002 [23] which mention that there are several factors to consider, the most important is the dose used; doses greater than 400 mg/day of hydroxychloroquine or greater than 6.5 mg/kg of ideal body weight for small people, and 250 mg/day or more than 3 mg/kg of ideal body weight for chloroquine are considered risky.
In 2011, the American Academy of Ophthalmology conducted a review of these guidelines [26] in which it was considered that the accumulated dose (greater than 1000 g for hydroxychloroquine and more than 460 g for chloroquine) is a more appropriate parameter for monitoring. In addition to the dose, treatment duration is also an important factor; although literature describes that the risk of retinal toxicity is increased starting from five years of use, there are some risk factors for early presentation: age more than 60 years, increased body fat, kidney or liver disease, or prior macular disease [26]. The most recent guidelines published in May 2016 [27] agree in considering the daily dose as the most important risk factor, but focusing in patient’s real weight; They suggest that doses greater than 5.0 mg/kg of hydroxychloroquine and greater than 2.3 mg/kg of chloroquine increase the risk dramatically, in addition to the previously described duration time and tamoxifen use that increases five times the risk of toxicity.
Other risk factors not included in the American Academy of Ophthalmology recommendations, have been described only in some small studies. Palma Sanchez et al. mention systemic arterial hypertension as a risk factor [6]. A study in Mexican patients (Araiza-Casillas et al.) found that the presence of chloroquine deposits in the cornea increases the risk of retinal toxicity five times, contrary to the general consensus of the presence of cornea verticilata caused by deposits of antimalarial drugs does not correlate with retinal toxicity, in addition of being a reversible process when stopping the medication [28],[29].
Diagnostic approach of retinal toxicity associated with antimalarial drugs
Current recommendations advise testing within the first six months of treatment and annually if there are clinical findings suggestive of imminent toxicity or no later than five years of continuous therapy [26].
New ophthalmology tools allow to detect early the first changes of retinopathy with multifocal electroretinography, spectral domain optical coherence tomography or fundus autofluorescence [22]. The objective of testing asymptomatic patients is due to the fact that structural alterations precede symptoms and visual loss in advanced stages is irreversible. Until now the only treatment is the interruption of the medication. While retinal alterations can progress even after stopping the drug, progression after the interruption is much lower if diagnosed in early stages when there is no damage to the retinal pigment epithelium versus late stages [22].
Ancillary studies
In addition to medical history and ophthalmologic examination, ancillary studies have been evaluated for monitoring and early diagnosis of disease. According to studies prior to 2002, the American Academy of Ophthalmology recommended the use of fundus photographs, Amsler grid, Humphrey automated campimetry 10-2 and color vision tests. Many of these studies have been replaced by new equipment able to evaluate subtler changes than previously used studies.
Subjective studies
The Humphrey campimetry continues to be an appropriate strategy for screening; It is important to use the Central 10° (10-2) evaluation strategy because if the usual strategies for the study of glaucoma patients (24-2 or 30-2) are used, the amount of points evaluated at foveal level is very small which decreases significantly its sensitivity. Initially, studies suggested the use of red stimulus for having higher sensitivity [30], with the disadvantage of being less specific and requires a learning curve for proper evaluation, while the use of white stimulus is widely known because of its use in patients with glaucoma and is the one currently recommended by the American Academy of Ophthalmology [26],[27]. The presence of scotomas in the two to eight central degrees of campimetry may precede the clinical findings or the symptoms of the patient. However, Browing and Lee [31], compared the campimetric findings in 10 patients with hydroxychloroquine retinopathy with the ones of other 21 users of the medication but without clinical or paraclinical data of retinopathy (perimetry, spectral domain optical coherence tomography (SD-OCT) and multifocal electroretinogram (ERGm)), and found that all patients without retinopathy had isolated and evanescent scotomas during the evaluation, so that their presence must be corroborated with further studies.
In early stages, the only finding may be a decrease in foveal sensitivity of more than 5 dB, that will evolve to a paracentral scotoma between the two and eight degrees of the fixing point if exposure to the antimalarial drugs continues [31], and may progress to even more advanced scotomas.
Because of the variability of results and the strong dependence of correct performance, Amsler grid has been removed as a test method from the American Academy of Ophthalmology 2011 recommendations [26].
Objective studies
The introduction of this type of study is the biggest advance in the evaluation of retinal toxicity. Since 2002 recommendations, guidelines have focused in studies that do not depend on patient’s response. However, the use of electroretinogram or the electrooculogram didn’t show good results because the initial damage was focused in the macula, which prevented detecting it when averaging the electrical response of the entire retina, for which reason their results were only evident at late stages of damage [23],[32]. This problem was solved with the development of the multifocal electroretinogram which, although was initially described in 1992 by Sutter et al. [33], began to be used for the monitoring of patients with antimalarial treatment until the year 2000 [34] and used more frequently since 2003 [35],[36]. This occurred because multifocal electroretinogram can detect retinal toxicity even before clinical signs are present [37] and any damage seen in 10-2 automated campimetry. As an initial finding there could be paracentral electrical response suppression that may progress to total macular suppression in more advanced cases. A systematic review by Tsang et al. [38] published in mid-2015, showed that when using automated campimetry as a gold standard, the multifocal electroretinogram has sensitivity of 0.90 (CI of 95% 0.62 to 0.98) and specificity of 0.52 (CI of 95% 0.29 to 0.74).
Spectral domain optical coherence tomography (SD-OCT) has also been used recently as a follow up method. In early stages, perifoveal thinning of the inner layers can be found (ganglion cells and internal plexiform layer) [39],[40], that may progress to generalized thinning [41]. In addition, it is possible to assess damage at photoreceptor level identified by loss of the union line of the internal and external segments [26] or the loss of the ellipsoid line in the parafoveal region, known as the flying saucer sign [42],[43],[44]. Like multifocal electroretinogram, it is possible to detect early changes in the spectral domain optical coherence tomography before noticing any alterations in the automated campimetry. Nevertheless, ring shaped scotomas can be found in 10% of cases with a normal spectral domain optical coherence [45]. For this reason, both studies must be complemented. The sensitivity of this test has been reported to be 78.6% and specificity 98% [46]. Besides its use for early diagnosis, spectral domain optical coherence tomography can be helpful in the follow up of patients with detected retinal toxicity, since some cases may be complicated with the development of cystoid macular edema or epiretinal membranes [47].
The most recent tool included for diagnostic testing is the fundus autofluorescence. It is a non-invasive diagnostic procedure that allows to evaluate lipofuscin and other fluorophores distribution in the retinal pigment epithelium. In early stages, when damage to the retinal pigment epithelium is subtle, the test can demonstrate it through a reduction in its autofluorescence, while if the damage is at photoreceptor level, there would be an increase in autofluorescence due to accumulation of debris of the outer segments [48],[49]. Like the spectral domain optical coherence tomography and multifocal electroretinogram, findings in fundus autofluorescence may precede the damage seen in the automated campimetry.
Screening recommendations
Although the American Academy of Ophthalmology has published guidelines for the screening of patients who used antimalarial drugs, these recommendations must be individualized and take into account how many risk factors the patient presents (Table 1). Ideally, a complete ophthalmological evaluation should be performed before starting the use of antimalarial drugs since the presence of retinal or macular disease is a relative contraindication for the use of these medications. In addition, since the first sign may be a macular thinning, it is recommended to test with basal ancillary methods including the automated campimetry and one or more objective studies (spectral domain optical coherence tomography, multifocal electroretinogram or fundus autofluorescence). Finally, personalized advice should be given about the need for periodic ophthalmological assessment and inform the patient about initial symptoms of retinal toxicity like a decrease in visual acuity sensitivity, difficulties to read or the presence of scotomas in visual field.
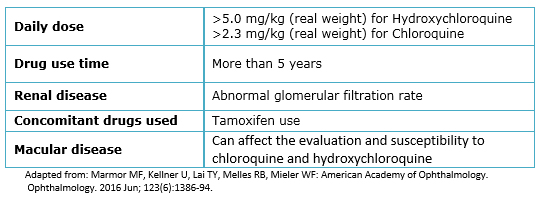
Table 1. Risks factors for chloroquine/hydroxychloroquine related retinal toxicity.
As previously said, the main risk factor for developing retinal toxicity is the cumulative dose, and because it has been estimated that prior to five years of use the risk is low, the guidelines of the American Academy of Ophthalmology suggest that annual screening should begin after five years of use in patients without other risk factors. However, a study in Mexico reported by Araiza-Casillas et al. [50] found that the time of chloroquine use in 16 patients with toxicity detected by automated campimetry or angiography was on average 54.1 ± 27.8 months (range 30 to 197 months), the average dose was 212.3 mg (range 122 to 272 mg) and the cumulative mean dose was less than 288 g (range of 178 to 909 g). All these values are lower than those commonly reported as risk factors, with 50% of the patients asymptomatic. Palma-Sánchez et al. [6] conducted another study in Spain in which they studied 40 patients using antimalarial drugs and found retinal toxicity in 13.1% (CI 95%, 5 to 21%) of them, with an average daily dose of 254 mg and cumulative mean dose of 251.3 ± 182.2 g for hydroxychloroquine and an average daily dose of 250 mg for chloroquine and a cumulative mean dose of 371.5 ± 377.2 g. We consider that it is not possible to extrapolate the recommendations described in other populations, which is why it should be discussed with the patient about the pros and cons of waiting five years for the next revision.
Treatment
Currently, there is no treatment to revert the retinal damage caused by antimalarial drugs, so early diagnosis is vital to diminish the risk of serious visual loss. Once the diagnosis is made the next step is the immediate suspension of the drug. However, even after the medication is suspended, retinal toxicity can continue for several months or years in some cases. This occurs because antimalarial drugs are bind to the melanin present in pigmented tissues throughout the body [51] including the eyeball and are kept there even after the drug is suspended [52]. The follow-up must be continued until having stabilization of the toxicity.
In cases in which a test reveals initial evidence of toxicity, it is recommended to repeat the studies to confirm the result and perform a careful examination to rule out other etiologies. If abnormal results are reported a second time, the patient should be informed about the risks and benefits of continuing the medication; in which case clinical follow-up and ancillary studies should be done more frequently at least every three months to evaluate for toxicity. A different treatment for the underlying disease should be discussed with the primary physician once retinal toxicity is identified.
Clinical case
A 56-year-old female attends to ophthalmologic consultation presenting decreased visual acuity for three years, which has been slowly progressive and gets worse in low light environments, accompanied by vision with colored halos. The patient denies eye pain, scotomas and flickering lights. She has a diagnosis of systemic lupus erythematosus for nine years, treated initially with prednisone, methotrexate (7.5 mg/week), folic acid (5 mg/day) and chloroquine (Aralen®, Sanofi-Aventis: Mexico; variable dose since the beginning, see Table 2) for six years. Prior to the start of treatment, the patient was evaluated by an ophthalmologist who did not found any contraindication for the use of chloroquine. After six years of treatment, the patient goes to the ophthalmology clinic presenting a decrease in vision. The physician suggested stopping the drug, indication commented and supported by the rheumatologist. Despite this, the patient mentions that in the past three years, decrease in visual acuity has continued.
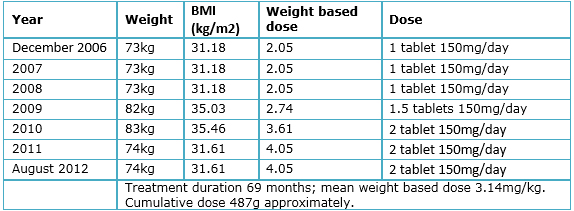
Table 2. Patient’s weight and drug dose variation.
The ophthalmologic examination shows visual acuity of 20/200 in both eyes without improvement with stenopeic glasses, and a vision of 20/30 with lenses in both eyes. Eye movements and pupillary reflexes preserved. Intraocular pressure of 12 mmHg in the right eye and 11mmHg in left eye. In biomicroscopy, is observed in both eyes: conjunctiva with pigmented lesion in limbo, transparent cornea with retrokeratic pigment deposits and no signs of drug accumulation, deep anterior chamber with no cells and clear lens. The following signs are observed in funduscopic exam (both eyes): transparent vitreous, retina with no lesions, optic nerve with well-defined borders and central vessels and an excavation of 0.6, macula with pigmentary alteration (Figure 1a and 1b). Fundus autofluorescence (Topcon TRCNW8S Plus, Tokyo, Japan), shows a hypofluorescent lesion ring around the fovea surrounded by a hyperfluorescent area caused by debris accumulation from outer segments, known as "bulls-eye” sign (Figure 1 c and 1 d). With these findings, the diagnosis of chloroquine related macular toxicity is made.
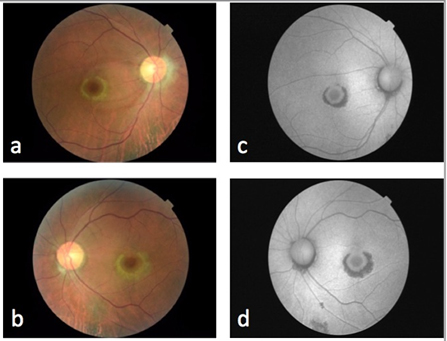
Figure 1. Fundus photograph showing macular changes in right eye (a) and left eye (b). Autofluorescence of rigth eye (c) and left eye (d).
Auxiliary studies were conducted to determine the degree of retinal damage: 10-2 Humphrey automated campimetry with white stimulus and SITA-Standard strategy, with reliability, showing a paracentral scotoma predominantly in superior quadrants of right eye and an ring shaped central scotoma in the left eye (Figure 2a and 2b).
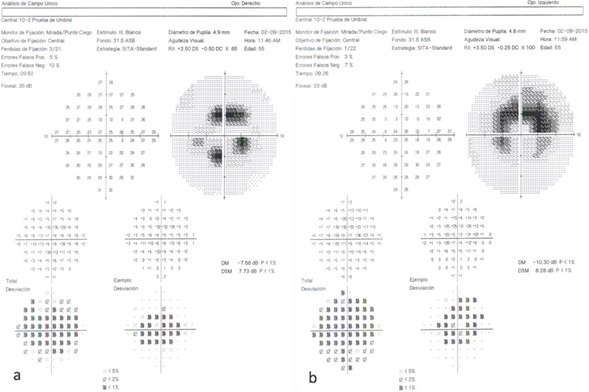
Figure 2. 10-2 Humphrey visual fields right eye (a) and left eye (b)
Table 2 shows the variations in drug dose and patient’s weight throughout the duration of treatment. The patient's height is 1.53 m. Accumulated dose was approximately 487g and the average weight based dose was 3.14 mg/kg. The patient did not have neither liver nor kidney damage secondary to systemic lupus erythematous, as stated by laboratory studies.
Spectral domain optical coherence tomography (CIRRUS Version 7; Zeiss Jena, Germany) with macular cube of 512 x 128 (intensity 9/10) shows areas of retinal thinning and loss of the inner/outer segment junction (high definition images, Figure 3a and 3b).
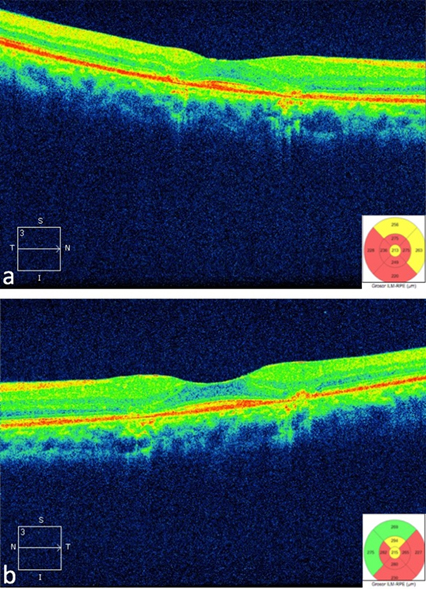
Figure 3. Optical Coherence Tomography showing effacement of the inner/outer segment junction in rigth eye (a) and left eye (b)
Discussion
The patient presents classic findings of chloroquine toxicity like bull's-eye maculopathy, despite having borderline risk factors as described by the American Academy of Ophthalmology [26],[27], given that the dose used for the first 25 months of treatment was lower than the described for toxicity and the time using a higher dose was 54 months, with a cumulative chloroquine dose of 487g (very close to the 460g suggested by the American Academy of ophthalmology). The total duration of drug use (69 months) is also very close to the 60 months (5 years) described as safe time in the guidelines. The patient’s age (56 years) is less than 60 years. The patient did not have kidney damage, there was no concomitant tamoxifen use and, according to the initial ophthalmologic exam described by the patient, there was no macular or retinal disease prior to the beginning of the treatment with chloroquine. All of this matches that described by Araiza-Casillas et al. [50] in Mexican patients, finding retinal toxicity even in patients without risk factors, or with borderline values, suggesting a racial predisposition or another not well studied factor that rises the need for a closer follow-up on our population.
In addition to risk factors, the patient shows findings previously described for ancillary studies that enable us to make a definitive diagnosis, like the loss of the inner/outer segment junction found in optical coherence tomography [26], as well as a generalized macular thinning [41]. Autofluorescence shows alternating areas increased signal corresponding to damage at photoreceptor level with areas of decreased signal caused by damage to the retinal pigment epithelium [48],[49]. Finally, automated campimetry showed an absolute paracentral scotoma predominantly in superior quadrants of the right eye and a ring shaped scotoma in the central two to eight degrees of left eye axis.
Conclusion
Retinal toxicity secondary to chloroquine/hydroxychloroquine use is a condition that can cause serious and irreversible visual loss. Until today, the best treatment is early diagnosis therefore screening test should be made to all patients that use this drugs, since as exemplified in this clinical case, visual disturbances can continue even after treatment interruption.
Notes
From the authors
The purpose of publishing this clinical case is to mention the changes in the recommendations of the American Academy of Ophthalmology for the screening in this group of patients, and to emphasize that even though the guidelines can be a very good orientation tool, it is important to give a personalized approach and discuss with the patient about it.
From the editor
The authors originally submitted this article in Spanish and subsequently translated it into English. The Journal has not copyedited this version.
Conflicts of interest
The authors have completed the ICMJE conflict of interest declaration form and declare they did not receive any financing for the making of this report; there are no financing relationships with any organization that could have interest in the published article, in the last three years; and not having other kind of relationships or activities that could influence the article. The form can be requested contacting the responsible author or the Journal´s editorial direction.
Funding
The authors declare no external funding sources for this study.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Los antipalúdicos son fármacos ampliamente utilizados en varios países para el control de enfermedades reumatológicas como el lupus eritematoso sistémico y la artritis reumatoide. En México continúan siendo muy aceptados por su bajo costo y pocos efectos adversos, la mayoría de ellos no graves y reversibles. Sin embargo, a nivel oftalmológico se produce acúmulo de los metabolitos de estos medicamentos que provoca un daño visual que puede llegar a ser irreversible. Por esta razón es importante una evaluación oftalmológica y un seguimiento adecuado de estos pacientes. A través de un caso clínico ejemplificamos los hallazgos oftalmológicos característicos así como los factores de riesgo de la toxicidad retiniana y finalmente se discuten los lineamientos actuales para el diagnóstico y seguimiento en pacientes que utilizan estos medicamentos.
 Authors:
Manuel Garza-Leon [1 ], Diana Elsa Flores-Alvarado [2 ], Juan Manuel Muñoz-Bravo [3 ]
Authors:
Manuel Garza-Leon [1 ], Diana Elsa Flores-Alvarado [2 ], Juan Manuel Muñoz-Bravo [3 ]
Affiliation:
[1] Departamento de Ciencias Clínicas, División de Ciencias de la Salud, Universidad de Monterrey, Monterrey, Nuevo León, México
[2] Departamento de Reumatología, Hospital Universitario, Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, México
[3] Fundación Destellos de Luz, Nuevo León, México
E-mail: dr.manuel.garza@gmail.com
Author address:
[1] Centro Médico Hidalgo
Avenida Hidalgo 2425 Poniente
Séptimo piso
Consultorio 706
Colonia Obispado
Monterrey
Nuevo León
México

Citation: Garza-Leon M , Flores-Alvarado DE, Muñoz-Bravo JM . Retinal toxicity induced by antimalarial drugs: literature review and case report. Medwave 2016 Jun;16(5):e6471 doi: 10.5867/medwave.2016.05.6471
Submission date: 6/4/2016
Acceptance date: 3/6/2016
Publication date: 17/6/2016
Origin: not requested
Type of review: reviewed by four external peer reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Jiménez Palop M. [Antimalarials: an update in rheumatic diseases]. Reumatol Clin. 2006 Jul;2(4):190-201. | CrossRef | PubMed |
- Jover JA, Leon L, Pato E, Loza E, Rosales Z, Matias MA, et al. Long-term use of antimalarial drugs in rheumatic diseases. Clin Exp Rheumatol. 2012 May-Jun;30(3):380-7. | PubMed |
- Albrecht K, Huscher D, Richter J, Backhaus M, Bischoff S, Kötter I, et al. Changes in referral, treatment and outcomes in patients with systemic lupus erythematosus in Germany in the 1990s and the 2000s. Lupus Sci Med. 2014 Dec 12;1(1):e000059. | CrossRef | PubMed |
- Felson DT, Anderson JJ, Meenan RF. Use of short-term efficacy/toxicity tradeoffs to select second-line drugs in rheumatoid arthritis. A metaanalysis of published clinical trials. Arthritis Rheum. 1992 Oct;35(10):1117-25. | PubMed |
- Choy EH, Smith C, Doré CJ, Scott DL. A meta-analysis of the efficacy and toxicity of combining disease-modifying anti-rheumatic drugs in rheumatoid arthritis based on patient withdrawal. Rheumatology (Oxford). 2005 Nov;44(11):1414-21. | PubMed |
- Palma Sánchez D, Rubio Velazquez E, Soro Marín S, Reyes García R. Retinal toxicity due to antimalarials: frequency and risk factors. Reumatol Clin. 2013 Sep-Oct;9(5):259-62. doi: http://dx.doi.org/10.1016/j.reuma.2012.10.011 | PubMed |
- Bernstein HN. Chloroquine ocular toxicity. Surv Ophthalmol. 1967 Oct;12(5):415-47. 4980087
- Bernstein HN. Ophthalmologic considerations and testing in patients receiving long-term antimalarial therapy. Am J Med. 1983 Jul 18;75(1A):25-34. | PubMed |
- Navajas EV, Krema H, Hammoudi DS, Lipton JH, Simpson ER, Boyd S, et al. Retinal toxicity of high-dose hydroxychloroquine in patients with chronic graft-versus-host disease. Can J Ophthalmol. 2015 Dec;50(6):442-50. | CrossRef | PubMed |
- Leung LS, Neal JW, Wakelee HA, Sequist LV, Marmor MF. Rapid Onset of Retinal Toxicity From High-Dose Hydroxychloroquine Given for Cancer Therapy. Am J Ophthalmol. 2015 Oct;160(4):799-805.e1. | CrossRef | PubMed |
- Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010 Jun;62(6):775-84. | CrossRef | PubMed |
- Hobbs HE, Sorsby A, Freedman A. Retinopathy following chloroquine therapy. Lancet. 1959 Oct 3;2(7101):478-80. | PubMed |
- Shearer RV, Dubois EL. Ocular changes induced by long-term hydroxychloroquine (plaquenil) therapy. Am J Ophthalmol. 1967 Aug;64(2):245-52. | PubMed |
- Levy GD, Munz SJ, Paschal J, Cohen HB, Pince KJ, Peterson T. Incidence of hydroxychloroquine retinopathy in 1,207 patients in a large multicenter outpatient practice. Arthritis Rheum. 1997 Aug;40(8):1482-6. | PubMed |
- Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology. 2003 Jul;110(7):1321-6. | PubMed |
- Michihara A, Toda K, Kubo T, Fujiwara Y, Akasaki K, Tsuji H. Disruptive effect of chloroquine on lysosomes in cultured rat hepatocytes. Biol Pharm Bull. 2005 Jun;28(6):947-51. | PubMed |
- Mahon GJ, Anderson HR, Gardiner TA, McFarlane S, Archer DB, Stitt AW. Chloroquine causes lysosomal dysfunction in neural retina and RPE: implications for retinopathy. Curr Eye Res. 2004 Apr;28(4):277-84. | PubMed |
- Sundelin SP, Terman A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. APMIS. 2002 Jun;110(6):481-9. | PubMed |
- Suter M, Remé C, Grimm C, Wenzel A, Jäättela M, Esser P, et al. The lipofusion component N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells.J Biol Chem. 2000 Dec 15;275(50):39625-30. | PubMed |
- Radu RA, Yuan Q, Hu J, Peng JH, Lloyd M, Nusinowitz S, et al. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Invest Ophthalmol Vis Sci. 2008 Sep;49(9):3821-9. | CrossRef | PubMed |
- Gregory MH, Rutty DA, Wood RD. Differences in the retinotoxic action of chloroquine and phenothiazine derivatives. J Pathol. 1970 Nov;102(3):139-50. | PubMed |
- de Sisternes L, Hu J, Rubin DL, Marmor MF. Localization of damage in progressive hydroxychloroquine retinopathy on and off the drug: inner versus outer retina, parafovea versus peripheral fovea. Invest Ophthalmol Vis Sci. 2015 May;56(5):3415-26. | CrossRef | PubMed |
- Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF; American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002 Jul;109(7):1377-82. | PubMed |
- Lee DH, Melles RB, Joe SG, Lee JY, Kim JG, Lee CK, et al. Pericentral hydroxychloroquine retinopathy in Korean patients. Ophthalmology. 2015 Jun;122(6):1252-6. doi: | CrossRef | PubMed |
- Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015 Jan;122(1):110-6. | CrossRef | PubMed |
- Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011 Feb;118(2):415-22. doi: | CrossRef | PubMed |
- Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF; American Academy of Ophthalmology. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology. 2016 Jun;123(6):1386-94. | CrossRef | PubMed |
- Tehrani R, Ostrowski RA, Hariman R, Jay WM. Ocular toxicity of hydroxychloroquine. Semin Ophthalmol. 2008 May-Jun;23(3):201-9. | CrossRef | PubMed |
- Easterbrook M. Is corneal deposition of antimalarial any indication of retinal toxicity? Can J Ophthalmol. 1990 Aug;25(5):249-51. | PubMed |
- Easterbrook M, Trope G. Value of Humphrey perimetry in the detection of early chloroquine retinopathy. Lens Eye Toxic Res. 1989;6(1-2):255-68. | PubMed |
- Browning DJ, Lee C. Scotoma analysis of 10-2 visual field testing with a white target in screening for hydroxychloroquine retinopathy. Clin Ophthalmol. 2015 May 27;9:943-52. | CrossRef | PubMed |
- Reijmer CN, Tijssen JG, Kok GA, van Lith GH. Interpretation of the electro-oculogram of patients taking chloroquine. Doc Ophthalmol. 1980 Apr 15;48(2):273-6. | PubMed |
- Sutter EE, Tran D. The field topography of ERG components in man--I. The photopic luminance response. Vision Res. 1992 Mar;32(3):433-46. | PubMed |
- Kellner U, Kraus H, Foerster MH. Multifocal ERG in chloroquine retinopathy: regional variance of retinal dysfunction. Graefes Arch Clin Exp Ophthalmol. 2000 Jan;238(1):94-7. | PubMed |
- Penrose PJ, Tzekov RT, Sutter EE, Fu AD, Allen AW Jr, Fung WE, et al. Multifocal electroretinography evaluation for early detection of retinal dysfunction in patients taking hydroxychloroquine. Retina. 2003 Aug;23(4):503-12. | PubMed |
- So SC, Hedges TR, Schuman JS, Quireza ML. Evaluation of hydroxychloroquine retinopathy with multifocal electroretinography. Ophthalmic Surg Lasers Imaging. 2003 May-Jun;34(3):251-8. | PubMed |
- Teoh SC, Lim J, Koh A, Lim T, Fu E. Abnormalities on the multifocal electroretinogram may precede clinical signs of hydroxychloroquine retino-toxicity. Eye (Lond). 2006 Jan;20(1):129-32. | PubMed |
- Tsang AC, Ahmadi Pirshahid S, Virgili G, Gottlieb CC, Hamilton J, Coupland SG. Hydroxychloroquine and chloroquine retinopathy: a systematic review evaluating the multifocal electroretinogram as a screening test. Ophthalmology.2015 Jun;122(6):1239-1251.e4. | CrossRef | PubMed |
- Pasadhika S, Fishman GA, Choi D, Shahidi M. Selective thinning of the perifoveal inner retina as an early sign of hydroxychloroquine retinal toxicity. Eye (Lond). 2010 May;24(5):756-62; quiz 763. doi: | CrossRef | PubMed |
- Ulviye Y, Betul T, Nur TH, Selda C. Spectral domain optical coherence tomography for early detection of retinal alterations in patients using hydroxychloroquine. Indian J Ophthalmol. 2013 Apr;61(4):168-71. doi: | CrossRef | PubMed |
- Kahn JB, Haberman ID, Reddy S. Spectral-domain optical coherence tomography as a screening technique for chloroquine and hydroxychloroquine retinal toxicity. Ophthalmic Surg Lasers Imaging. 2011 Nov-Dec;42(6):493-7. | CrossRef | PubMed |
- Ascaso FJ, Rodríguez NA, San Miguel R, Huerva V. The "flying saucer" sign on spectral domain optical coherence tomography in chloroquine retinopathy.Arthritis Rheum. 2013 Sep;65(9):2322. doi: | CrossRef | PubMed |
- Chen E, Brown DM, Benz MS, Fish RH, Wong TP, Kim RY, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the "flying saucer" sign). Clin Ophthalmol. 2010 Oct 21;4:1151-8. | CrossRef | PubMed |
- Asensio-Sánchez VM. [SD-OCT As screening test for hydroxychloroquine retinopathy: The «flying saucer» sign]. Arch Soc Esp Oftalmol. 2015 Jul;90(7):338-40. | CrossRef | PubMed |
- Marmor MF, Melles RB. Disparity between visual fields and optical coherence tomography in hydroxychloroquine retinopathy. Ophthalmology. 2014 Jun;121(6):1257-62. | CrossRef | PubMed |
- Allam RS, Abd-Elmohsen MN, Khafagy MM, Raafat KA, Sheta SM. Spectral-Domain Optical Coherence Tomography of Preclinical Chloroquine Maculopathy in Egyptian Rheumatoid Arthritis Patients. J Ophthalmol. 2015;2015:292357. | CrossRef | PubMed |
- Kellner S, Weinitz S, Farmand G, Kellner U. Cystoid macular oedema and epiretinal membrane formation during progression of chloroquine retinopathy after drug cessation. Br J Ophthalmol. 2014 Feb;98(2):200-6. | CrossRef | PubMed |
- Kellner S, Weinitz S, Kellner U. Spectral domain optical coherence tomography detects early stages of chloroquine retinopathy similar to multifocal electroretinography, fundus autofluorescence and near-infrared autofluorescence. Br J Ophthalmol. 2009 Nov;93(11):1444-7. doi: | CrossRef | PubMed |
- Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006 Aug;47(8):3531-8. | PubMed |
- Araiza-Casillas R, Cárdenas F, Morales Y, Cardiel MH. Factors associated with chloroquine-induced retinopathy in rheumatic diseases. Lupus. 2004;13(2):119-24. | PubMed |
- Lindquist NG, Ullberg S. The melanin affinity of chloroquine and chlorpromazine studied by whole body autoradiography. Acta Pharmacol Toxicol (Copenh). 1972;2:Suppl 2:1-32. | PubMed |
- Bernstein H, Zvaifler N, Rubin M, Mansour AM. The ocular deposition of chloroquine. Invest Ophthalmol. 1963 Aug;2:384-92. | PubMed |
 Jiménez Palop M. [Antimalarials: an update in rheumatic diseases]. Reumatol Clin. 2006 Jul;2(4):190-201. | CrossRef | PubMed |
Jiménez Palop M. [Antimalarials: an update in rheumatic diseases]. Reumatol Clin. 2006 Jul;2(4):190-201. | CrossRef | PubMed | Jover JA, Leon L, Pato E, Loza E, Rosales Z, Matias MA, et al. Long-term use of antimalarial drugs in rheumatic diseases. Clin Exp Rheumatol. 2012 May-Jun;30(3):380-7. | PubMed |
Jover JA, Leon L, Pato E, Loza E, Rosales Z, Matias MA, et al. Long-term use of antimalarial drugs in rheumatic diseases. Clin Exp Rheumatol. 2012 May-Jun;30(3):380-7. | PubMed | Albrecht K, Huscher D, Richter J, Backhaus M, Bischoff S, Kötter I, et al. Changes in referral, treatment and outcomes in patients with systemic lupus erythematosus in Germany in the 1990s and the 2000s. Lupus Sci Med. 2014 Dec 12;1(1):e000059. | CrossRef | PubMed |
Albrecht K, Huscher D, Richter J, Backhaus M, Bischoff S, Kötter I, et al. Changes in referral, treatment and outcomes in patients with systemic lupus erythematosus in Germany in the 1990s and the 2000s. Lupus Sci Med. 2014 Dec 12;1(1):e000059. | CrossRef | PubMed | Felson DT, Anderson JJ, Meenan RF. Use of short-term efficacy/toxicity tradeoffs to select second-line drugs in rheumatoid arthritis. A metaanalysis of published clinical trials. Arthritis Rheum. 1992 Oct;35(10):1117-25. | PubMed |
Felson DT, Anderson JJ, Meenan RF. Use of short-term efficacy/toxicity tradeoffs to select second-line drugs in rheumatoid arthritis. A metaanalysis of published clinical trials. Arthritis Rheum. 1992 Oct;35(10):1117-25. | PubMed | Choy EH, Smith C, Doré CJ, Scott DL. A meta-analysis of the efficacy and toxicity of combining disease-modifying anti-rheumatic drugs in rheumatoid arthritis based on patient withdrawal. Rheumatology (Oxford). 2005 Nov;44(11):1414-21. | PubMed |
Choy EH, Smith C, Doré CJ, Scott DL. A meta-analysis of the efficacy and toxicity of combining disease-modifying anti-rheumatic drugs in rheumatoid arthritis based on patient withdrawal. Rheumatology (Oxford). 2005 Nov;44(11):1414-21. | PubMed | Palma Sánchez D, Rubio Velazquez E, Soro Marín S, Reyes García R. Retinal toxicity due to antimalarials: frequency and risk factors. Reumatol Clin. 2013 Sep-Oct;9(5):259-62. doi: http://dx.doi.org/10.1016/j.reuma.2012.10.011 | PubMed |
Palma Sánchez D, Rubio Velazquez E, Soro Marín S, Reyes García R. Retinal toxicity due to antimalarials: frequency and risk factors. Reumatol Clin. 2013 Sep-Oct;9(5):259-62. doi: http://dx.doi.org/10.1016/j.reuma.2012.10.011 | PubMed | Bernstein HN. Chloroquine ocular toxicity. Surv Ophthalmol. 1967 Oct;12(5):415-47. 4980087
Bernstein HN. Chloroquine ocular toxicity. Surv Ophthalmol. 1967 Oct;12(5):415-47. 4980087  Bernstein HN. Ophthalmologic considerations and testing in patients receiving long-term antimalarial therapy. Am J Med. 1983 Jul 18;75(1A):25-34. | PubMed |
Bernstein HN. Ophthalmologic considerations and testing in patients receiving long-term antimalarial therapy. Am J Med. 1983 Jul 18;75(1A):25-34. | PubMed | Navajas EV, Krema H, Hammoudi DS, Lipton JH, Simpson ER, Boyd S, et al. Retinal toxicity of high-dose hydroxychloroquine in patients with chronic graft-versus-host disease. Can J Ophthalmol. 2015 Dec;50(6):442-50. | CrossRef | PubMed |
Navajas EV, Krema H, Hammoudi DS, Lipton JH, Simpson ER, Boyd S, et al. Retinal toxicity of high-dose hydroxychloroquine in patients with chronic graft-versus-host disease. Can J Ophthalmol. 2015 Dec;50(6):442-50. | CrossRef | PubMed | Leung LS, Neal JW, Wakelee HA, Sequist LV, Marmor MF. Rapid Onset of Retinal Toxicity From High-Dose Hydroxychloroquine Given for Cancer Therapy. Am J Ophthalmol. 2015 Oct;160(4):799-805.e1. | CrossRef | PubMed |
Leung LS, Neal JW, Wakelee HA, Sequist LV, Marmor MF. Rapid Onset of Retinal Toxicity From High-Dose Hydroxychloroquine Given for Cancer Therapy. Am J Ophthalmol. 2015 Oct;160(4):799-805.e1. | CrossRef | PubMed | Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010 Jun;62(6):775-84. | CrossRef | PubMed |
Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010 Jun;62(6):775-84. | CrossRef | PubMed | Hobbs HE, Sorsby A, Freedman A. Retinopathy following chloroquine therapy. Lancet. 1959 Oct 3;2(7101):478-80. | PubMed |
Hobbs HE, Sorsby A, Freedman A. Retinopathy following chloroquine therapy. Lancet. 1959 Oct 3;2(7101):478-80. | PubMed | Shearer RV, Dubois EL. Ocular changes induced by long-term hydroxychloroquine (plaquenil) therapy. Am J Ophthalmol. 1967 Aug;64(2):245-52. | PubMed |
Shearer RV, Dubois EL. Ocular changes induced by long-term hydroxychloroquine (plaquenil) therapy. Am J Ophthalmol. 1967 Aug;64(2):245-52. | PubMed | Levy GD, Munz SJ, Paschal J, Cohen HB, Pince KJ, Peterson T. Incidence of hydroxychloroquine retinopathy in 1,207 patients in a large multicenter outpatient practice. Arthritis Rheum. 1997 Aug;40(8):1482-6. | PubMed |
Levy GD, Munz SJ, Paschal J, Cohen HB, Pince KJ, Peterson T. Incidence of hydroxychloroquine retinopathy in 1,207 patients in a large multicenter outpatient practice. Arthritis Rheum. 1997 Aug;40(8):1482-6. | PubMed | Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology. 2003 Jul;110(7):1321-6. | PubMed |
Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology. 2003 Jul;110(7):1321-6. | PubMed | Michihara A, Toda K, Kubo T, Fujiwara Y, Akasaki K, Tsuji H. Disruptive effect of chloroquine on lysosomes in cultured rat hepatocytes. Biol Pharm Bull. 2005 Jun;28(6):947-51. | PubMed |
Michihara A, Toda K, Kubo T, Fujiwara Y, Akasaki K, Tsuji H. Disruptive effect of chloroquine on lysosomes in cultured rat hepatocytes. Biol Pharm Bull. 2005 Jun;28(6):947-51. | PubMed | Mahon GJ, Anderson HR, Gardiner TA, McFarlane S, Archer DB, Stitt AW. Chloroquine causes lysosomal dysfunction in neural retina and RPE: implications for retinopathy. Curr Eye Res. 2004 Apr;28(4):277-84. | PubMed |
Mahon GJ, Anderson HR, Gardiner TA, McFarlane S, Archer DB, Stitt AW. Chloroquine causes lysosomal dysfunction in neural retina and RPE: implications for retinopathy. Curr Eye Res. 2004 Apr;28(4):277-84. | PubMed | Sundelin SP, Terman A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. APMIS. 2002 Jun;110(6):481-9. | PubMed |
Sundelin SP, Terman A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. APMIS. 2002 Jun;110(6):481-9. | PubMed | Suter M, Remé C, Grimm C, Wenzel A, Jäättela M, Esser P, et al. The lipofusion component N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells.J Biol Chem. 2000 Dec 15;275(50):39625-30. | PubMed |
Suter M, Remé C, Grimm C, Wenzel A, Jäättela M, Esser P, et al. The lipofusion component N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells.J Biol Chem. 2000 Dec 15;275(50):39625-30. | PubMed | Radu RA, Yuan Q, Hu J, Peng JH, Lloyd M, Nusinowitz S, et al. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Invest Ophthalmol Vis Sci. 2008 Sep;49(9):3821-9. | CrossRef | PubMed |
Radu RA, Yuan Q, Hu J, Peng JH, Lloyd M, Nusinowitz S, et al. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Invest Ophthalmol Vis Sci. 2008 Sep;49(9):3821-9. | CrossRef | PubMed | Gregory MH, Rutty DA, Wood RD. Differences in the retinotoxic action of chloroquine and phenothiazine derivatives. J Pathol. 1970 Nov;102(3):139-50. | PubMed |
Gregory MH, Rutty DA, Wood RD. Differences in the retinotoxic action of chloroquine and phenothiazine derivatives. J Pathol. 1970 Nov;102(3):139-50. | PubMed | de Sisternes L, Hu J, Rubin DL, Marmor MF. Localization of damage in progressive hydroxychloroquine retinopathy on and off the drug: inner versus outer retina, parafovea versus peripheral fovea. Invest Ophthalmol Vis Sci. 2015 May;56(5):3415-26. | CrossRef | PubMed |
de Sisternes L, Hu J, Rubin DL, Marmor MF. Localization of damage in progressive hydroxychloroquine retinopathy on and off the drug: inner versus outer retina, parafovea versus peripheral fovea. Invest Ophthalmol Vis Sci. 2015 May;56(5):3415-26. | CrossRef | PubMed | Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF; American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002 Jul;109(7):1377-82. | PubMed |
Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF; American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002 Jul;109(7):1377-82. | PubMed | Lee DH, Melles RB, Joe SG, Lee JY, Kim JG, Lee CK, et al. Pericentral hydroxychloroquine retinopathy in Korean patients. Ophthalmology. 2015 Jun;122(6):1252-6. doi: | CrossRef | PubMed |
Lee DH, Melles RB, Joe SG, Lee JY, Kim JG, Lee CK, et al. Pericentral hydroxychloroquine retinopathy in Korean patients. Ophthalmology. 2015 Jun;122(6):1252-6. doi: | CrossRef | PubMed | Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015 Jan;122(1):110-6. | CrossRef | PubMed |
Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015 Jan;122(1):110-6. | CrossRef | PubMed | Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011 Feb;118(2):415-22. doi: | CrossRef | PubMed |
Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011 Feb;118(2):415-22. doi: | CrossRef | PubMed | Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF; American Academy of Ophthalmology. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology. 2016 Jun;123(6):1386-94. | CrossRef | PubMed |
Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF; American Academy of Ophthalmology. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology. 2016 Jun;123(6):1386-94. | CrossRef | PubMed | Tehrani R, Ostrowski RA, Hariman R, Jay WM. Ocular toxicity of hydroxychloroquine. Semin Ophthalmol. 2008 May-Jun;23(3):201-9. | CrossRef | PubMed |
Tehrani R, Ostrowski RA, Hariman R, Jay WM. Ocular toxicity of hydroxychloroquine. Semin Ophthalmol. 2008 May-Jun;23(3):201-9. | CrossRef | PubMed | Easterbrook M. Is corneal deposition of antimalarial any indication of retinal toxicity? Can J Ophthalmol. 1990 Aug;25(5):249-51. | PubMed |
Easterbrook M. Is corneal deposition of antimalarial any indication of retinal toxicity? Can J Ophthalmol. 1990 Aug;25(5):249-51. | PubMed | Easterbrook M, Trope G. Value of Humphrey perimetry in the detection of early chloroquine retinopathy. Lens Eye Toxic Res. 1989;6(1-2):255-68. | PubMed |
Easterbrook M, Trope G. Value of Humphrey perimetry in the detection of early chloroquine retinopathy. Lens Eye Toxic Res. 1989;6(1-2):255-68. | PubMed | Browning DJ, Lee C. Scotoma analysis of 10-2 visual field testing with a white target in screening for hydroxychloroquine retinopathy. Clin Ophthalmol. 2015 May 27;9:943-52. | CrossRef | PubMed |
Browning DJ, Lee C. Scotoma analysis of 10-2 visual field testing with a white target in screening for hydroxychloroquine retinopathy. Clin Ophthalmol. 2015 May 27;9:943-52. | CrossRef | PubMed | Reijmer CN, Tijssen JG, Kok GA, van Lith GH. Interpretation of the electro-oculogram of patients taking chloroquine. Doc Ophthalmol. 1980 Apr 15;48(2):273-6. | PubMed |
Reijmer CN, Tijssen JG, Kok GA, van Lith GH. Interpretation of the electro-oculogram of patients taking chloroquine. Doc Ophthalmol. 1980 Apr 15;48(2):273-6. | PubMed | Sutter EE, Tran D. The field topography of ERG components in man--I. The photopic luminance response. Vision Res. 1992 Mar;32(3):433-46. | PubMed |
Sutter EE, Tran D. The field topography of ERG components in man--I. The photopic luminance response. Vision Res. 1992 Mar;32(3):433-46. | PubMed | Kellner U, Kraus H, Foerster MH. Multifocal ERG in chloroquine retinopathy: regional variance of retinal dysfunction. Graefes Arch Clin Exp Ophthalmol. 2000 Jan;238(1):94-7. | PubMed |
Kellner U, Kraus H, Foerster MH. Multifocal ERG in chloroquine retinopathy: regional variance of retinal dysfunction. Graefes Arch Clin Exp Ophthalmol. 2000 Jan;238(1):94-7. | PubMed | Penrose PJ, Tzekov RT, Sutter EE, Fu AD, Allen AW Jr, Fung WE, et al. Multifocal electroretinography evaluation for early detection of retinal dysfunction in patients taking hydroxychloroquine. Retina. 2003 Aug;23(4):503-12. | PubMed |
Penrose PJ, Tzekov RT, Sutter EE, Fu AD, Allen AW Jr, Fung WE, et al. Multifocal electroretinography evaluation for early detection of retinal dysfunction in patients taking hydroxychloroquine. Retina. 2003 Aug;23(4):503-12. | PubMed | So SC, Hedges TR, Schuman JS, Quireza ML. Evaluation of hydroxychloroquine retinopathy with multifocal electroretinography. Ophthalmic Surg Lasers Imaging. 2003 May-Jun;34(3):251-8. | PubMed |
So SC, Hedges TR, Schuman JS, Quireza ML. Evaluation of hydroxychloroquine retinopathy with multifocal electroretinography. Ophthalmic Surg Lasers Imaging. 2003 May-Jun;34(3):251-8. | PubMed | Teoh SC, Lim J, Koh A, Lim T, Fu E. Abnormalities on the multifocal electroretinogram may precede clinical signs of hydroxychloroquine retino-toxicity. Eye (Lond). 2006 Jan;20(1):129-32. | PubMed |
Teoh SC, Lim J, Koh A, Lim T, Fu E. Abnormalities on the multifocal electroretinogram may precede clinical signs of hydroxychloroquine retino-toxicity. Eye (Lond). 2006 Jan;20(1):129-32. | PubMed | Tsang AC, Ahmadi Pirshahid S, Virgili G, Gottlieb CC, Hamilton J, Coupland SG. Hydroxychloroquine and chloroquine retinopathy: a systematic review evaluating the multifocal electroretinogram as a screening test. Ophthalmology.2015 Jun;122(6):1239-1251.e4. | CrossRef | PubMed |
Tsang AC, Ahmadi Pirshahid S, Virgili G, Gottlieb CC, Hamilton J, Coupland SG. Hydroxychloroquine and chloroquine retinopathy: a systematic review evaluating the multifocal electroretinogram as a screening test. Ophthalmology.2015 Jun;122(6):1239-1251.e4. | CrossRef | PubMed | Pasadhika S, Fishman GA, Choi D, Shahidi M. Selective thinning of the perifoveal inner retina as an early sign of hydroxychloroquine retinal toxicity. Eye (Lond). 2010 May;24(5):756-62; quiz 763. doi: | CrossRef | PubMed |
Pasadhika S, Fishman GA, Choi D, Shahidi M. Selective thinning of the perifoveal inner retina as an early sign of hydroxychloroquine retinal toxicity. Eye (Lond). 2010 May;24(5):756-62; quiz 763. doi: | CrossRef | PubMed | Ulviye Y, Betul T, Nur TH, Selda C. Spectral domain optical coherence tomography for early detection of retinal alterations in patients using hydroxychloroquine. Indian J Ophthalmol. 2013 Apr;61(4):168-71. doi: | CrossRef | PubMed |
Ulviye Y, Betul T, Nur TH, Selda C. Spectral domain optical coherence tomography for early detection of retinal alterations in patients using hydroxychloroquine. Indian J Ophthalmol. 2013 Apr;61(4):168-71. doi: | CrossRef | PubMed | Kahn JB, Haberman ID, Reddy S. Spectral-domain optical coherence tomography as a screening technique for chloroquine and hydroxychloroquine retinal toxicity. Ophthalmic Surg Lasers Imaging. 2011 Nov-Dec;42(6):493-7. | CrossRef | PubMed |
Kahn JB, Haberman ID, Reddy S. Spectral-domain optical coherence tomography as a screening technique for chloroquine and hydroxychloroquine retinal toxicity. Ophthalmic Surg Lasers Imaging. 2011 Nov-Dec;42(6):493-7. | CrossRef | PubMed | Ascaso FJ, Rodríguez NA, San Miguel R, Huerva V. The "flying saucer" sign on spectral domain optical coherence tomography in chloroquine retinopathy.Arthritis Rheum. 2013 Sep;65(9):2322. doi: | CrossRef | PubMed |
Ascaso FJ, Rodríguez NA, San Miguel R, Huerva V. The "flying saucer" sign on spectral domain optical coherence tomography in chloroquine retinopathy.Arthritis Rheum. 2013 Sep;65(9):2322. doi: | CrossRef | PubMed | Chen E, Brown DM, Benz MS, Fish RH, Wong TP, Kim RY, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the "flying saucer" sign). Clin Ophthalmol. 2010 Oct 21;4:1151-8. | CrossRef | PubMed |
Chen E, Brown DM, Benz MS, Fish RH, Wong TP, Kim RY, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the "flying saucer" sign). Clin Ophthalmol. 2010 Oct 21;4:1151-8. | CrossRef | PubMed | Asensio-Sánchez VM. [SD-OCT As screening test for hydroxychloroquine retinopathy: The «flying saucer» sign]. Arch Soc Esp Oftalmol. 2015 Jul;90(7):338-40. | CrossRef | PubMed |
Asensio-Sánchez VM. [SD-OCT As screening test for hydroxychloroquine retinopathy: The «flying saucer» sign]. Arch Soc Esp Oftalmol. 2015 Jul;90(7):338-40. | CrossRef | PubMed | Marmor MF, Melles RB. Disparity between visual fields and optical coherence tomography in hydroxychloroquine retinopathy. Ophthalmology. 2014 Jun;121(6):1257-62. | CrossRef | PubMed |
Marmor MF, Melles RB. Disparity between visual fields and optical coherence tomography in hydroxychloroquine retinopathy. Ophthalmology. 2014 Jun;121(6):1257-62. | CrossRef | PubMed | Allam RS, Abd-Elmohsen MN, Khafagy MM, Raafat KA, Sheta SM. Spectral-Domain Optical Coherence Tomography of Preclinical Chloroquine Maculopathy in Egyptian Rheumatoid Arthritis Patients. J Ophthalmol. 2015;2015:292357. | CrossRef | PubMed |
Allam RS, Abd-Elmohsen MN, Khafagy MM, Raafat KA, Sheta SM. Spectral-Domain Optical Coherence Tomography of Preclinical Chloroquine Maculopathy in Egyptian Rheumatoid Arthritis Patients. J Ophthalmol. 2015;2015:292357. | CrossRef | PubMed | Kellner S, Weinitz S, Farmand G, Kellner U. Cystoid macular oedema and epiretinal membrane formation during progression of chloroquine retinopathy after drug cessation. Br J Ophthalmol. 2014 Feb;98(2):200-6. | CrossRef | PubMed |
Kellner S, Weinitz S, Farmand G, Kellner U. Cystoid macular oedema and epiretinal membrane formation during progression of chloroquine retinopathy after drug cessation. Br J Ophthalmol. 2014 Feb;98(2):200-6. | CrossRef | PubMed | Kellner S, Weinitz S, Kellner U. Spectral domain optical coherence tomography detects early stages of chloroquine retinopathy similar to multifocal electroretinography, fundus autofluorescence and near-infrared autofluorescence. Br J Ophthalmol. 2009 Nov;93(11):1444-7. doi: | CrossRef | PubMed |
Kellner S, Weinitz S, Kellner U. Spectral domain optical coherence tomography detects early stages of chloroquine retinopathy similar to multifocal electroretinography, fundus autofluorescence and near-infrared autofluorescence. Br J Ophthalmol. 2009 Nov;93(11):1444-7. doi: | CrossRef | PubMed | Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006 Aug;47(8):3531-8. | PubMed |
Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006 Aug;47(8):3531-8. | PubMed | Araiza-Casillas R, Cárdenas F, Morales Y, Cardiel MH. Factors associated with chloroquine-induced retinopathy in rheumatic diseases. Lupus. 2004;13(2):119-24. | PubMed |
Araiza-Casillas R, Cárdenas F, Morales Y, Cardiel MH. Factors associated with chloroquine-induced retinopathy in rheumatic diseases. Lupus. 2004;13(2):119-24. | PubMed | Lindquist NG, Ullberg S. The melanin affinity of chloroquine and chlorpromazine studied by whole body autoradiography. Acta Pharmacol Toxicol (Copenh). 1972;2:Suppl 2:1-32. | PubMed |
Lindquist NG, Ullberg S. The melanin affinity of chloroquine and chlorpromazine studied by whole body autoradiography. Acta Pharmacol Toxicol (Copenh). 1972;2:Suppl 2:1-32. | PubMed | Bernstein H, Zvaifler N, Rubin M, Mansour AM. The ocular deposition of chloroquine. Invest Ophthalmol. 1963 Aug;2:384-92. | PubMed |
Bernstein H, Zvaifler N, Rubin M, Mansour AM. The ocular deposition of chloroquine. Invest Ophthalmol. 1963 Aug;2:384-92. | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








