Key Words: COVID-19, severe acute respiratory syndrome coronavirus 2, coronavirus infections, systematic review, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers
Abstract
Objective
This living systematic review aims to provide a timely, rigorous, and continuously updated summary of the evidence available on the role of angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) in the treatment of patients with COVID-19.
Data sources
We conducted searches in PubMed/Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), grey literature and in a centralized repository in L·OVE (Living OVerview of Evidence), which retrieves articles from multiple sources such as PubMed/MEDLINE, Cochrane Central Register of Controlled Trials, Embase, among other pre-print and protocols repositories. In response to the COVID-19 emergency, L·OVE (Living OVerview of Evidence) was adapted to expand the range of evidence and customized to group all COVID-19 evidence in one place on a daily search basis. The search covered a period of time up to July 31, 2020.
Eligibility criteria for selecting studies and methods
We adapted an already published standard protocol for multiple parallel living systematic reviews to this question's specificities. We included randomized trials evaluating the effect of either suspension or indication of angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers as monotherapy, or in combination versus placebo or no treatment in patients with COVID-19. We searched for randomized trials evaluating the effect of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers versus placebo or no treatment in patients with COVID-19. Two reviewers independently screened each study for eligibility, extracted data, and assessed the risk of bias. We pooled the results using meta-analysis and applied the GRADE system to assess the certainty of the evidence for each outcome. We will resubmit results every time the conclusions change or whenever there are substantial updates.
Results
We screened 772 records, but none was considered for eligibility. We identified 55 ongoing studies, including 41 randomized trials evaluating angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers for patients with COVID-19.
Conclusions
We did not find a randomized clinical trial meeting our inclusion criteria, and hence there is no evidence for supporting the role of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in the treatment of patients with COVID-19. A substantial number of ongoing studies would provide valuable evidence to inform researchers and decision-makers in the near future.
PROSPERO registration number
CRD42020182495
Protocol preprint DOI
10.31219/osf.io/vp9nj
|
Main messages
|
Introduction
COVID-19 is an infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1]. It was first identified in Wuhan, China, on December 31, 2019[2]; six months later, more than 16 million contagion cases have been identified across 215 countries, and more than 650 000 people have died[3]. On March 11, 2020, the World Health Organization (WHO) characterized the COVID-19 outbreak as a pandemic[1].
While most cases result in mild symptoms, some of them progress to pneumonia, acute respiratory distress syndrome, and death[4],[5],[6]. The case fatality rate reported across countries, settings, and age groups is highly variable but ranges from about 0.5% to 10%[7]. In hospitalized patients, the case fatality rate in some centers has been reported to be higher than 10%[8].
Several studies confirm that severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)—similar to severe acute respiratory syndrome coronavirus (SARS-CoV)—uses the angiotensin‐converting enzyme 2 (ACE2) receptor for host cell entry[9],[10],[11]. So, the potential role for angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers has been the subject of much debate.
It has been theorized that patients with COVID-19 comorbid to cardiovascular diseases (such as diabetes or arterial hypertension) might present an angiotensin‐converting enzyme 2 overexpression[12]. Moreover, it has been thought that angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors drugs—generally used as anti-hypertensive therapy—could cause an up-regulation effect on angiotensin‐converting enzyme 2, leading to the possibility of severe forms of COVID-19[12]. Indeed, some observational studies show both hypertension and diabetes mellitus as independent risk factors of mortality for patients with COVID-19 admitted to the hospital[13],[14]. However, the underlying mechanisms seem to be much more complex than initially thought[15]. In fact, some authors propose that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) could down-regulate the presence of angiotensin‐converting enzyme 2 receptors in lung, kidney, and heart and trigger a harmful hyperactivation of the renin-angiotensin system[10],[16],[17] just as severe acute respiratory syndrome coronavirus (SARS-CoV) has shown[18],[19].
Several comparative observational studies have been conducted attempting to elucidate the effect of angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors in patients with COVID-19. Nevertheless, the lack of randomized trials and the growing amount of non-experimental studies with heterogeneous quality in reporting and methods have not facilitated a complete appraisal through systematic reviews. Indeed, up-to-date and good-quality systematic reviews are lacking. Thus, the therapeutic scope of angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors in patients' clinical condition with COVID-19 remains unclear.
Using innovative and agile processes, taking advantage of technological tools, and resorting to the collective effort of several research groups, this living systematic review aims to provide a timely, rigorous, and continuously updated summary of the evidence available on the role of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with COVID-19.
Methods
Protocol and registration
This manuscript complies with the ‘Preferred Reporting Items for Systematic reviews and Meta-Analyses’ (PRISMA) guidelines for reporting systematic reviews and meta-analyses[20].
A protocol stating the shared objectives and methodology of multiple evidence syntheses (systematic reviews and overviews of systematic reviews) to be conducted in parallel for different questions relevant to COVID-19 was published elsewhere[21]. This protocol was adapted to the specificities of the question assessed in this review[22] and registered in PROSPERO (CRD42020182495).
Search strategies
Our literature search was devised by the team maintaining the L·OVE (Living OVerview of Evidence) platform, using the following approach:
- Identification of terms relevant to the population and intervention components of the search strategy, using Word2vec technology[23] to the corpus of documents available in Epistemonikos Database[24].
- Discussion of terms with content and methods experts to identify relevant, irrelevant, and missing terms.
- Creation of a sensitive Boolean strategy encompassing all the relevant terms.
- Iterative analysis of articles missed by the Boolean strategy and refinement of the strategy accordingly.
We conducted searches using L·OVE (Living OVerview of Evidence) platform for COVID-19, a system that maps PICO questions to a repository, maintained through regular searches in 27 databases, preprint servers, trial registries, and websites relevant to COVID-19. All the searches covered a period until July 31, 2020. No date or language restrictions were applied.
All the platform information comes from a repository developed and maintained by Epistemonikos Foundation through the screening of different sources relevant to COVID-19[25]. At the time of releasing this article, this repository included more than 65 000 articles relevant to the Coronavirus disease, coming from the following databases, trial registries, preprint servers and websites relevant to COVID-19: Epistemonikos database, Pubmed, EMBASE, ICTRP Search Portal, Clinicaltrials.gov, ISRCTN registry, Chinese Clinical Trial Registry, IRCT - Iranian Registry of Clinical Trials, EU Clinical Trials Register: Clinical trials for covid-19, NIPH Clinical Trials Search (Japan) - Japan Primary Registries Network (JPRN) (JapicCTI, JMACCT CTR, jRCT, UMIN CTR), UMIN-CTR - UMIN Clinical Trials Registry, JRCT - Japan Registry of Clinical Trials, JAPIC Clinical Trials Information, Clinical Research Information Service (CRiS), Republic of Korea, ANZCTR - Australian New Zealand Clinical Trials Registry, ReBec - Brazilian Clinical Trials Registry, CTRI - Clinical Trials Registry - India, DRKS - German Clinical Trials Register, LBCTR - Lebanese Clinical Trials Registry, TCTR - Thai Clinical Trials Registry, NTR - The Netherlands National Trial Register, PACTR - Pan African Clinical Trial Registry, REPEC - Peruvian Clinical Trial Registry, SLCTR - Sri Lanka Clinical Trials Registry, medRxiv Preprints, bioRxiv Preprints, SSRN Preprints, WHO COVID-19 database.
The database[24] acts as a central repository. Only articles fulfilling Epistemonikos criteria are visible to users. The remaining articles are only accessible for members of COVID-19 L·OVE (Living OVerview of Evidence) Working Group.
The searches covered from the inception date of each database until the day before submission. No study design, publication status, or language restriction were applied to the searches in Epistemonikos or the additional searches.
The following strategy was used to search in Epistemonikos Database[24]. We adapted it to the syntax of other databases.
(coronavir* OR coronovirus* OR "corona virus" OR "virus corona" OR "corono virus" OR "virus corono" OR hcov* OR "covid-19" OR covid19* OR "covid 19" OR "2019-nCoV" OR cv19* OR "cv-19" OR "cv 19" OR "n-cov" OR ncov* OR "sars-cov-2" OR "sars-cov2" OR "SARS-Coronavirus-2" OR "SARS-Coronavirus2" OR (wuhan* AND (virus OR viruses OR viral)) OR (covid* AND (virus OR viruses OR viral)) OR "sars-cov" OR "sars cov" OR "sars-coronavirus" OR "severe acute respiratory syndrome" OR "mers-cov" OR "mers cov" OR "middle east respiratory syndrome" OR "middle-east respiratory syndrome" OR "covid-19-related" OR "SARS-CoV-2-related" OR "SARS-CoV2-related" OR "2019-nCoV-related" OR "cv-19-related" OR "n-cov-related") AND (((("renin-angiotensin" OR "renin angiotensin" OR (renin* AND angiotensin*) OR "renin-angiotensin-aldosterone") AND (inhibit* OR block* OR antag* OR anti)) OR RAAS OR RAAB) OR ((("angiotensin-converting" OR (angiotensin* AND converting*) OR ACE OR "angiotensin-converting-enzyme") AND (inhibit* OR block* OR antag* OR anti)) OR aceis* OR "ace-inhibitor" OR "ace-inhibitors" OR "ace-i" OR "ace-is" OR (captopril* OR Capoten*) OR (enalapril* OR Vasotec* OR Renitec* OR Enacard*) OR (lisinopril* OR Prinivil* OR Zestril*) OR (perindopril* OR Coversyl* OR Coversum* OR Aceon*) OR (ramipril* OR Altace*) OR (quinapril* OR Accupril*) OR (benazepril* OR Lotensin*) OR cilazapril* OR (fosinopril* OR Monopril*) OR (trandolapril* OR Mavik*) OR (spirapril* OR Renormax*) OR (delapril* OR alindapril*) OR (moexipril* OR Univasc) OR temocapril* OR (zofenopril* OR Zocardis*) OR (imidapril* OR Tanatril*) OR alacepril*) OR ((("angiotensin-receptor" OR (angiotensin* AND receptor*) OR "angiotensin-ii" OR "angiotensin ii" OR "angiotensin ii-receptor") AND (inhibit* OR block* OR antag* OR anti)) OR arbs* OR "angiotensin-receptor-blocker" OR "angiotensin-receptor-blockers" OR aiira* OR (losartan* OR Cozaar*) OR (eprosartan* OR Teveten*) OR (valsartan* OR Diovan*) OR (irbesartan* OR Avapro*) OR tasosartan* OR (candesartan* OR Atacand*) OR (telmisartan* OR Micardis* OR Actavis*) OR (olmesartan* OR Benicar*) OR (azilsartan* OR Edarbi* OR Azilva* OR "TAK-536"* OR "TAK 536"* OR TAK536* OR "TAK-491"* OR "TAK 491"* OR TAK491*) OR (fimasartan* OR Kanarb*) OR abitesartan* OR elisartan* OR embusartan* OR (forasartan* OR "SC-52458" OR "SC-52458" OR SC52458*) OR milfasartan* OR saprisartan* OR zolasartan*))
Eligibility criteria
Types of studies
We planned to include randomized trials. We excluded information from non-randomized studies, post-trial analyses, and studies evaluating animal models' effects or in vitro conditions.
Types of participants
We planned to include trials assessing participants with COVID-19, as defined by the authors of the trials. Whenever we find substantial clinical heterogeneity on how the condition was defined, we planned to explore it using a sensitivity analysis.
Type of interventions
The interventions of interest were angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. We did not restrict our criteria to any dosage, duration, timing, or route of administration. The comparison of interest was placebo (angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers plus optimal treatment versus placebo plus optimal treatment) or no treatment (angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers plus optimal treatment versus optimal treatment).
Trials evaluating angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in combination versus placebo (angiotensin-converting enzyme inhibitors plus angiotensin II receptor blockers plus optimal treatment versus placebo plus optimal treatment) or no treatment (angiotensin-converting enzyme inhibitors plus angiotensin II receptor blockers plus optimal treatment versus optimal treatment) were eligible. Trials assessing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers suspension were also eligible. Trials assessing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers combination plus other drugs were eligible if the co-interventions were identical in both intervention and comparison groups.
Type of outcomes
We did not use the outcomes as an inclusion criterion during the selection process. Any article meeting all the criteria, except for the outcome criterion, was preliminarily included and assessed in full text.
We used the Core Outcome Sets for COVID-19 (COS-COVID)[26], the existing guidelines and reviews, and the authors' judgment as an input for selecting the primary and secondary outcomes, as well as to decide upon inclusion. The review team revised this list of outcomes to incorporate ongoing efforts to define Core Outcomes Sets (e.g., COVID-19 Core Outcomes)[27].
Primary outcome
● All-cause mortality
Secondary outcomes
● Mechanical ventilation
● Extracorporeal membrane oxygenation
● Length of hospital stay
● Respiratory failure
● Serious adverse events
● Time to reverse transcription-polymerase chain reaction for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 RT-PCR) negativity
Other outcomes
● Acute respiratory distress syndrome
● Total adverse events
We planned to present primary and secondary outcomes into GRADE ‘Summary of Findings’ tables[28].
Selection of studies
The results of the literature search in the Epistemonikos database were automatically incorporated into the L·OVE (Living OVerview of Evidence) platform (automated retrieval), where they were de-duplicated by an algorithm comparing unique identifiers (database ID, DOI, trial registry ID), and citation details (i.e., author names, journal, year of publication, volume, number, pages, article title, and article abstract).
Two researchers independently screened the titles and abstracts yielded by the search against the inclusion criteria. We obtained the full reports for all titles that appeared to meet the inclusion criteria or require further analysis to decide their inclusion. We recorded the reasons for excluding trials in any stage of the search and outlined the study selection process in a ‘Preferred Reporting Items for Systematic reviews and Meta-Analyses’ (PRISMA) flow diagram adapted for this project.
Extraction and management of data
Two reviewers were considered independently to extract data from each included study and would use standardized forms. We planned to collect the following information: study design, setting, participant characteristics (including disease severity and age) and study eligibility criteria; details about the administered intervention and comparison, including dose and therapeutic scheme, duration, timing (i.e., time after diagnosis) and route of administration; the outcomes assessed and the time they were measured; the source of funding of the study and the conflicts of interest disclosed by the investigators; the risk of bias assessment for each study. We planned to resolve disagreements by discussion, and one arbiter adjudicated unresolved disagreements.
Risk of bias assessment
We planned to assess the risk of bias for each randomized trial using the 'risk of bias' tool (RoB 2.0: a revised tool to assess risk of bias in randomized trials)[29]. We planned to consider the effect of assignment to the intervention for this review. Two independent reviewers were considered to assess the five domains of bias for each outcome result of all reported outcomes and time points. These five domains are, bias due to (1) the randomization process, (2) deviations from intended interventions (effects of assignment to interventions at baseline), (3) missing outcome data, (4) measurement of the outcome, and (5) selection of reported results. Answers to signaling questions and collectively supporting information were considered to lead to a domain‐level judgment in the form of 'Low risk of bias,' 'Some concerns,' or 'High risk of bias.' These domain‐level judgments were considered to inform an overall 'risk of bias' judgment for each result. Discrepancies between review authors were considered to be resolved by discussion to reach consensus. If necessary, a third review author was considered for a consultation to achieve a decision.
We planned to consider the following factors as potential baseline confounders:
● Age
● Comorbidities (e.g., cardiovascular disease, renal disease, eye disease, liver disease)
● Co-interventions
● Severity, as defined by the authors (i.e., respiratory failure vs. respiratory distress syndrome vs. intensive care unit requirement)
Measures of treatment effect
For dichotomous outcomes, we planned to express the estimate of the treatment effect of an intervention as risk ratios or odds ratios along with 95% confidence intervals. We planned to use mean difference and standard deviation to summarize the data using 95% confidence intervals for continuous outcomes. Whenever continuous outcomes are measured using different scales, we planned to express the treatment effect as a standardized mean difference with 95% confidence intervals. When possible, we planned to multiply the standardized mean difference by a standard deviation from the pooled studies, as, for example, the standard deviation from a well-known scale used by several of the studies included in the analysis on which the result is based. In cases where the minimally important difference is known, we planned to present continuous outcomes as minimally important difference units or inform the results as the difference in the proportion of patients achieving a minimal important effect between intervention and control[30]. Then, we planned to display these results on the 'Summary of Findings Table' as a mean difference[30].
Strategy for data synthesis
If we included more than one trial, we planned to conduct a formal quantitative synthesis (meta-analysis) for clinically homogeneous studies using RevMan 5[31] and using the inverse variance method with the random-effects model. For any outcomes where data were insufficient to calculate an effect estimate, we planned to present a narrative synthesis, describing the studies in terms of the direction and the size of effects, and any available measure of precision.
Subgroup and sensitivity analysis
We planned to perform subgroup analysis according to the definition of severe COVID-19 infection (i.e., respiratory failure vs. respiratory distress syndrome vs. intensive care unit requirement). In case we identified significant differences between subgroups (test for interaction < 0.05), we considered reporting the results of individual subgroups separately.
We planned to perform sensitivity analysis excluding the high risk of bias studies; and, if non-randomized studies were used, excluding studies that did not report adjusted estimates. In cases where the primary analysis effect estimates and the sensitivity analysis effect estimates significantly differ, we considered presenting either the low risk of bias-adjusted sensitivity analysis estimates or the primary analysis estimates but downgrading the evidence's certainty of risk of bias.
Assessment of certainty of the evidence
We planned to judge the certainty of the evidence for all outcomes using the Grading of Recommendations Assessment, Development and Evaluation working group methodology (GRADE Working Group)[32] across the domains of risk of bias, consistency, directness, precision and reporting bias. Certainty was considered to be adjudicated as high, moderate, low or very low. For the main comparisons and outcomes, we planned to prepare Summary of Findings (SoF) tables[28],[30] as well as interactive Summary of Findings tables. A Summary of Findings table with all the comparisons and outcomes was considered to be presented as an appendix.
Living evidence synthesis
An artificial intelligence algorithm deployed in the Coronavirus/COVID-19 topic of the platform will provide instant notification of articles with a high likelihood of being eligible. The authors will review them, decide upon inclusion, and update the review's living web version accordingly. We will consider resubmission to a journal if there is a change in the direction of the effect on the critical outcomes or a substantial modification to the certainty of the evidence.
This review is part of a larger project set up to produce multiple parallel systematic reviews relevant to COVID-19[21].
Results
Results of the search
The search in the L·OVE (Living OVerview of Evidence) platform retrieved 772 records. We considered 480 as potentially eligible and retrieved and evaluated their full texts. However, none of the studies were eligible for inclusion. That being said, 88 records were observational studies and are awaiting assessment. The reasons for exclusion - List of included studies and excluded and ongoing studies - are described in the Appendices 1 and 2.
Ongoing studies
We identified 55 ongoing studies (41 randomized trials and 14 non-randomized studies). See Appendix 1 and 2 for a list of included, excluded, and ongoing studies. The study selection process is summarized in Figure 1 - PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) Flowchart.
Figure 1. PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) Flowchart.
Description of the studies
No study was considered eligible.
Discussion
After conducting a comprehensive search, we found no randomized trials evaluating the effect of angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers in patients with COVID-19. Given the growing body of evidence about this topic[33], we aimed to conduct a living systematic review of clinical trials and considering that our search included 41 ongoing randomized trials, future updates will be performed.
Systematic reviews are considered the gold standard for collecting and summarizing the available evidence regarding a clinical question. However, the traditional model for conducting reviews has several limitations, including high demand for time and resources[34], and rapid obsolescence[33].
In the wake of the COVID-19 crisis, researchers have made efforts to answer the urgent needs of health decision-makers during these months, although scientific rigor in some manner has been jeopardized[35]. Information is produced at a vertiginous speed[34]. Twenty-two systematic reviews have been produced—15 of them as preprints with no peer-review—aiming to provide synthesized and up-to-date evidence addressing the use of angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors drugs on patients with COVID-19[36],[37],[38],[39],[40],[41],[42],[43],[44],[45],[46],[47],[48],[49],[50],[51],[52],[53],[54],[55],[56],[57]. Nevertheless, these quicker alternatives risk losing efficiency, accuracy, and rigor. Thus, only three of the 22 systematic reviews have registered a protocol[40],[41],[43], half of which were published as preprints, and six reported no assessment of risk of bias of the included studies[36],[37],[48],[53],[54],[55]. Likewise, none included randomized trials, yet none used ROBINS-I tool (ROBINS-I: Risk Of Bias In Non-randomised Studies of Interventions)[58] for assessing the risk of bias in non-randomized studies, and only one of them[52] graded the certainty of evidence using the GRADE approach[32].
Our project's main limitation is the short period of time that has elapsed since the beginning of the pandemic, which does not allow the scientific community to produce enough evidence for inclusion. Despite what our review is promising for the near future, no high-quality evidence has yet been produced to inform decision making.
Identifying, appraising, and synthesizing health research requires careful attention to a rigorous methodology, considering that systematic reviews are not updated conventionally or updated intermittently, which leaves gaps between updates. The recent missing research may put them at risk of inaccuracy[59]. Our work's main strength is that living systematic reviews address the issue of obsolescence and inaccuracy[59],[60], and help prevent waste in the contribution of new research, making them more accurate. On the other hand, integrating human-machine integration allows us to search daily and gives us high confidence about including relevant new research[60].
A living systematic review and network meta-analysis about drug treatments for COVID-19 has recently been published[61]. According to its protocol, the authors expect to include all those ongoing randomized clinical trials regarding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers for patients with COVID-19 that meet their inclusion criteria[61].
This present review is part of a larger project set up to put such an approach into practice. This project aims to produce multiple parallel living systematic reviews relevant to COVID-19 following the higher quality standards in evidence synthesis production[17]. We believe that our methods are well suited to handle the abundance of evidence to come, including evidence on the role of angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers for COVID-19. We have identified multiple ongoing studies addressing this question, including 41 randomized trials, which will provide valuable evidence to inform researchers and decision-makers in the near future.
Conclusion
We found no randomized clinical trial meeting our inclusion criteria, and hence there is no evidence for supporting the role of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in the treatment of patients with COVID-19. A substantial number of ongoing studies would provide valuable evidence to inform researchers and decision-makers in the near future.
During the COVID-19 pandemic, we will maintain a living, web-based, openly available version of this review, and we will re-submit the review every time the conclusions change or whenever there are substantial updates. Our systematic review aims to provide high-quality, up-to-date synthesis of the evidence useful for clinicians and other decision-makers.
Notes
Acknowledgments
The COVID-19 L·OVE Working Group and Epistemonikos Foundation have made it possible to build the systems and compile the information needed by this project. Epistemonikos is a collaborative effort based on the ongoing volunteer work of over a thousand contributors since 2012.
Roles and contributions
GR: conceived the standard protocol for all the reviews being conducted by the COVID-19 L·OVE Working Group. NM, EM: performed complementary searches through reference list screening, the scanning reference list of grey literature and narrative reviews, and conducting cross-citation and performed full-text screening; drafted the manuscript, to which all other authors contributed. IP, CV: performed the initial title/abstract screening in Collaboratron™ and L·OVE (Living OVerview of Evidence) platform, and maintained the live screening until submission. JP-B: performed full-text screening. All authors participated in writing the discussion section and conclusions and reviewed the final version.
The corresponding author is the guarantor and declares that all authors meet authorship criteria and that no other authors meeting the criteria have been omitted.
The COVID-19 L·OVE (Living OVerview of Evidence) Working Group was created by Epistemonikos and a number of expert teams to provide decision-makers with the best evidence related to COVID-19. Up-to-date information about the group and its member organizations is available here:
Competing interests
All authors declare no financial relationships with any organization that might have a real or perceived interest in this work. There are no other relationships or activities that might have influenced the submitted work.
Funding
This project was not commissioned by any organization and did not receive external funding. Epistemonikos Foundation provides training, support, and tools at no cost for all the members of the COVID-19 L·OVE (Living OVerview of Evidence) Working Group.
PROSPERO registration number:
CRD42020182495
Protocol preprint DOI:
10.31219/osf.io/vp9nj
Ethics
As researchers will not access information that could lead to identifying an individual participant, obtaining ethical approval was waived.
Data sharing
All data related to the project will be available. Epistemonikos Foundation will grant access to data.
Appendix 1
Appendix 1.

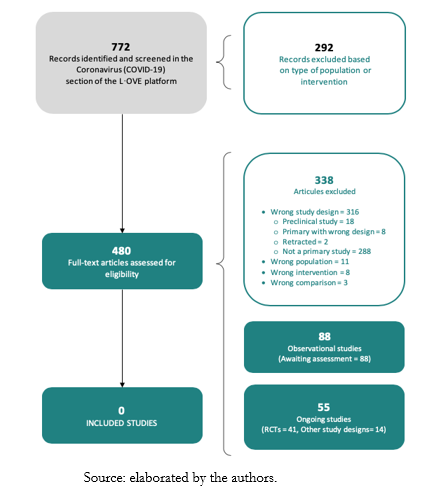 Figure 1. PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) Flowchart.
Figure 1. PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) Flowchart.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Objetivo
Esta revisión sistemática viva tiene como objetivo proporcionar un resumen oportuno, riguroso y continuamente actualizado de la evidencia disponible sobre el rol de los inhibidores de la enzima convertidora de angiotensina (iECA) y los bloqueadores del receptor de angiotensina II (ARA-II) en el tratamiento de pacientes con COVID-19.
Fuentes de datos
Realizamos búsquedas en PubMed/Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), literatura gris y en el repositorio centralizado L·OVE (Living OVerview of Evidence) que recupera artículos de múltiples fuentes como PubMed/MEDLINE, Cochrane Central Register of Controlled Trials, Embase, entre otros repositorios de preprints y protocolos. En respuesta a la emergencia de COVID-19, L·OVE (Living OVerview of Evidence) se adaptó para ampliar el rango de información que cubre y se personalizó para agrupar toda la evidencia en torno a COVID-19 en un solo lugar, en una base de búsqueda diaria. La búsqueda cubrió el período hasta el 31 de julio de 2020.
Criterios de elegibilidad para la selección de estudios y métodos
Adaptamos un protocolo común ya publicado para múltiples revisiones sistemáticas vivas paralelas a las especificidades de esta pregunta. Se incluyeron ensayos aleatorizados que evaluaban el efecto de la suspensión o la indicación de inhibidores de la enzima convertidora de angiotensina o bloqueadores de los receptores de angiotensina II, como monoterapia o en combinación, versus placebo o ningún tratamiento, en pacientes con COVID-19. Se buscaron ensayos aleatorizados que evaluaran el efecto de los inhibidores de la enzima convertidora de angiotensina/bloqueadores del receptor de angiotensina II versus placebo o ningún tratamiento en pacientes con COVID-19. Dos revisores examinaron de forma independiente la elegibilidad de cada estudio, extrajeron los datos y evaluaron el riesgo de sesgo. Los resultados se agruparon mediante un metanálisis y se aplicó GRADE para evaluar la certeza de la evidencia para cada resultado. Cada vez que cambien las conclusiones o hayan actualizaciones sustanciales, volveremos a enviar un reporte.
Resultados
Analizamos 772 artículos, pero ninguno cumplió con los criterios de inclusión. Identificamos 55 estudios en curso, incluidos 41 ensayos aleatorizados que evaluaban inhibidores de la enzima convertidora de angiotensina/bloqueadores del receptor de angiotensina II para pacientes con COVID-19.
Conclusiones
No encontramos ningún ensayo clínico aleatorizado que cumpliera con nuestros criterios de inclusión y, por lo tanto, no hay pruebas que respalden el papel de los inhibidores de la enzima convertidora de angiotensina y los bloqueadores de los receptores de angiotensina II en el tratamiento de pacientes con COVID-19. Identificamos un número considerable de estudios en curso que podría proporcionar evidencia valiosa para informar a los investigadores y a los responsables de la toma de decisiones en un futuro próximo.
 Authors:
Nicolás Meza[1], Javier Pérez-Bracchiglione[1], Ignacio Pérez[2], Cristhian Carvajal [2], Luis Ortiz-Muñoz[3], Pablo Olguín [2], Gabriel Rada[3,4,5], Eva Madrid[1], COVID-19 L·OVE Working Group
Authors:
Nicolás Meza[1], Javier Pérez-Bracchiglione[1], Ignacio Pérez[2], Cristhian Carvajal [2], Luis Ortiz-Muñoz[3], Pablo Olguín [2], Gabriel Rada[3,4,5], Eva Madrid[1], COVID-19 L·OVE Working Group
Colaboradores:
Affiliation:
[1] Interdisciplinary Centre for Health Studies (CIESAL), Universidad de Valparaíso, Cochrane Chile Associate Centre, Viña del Mar, Chile
[2] School of Medicine, Cochrane Chile Associate Centre, Universidad de Valparaíso, Viña del Mar, Chile
[3] UC Evidence Center, Cochrane Chile Associate Center, Pontificia Universidad Católica de Chile, Santiago, Chile
[4] Fundación Epistemonikos, Santiago, Chile
[5] Internal Medicine Department, Faculty of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: eva.madrid@uv.cl
Author address:
[1] Angamos 655 Reñaca, Viña del Mar, Chile

Citation: Meza N, Pérez-Bracchiglione J, Pérez I, Carvajal C, Ortiz-Muñoz L, Olguín P, et al. Angiotensin-converting-enzyme inhibitors and angiotensin II receptor blockers for COVID-19: A living systematic review of randomized clinical trials. Medwave 2021;21(02):e8105 doi: 10.5867/medwave.2021.02.8105
Submission date: 5/8/2020
Acceptance date: 11/12/2020
Publication date: 3/3/2021
Origin: Not commissioned
Type of review: Externally peer-reviewed by three reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- World Health Organization. Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. World Health Organization; 2020. [On line] | Link |
- Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health -The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020 Feb;91:264-266. | CrossRef | PubMed |
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533-534. | CrossRef | PubMed |
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed |
- Tavakoli, Ahmad, Vahdat, Katayon, Keshavarz, Mohsen. Novel Coronavirus Disease 2019 (COVID 19): An Emerging Infectious Disease in the 21st Century. BPUMS. 2020;22(6):432-450. | CrossRef |
- Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 Jun;92(6):577-583. | CrossRef | PubMed |
- Global Covid-19 Case Fatality Rates. UK: Centre for Evidence-Based Medicine. [On line] | Link |
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020 Mar-Apr;34:101623. | CrossRef | PubMed |
- Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020 Feb 17;525(1):135–40. | CrossRef | PubMed |
- Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020 Apr;46(4):586-590. | CrossRef | PubMed |
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al.SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr 16;181(2):271-280.e8. | CrossRef | PubMed |
- Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020 Apr;8(4):e21. | CrossRef | PubMed |
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. | CrossRef | PubMed |
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 Jul 1;180(7):934-943. | CrossRef | PubMed |
- Guo J, Huang Z, Lin L, Lv J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin- Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc. 2020 Apr 7;9(7):e016219. | CrossRef | PubMed |
- Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. 2020 Apr 23;382(17):1653-1659. | CrossRef | PubMed |
- de Simone G, Mancusi C. Speculation is not evidence: antihypertensive therapy and COVID-19. Eur Heart J Cardiovasc Pharmacother. 2020 Jul 1;6(3):133-134. | CrossRef | PubMed |
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005 Aug;11(8):875-9. | CrossRef | PubMed |
- Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014 Jan;66:167-76. | CrossRef | PubMed |
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006-12. | CrossRef | PubMed |
- Rada G, Verdugo-Paiva F, Ávila C, Morel Marambio M, Bravo-Jeria R, Pesce F, et al. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020 Apr 1;20(3):e7868. English, Spanish. | CrossRef | PubMed |
- Meza N, Pérez-Bracchiglione J, Pérez I, Carvajal C, Olguín P, Rada G, Madrid E. (2020, June 29). Angiotensin-converting-enzyme inhibitors and angiotensin II receptor blockers for COVID-19: A living systematic review protocol. | CrossRef |
- Github repository. 2020. [On line] | Link |
- Epistemonikos Database Methods. Santiago: Epistemonikos Foundation. 2020. [On line] | Link |
- Methods for the special L·OVE of Coronavirus infection. Santiago: Epistemonikos Foundation. 2020. [On line] | Link |
- Jin X, Pang B, Zhang J, Liu Q, Yang Z, Feng J, et al. Core Outcome Set for Clinical Trials on Coronavirus Disease 2019 (COS-COVID). Engineering (Beijing). 2020 Oct;6(10):1147-1152. | CrossRef | PubMed |
- COVID-19 Core Outcomes. 2020. [On line] | Link |
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013 Feb;66(2):158 72. | CrossRef | PubMed |
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. | CrossRef | PubMed |
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013 Feb;66(2):173-83. | CrossRef | PubMed |
- Review Manager (RevMan) [Software]. Version 5.3.5 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr 26;336(7650):924-6. | CrossRef | PubMed |
- Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007 Aug 21;147(4):224-33. | CrossRef | PubMed |
- Borah R, Brown AW, Capers PL, Kaiser KA. Analysis of the time and workers needed to conduct systematic reviews of medical interventions using data from the PROSPERO registry. BMJ Open. 2017 Feb 27;7(2):e012545. | CrossRef | PubMed |
- Coronavirus and the risks of ‘speed science’. Reuters; 2020 [Accessed Jul 31, 2020]
- Aakash Garg, Amit Rout, Abhishek Sharma, Brittany Fiorello, John B. Kostis. Association of Renin Angiotensin System Blockers with Outcomes in Patients with Covid-19: A Systematic Review and Meta-analysis. medRxiv. 2020. | CrossRef |
- Abhinav Grover, Mansi Oberoi. A systematic review to evaluate the clinical outcomes in COVID -19 patients on angiotensin converting enzyme inhibitors or angiotensin receptor blockers. medRxiv. 2020. | CrossRef |
- Anna Ssentongo, Paddy Ssentongo, Emily S. Heilbrunn, Alain Lekoubou, Ping Du, Duanping Liao, et al. Renin-angiotensin-aldosterone system inhibitors and mortality in patients with hypertension hospitalized for COVID-19: a systematic review and meta-analysis. medRxiv. 2020. | CrossRef |
- Aref A. Bin Abdulhak, Tarek Kashour, Anas Noman, Haytham Tlayjeh, Ala Mohsen, Mouaz H. Al-Mallah, et al. Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers and Outcome of COVID-19 : A Systematic Review and Meta-analysis. medRxiv. 2020. | CrossRef |
- Carlos Diaz-Arocutipa, Jose Saucedo-Chinchay, Adrian V. Hernandez. Association Between ACEIs or ARBs Use and Clinical Outcomes in COVID-19 Patients: A Systematic Review and Meta-analysis. medRxiv 2020. | CrossRef |
- Dambha-Miller H, Albasri A, Hodgson S, Wilcox C, Islam N, Khan S, et al. Drug treatments affecting ACE2 in COVID-19 infection: a systematic review protocol. BJGP Open. 2020 Aug 25;4(3):bjgpopen20X101115. | CrossRef | PubMed |
- Flacco ME, Acuti Martellucci C, Bravi F, Parruti G, Cappadona R, Mascitelli A, et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: a meta-analysis. Heart. 2020 Oct;106(19):1519-1524. | CrossRef | PubMed |
- Greco A, Buccheri S, D'Arrigo P, Calderone D, Agnello F, Monte M, et al. Outcomes of renin-angiotensin- aldosterone system blockers in patients with COVID-19: a systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2020 Sep 1;6(5):335-337. | CrossRef | PubMed |
- Grover A, Oberoi M. A systematic review and meta-analysis to evaluate the clinical outcomes in COVID-19 patients on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Eur Heart J Cardiovasc Pharmacother. 2020 Jun 15:pvaa064. | CrossRef | PubMed |
- Guangbo Qu, Liqin Shu, Evelyn J Song, Dhiran Verghese, John Patrick Uy, Ce Cheng, et al. Association between angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers use and the risk of infection and clinical outcome of COVID-19: a comprehensive systematic review and meta-analysis. medRxiv. 2020. | CrossRef |
- Guo X, Zhu Y, Hong Y. Decreased Mortality of COVID-19 With Renin-Angiotensin-Aldosterone System Inhibitors Therapy in Patients With Hypertension: A Meta-Analysis. Hypertension. 2020 Aug;76(2):e13-e14. | CrossRef | PubMed |
- Barochiner J, Martinez R. Use of inhibitors of the renin angiotensin system and COVID-19 prognosis: a systematic review and meta-analysis. medRxiv. 2020. | CrossRef |
- Nunes JPL. Mortality and use of angiotensin converting enzyme inhibitors in Covid 19 disease - a systematic review. medRxiv. 2020. | CrossRef |
- Mackey K, King VJ, Gurley S, Kiefer M, Liederbauer E, Vela K, et al. Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults: A Living Systematic Review. Ann Intern Med. 2020 Aug 4;173(3):195-203. | CrossRef | PubMed |
- Mohitosh Biswas. Effects of ACEIs and ARBs on Clinical Outcomes in COVID-19 Patients: A Meta-Analysis. SSRN. 2020. | CrossRef |
- Pirola CJ, Sookoian S. Estimation of Renin Angiotensin-Aldosterone-System (RAAS)-Inhibitor effect on COVID-19 outcome: A Meta-analysis. J Infect. 2020 Aug;81(2):276-281. | CrossRef | PubMed |
- Pranata R, Permana H, Huang I, Lim MA, Soetedjo NNM, Supriyadi R, et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Diabetes Metab Syndr. 2020 Sep-Oct;14(5):983 990. | CrossRef | PubMed |
- Baral R, White M, Vassilios S. Impact of hospitalised patients with COVID-19 taking Renin-Angiotensin-Aldosterone System inhibitors: a systematic review and meta-analysis. medRxiv. 2020. | CrossRef |
- SAMIT GHOSAL, Jagat Jyoti Mukherjee, Binayak Sinha, Kalyan Kumar Gangopadhyay. The effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on death and severity of disease in patients with coronavirus disease 2019 (COVID-19): A meta-analysis. medRxiv. 2020. | CrossRef |
- Usman MS, Siddiqi TJ, Khan MS, Ahmed A, Ali SS, Michos ED, et al. A Meta-analysis of the Relationship Between Renin-Angiotensin-Aldosterone System Inhibitors and COVID-19. Am J Cardiol. 2020 Sep 1;130:159-161. | CrossRef | PubMed |
- Yihienew Mequanint Bezabih, Alemayehu Bezabih, Endalkachew Alamneh, Gregory M. Peterson, Woldesellassie M. Bezabhe. Comparison of renin-angiotensin-aldosterone system inhibitors with other antihypertensives in association with coronavirus disease-19 clinical outcomes: systematic review and meta-analysis. medRxiv. 2020. | CrossRef |
- Zhang X, Yu J, Pan LY, Jiang HY. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: A systematic review and meta-analysis. Pharmacol Res. 2020 Aug;158:104927. | CrossRef | PubMed |
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 Oct 12;355:i4919. | CrossRef | PubMed |
- Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al. Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol. 2017 Nov;91:23-30. | CrossRef | PubMed |
- Akl EA, Haddaway NR, Rada G, Lotfi T. Future of Evidence Ecosystem Series: Evidence synthesis 2.0: when systematic, scoping, rapid, living, and overviews of reviews come together. J Clin Epidemiol. 2020 Jul;123:162-165. | CrossRef | PubMed |
- Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020 Jul 30;370:m2980. | CrossRef | PubMed |
- Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. 2019 Jul;111:105-114. | CrossRef | PubMed |
- Collaboratron [Software]. Santiago: Epistemonikos Foundation, 2017.
 World Health Organization. Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. World Health Organization; 2020. [On line] | Link |
World Health Organization. Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. World Health Organization; 2020. [On line] | Link | Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic
threat of novel coronaviruses to global health -The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020 Feb;91:264-266. | CrossRef | PubMed |
Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic
threat of novel coronaviruses to global health -The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020 Feb;91:264-266. | CrossRef | PubMed | Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533-534. | CrossRef | PubMed |
Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533-534. | CrossRef | PubMed | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed |
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed | Tavakoli, Ahmad, Vahdat, Katayon, Keshavarz, Mohsen. Novel Coronavirus Disease 2019 (COVID 19): An Emerging Infectious Disease in the 21st Century. BPUMS. 2020;22(6):432-450. | CrossRef |
Tavakoli, Ahmad, Vahdat, Katayon, Keshavarz, Mohsen. Novel Coronavirus Disease 2019 (COVID 19): An Emerging Infectious Disease in the 21st Century. BPUMS. 2020;22(6):432-450. | CrossRef | Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 Jun;92(6):577-583. | CrossRef | PubMed |
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 Jun;92(6):577-583. | CrossRef | PubMed | Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020 Mar-Apr;34:101623. | CrossRef | PubMed |
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020 Mar-Apr;34:101623. | CrossRef | PubMed | Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020 Feb 17;525(1):135–40. | CrossRef | PubMed |
Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020 Feb 17;525(1):135–40. | CrossRef | PubMed | Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020 Apr;46(4):586-590. | CrossRef | PubMed |
Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020 Apr;46(4):586-590. | CrossRef | PubMed | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al.SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr 16;181(2):271-280.e8. | CrossRef | PubMed |
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al.SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr 16;181(2):271-280.e8. | CrossRef | PubMed | Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020 Apr;8(4):e21. | CrossRef | PubMed |
Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020 Apr;8(4):e21. | CrossRef | PubMed | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a
retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. | CrossRef | PubMed |
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a
retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. | CrossRef | PubMed | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 Jul 1;180(7):934-943. | CrossRef | PubMed |
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 Jul 1;180(7):934-943. | CrossRef | PubMed | Guo J, Huang Z, Lin L, Lv J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin- Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc. 2020 Apr 7;9(7):e016219. | CrossRef | PubMed |
Guo J, Huang Z, Lin L, Lv J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin- Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc. 2020 Apr 7;9(7):e016219. | CrossRef | PubMed | Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. 2020 Apr 23;382(17):1653-1659. | CrossRef | PubMed |
Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. 2020 Apr 23;382(17):1653-1659. | CrossRef | PubMed | de Simone G, Mancusi C. Speculation is not evidence: antihypertensive therapy and COVID-19. Eur Heart J Cardiovasc Pharmacother. 2020 Jul
1;6(3):133-134. | CrossRef | PubMed |
de Simone G, Mancusi C. Speculation is not evidence: antihypertensive therapy and COVID-19. Eur Heart J Cardiovasc Pharmacother. 2020 Jul
1;6(3):133-134. | CrossRef | PubMed | Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury.
Nat Med. 2005 Aug;11(8):875-9. | CrossRef | PubMed |
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury.
Nat Med. 2005 Aug;11(8):875-9. | CrossRef | PubMed | Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014 Jan;66:167-76. | CrossRef | PubMed |
Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014 Jan;66:167-76. | CrossRef | PubMed | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006-12. | CrossRef | PubMed |
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006-12. | CrossRef | PubMed | Rada G, Verdugo-Paiva F, Ávila C, Morel Marambio M, Bravo-Jeria R, Pesce F, et al. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020 Apr 1;20(3):e7868. English, Spanish. | CrossRef | PubMed |
Rada G, Verdugo-Paiva F, Ávila C, Morel Marambio M, Bravo-Jeria R, Pesce F, et al. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020 Apr 1;20(3):e7868. English, Spanish. | CrossRef | PubMed | Meza N, Pérez-Bracchiglione J, Pérez I, Carvajal C, Olguín P, Rada G, Madrid E. (2020, June 29). Angiotensin-converting-enzyme inhibitors and angiotensin II receptor blockers for COVID-19: A living systematic review protocol. | CrossRef |
Meza N, Pérez-Bracchiglione J, Pérez I, Carvajal C, Olguín P, Rada G, Madrid E. (2020, June 29). Angiotensin-converting-enzyme inhibitors and angiotensin II receptor blockers for COVID-19: A living systematic review protocol. | CrossRef | Methods for the special L·OVE of Coronavirus infection. Santiago: Epistemonikos Foundation. 2020. [On line] | Link |
Methods for the special L·OVE of Coronavirus infection. Santiago: Epistemonikos Foundation. 2020. [On line] | Link | Jin X, Pang B, Zhang J, Liu Q, Yang Z, Feng J, et al. Core Outcome Set for Clinical Trials on Coronavirus Disease 2019 (COS-COVID). Engineering (Beijing). 2020 Oct;6(10):1147-1152. | CrossRef | PubMed |
Jin X, Pang B, Zhang J, Liu Q, Yang Z, Feng J, et al. Core Outcome Set for Clinical Trials on Coronavirus Disease 2019 (COS-COVID). Engineering (Beijing). 2020 Oct;6(10):1147-1152. | CrossRef | PubMed | Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013 Feb;66(2):158 72. | CrossRef | PubMed |
Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013 Feb;66(2):158 72. | CrossRef | PubMed | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. | CrossRef | PubMed |
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. | CrossRef | PubMed | Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines:
13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013 Feb;66(2):173-83. | CrossRef | PubMed |
Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines:
13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013 Feb;66(2):173-83. | CrossRef | PubMed | Review Manager (RevMan) [Software]. Version 5.3.5 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Review Manager (RevMan) [Software]. Version 5.3.5 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.  Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr
26;336(7650):924-6. | CrossRef | PubMed |
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr
26;336(7650):924-6. | CrossRef | PubMed | Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007 Aug 21;147(4):224-33. | CrossRef | PubMed |
Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007 Aug 21;147(4):224-33. | CrossRef | PubMed | Borah R, Brown AW, Capers PL, Kaiser KA. Analysis of the time and workers needed to conduct systematic reviews of medical interventions using data from the PROSPERO registry. BMJ Open. 2017 Feb 27;7(2):e012545. | CrossRef | PubMed |
Borah R, Brown AW, Capers PL, Kaiser KA. Analysis of the time and workers needed to conduct systematic reviews of medical interventions using data from the PROSPERO registry. BMJ Open. 2017 Feb 27;7(2):e012545. | CrossRef | PubMed | Coronavirus and the risks of ‘speed science’. Reuters; 2020 [Accessed Jul 31, 2020]
Coronavirus and the risks of ‘speed science’. Reuters; 2020 [Accessed Jul 31, 2020]  Aakash Garg, Amit Rout, Abhishek Sharma, Brittany Fiorello, John B. Kostis. Association of Renin Angiotensin System Blockers with Outcomes in Patients with Covid-19: A Systematic Review and Meta-analysis. medRxiv. 2020. | CrossRef |
Aakash Garg, Amit Rout, Abhishek Sharma, Brittany Fiorello, John B. Kostis. Association of Renin Angiotensin System Blockers with Outcomes in Patients with Covid-19: A Systematic Review and Meta-analysis. medRxiv. 2020. | CrossRef | Abhinav Grover, Mansi Oberoi. A systematic review to evaluate the clinical outcomes in COVID -19 patients on angiotensin converting enzyme inhibitors or angiotensin receptor blockers. medRxiv. 2020. | CrossRef |
Abhinav Grover, Mansi Oberoi. A systematic review to evaluate the clinical outcomes in COVID -19 patients on angiotensin converting enzyme inhibitors or angiotensin receptor blockers. medRxiv. 2020. | CrossRef | Anna Ssentongo, Paddy Ssentongo, Emily S. Heilbrunn, Alain Lekoubou, Ping Du, Duanping Liao, et al. Renin-angiotensin-aldosterone system inhibitors and mortality in patients with hypertension hospitalized for COVID-19: a systematic review and meta-analysis. medRxiv. 2020. | CrossRef |
Anna Ssentongo, Paddy Ssentongo, Emily S. Heilbrunn, Alain Lekoubou, Ping Du, Duanping Liao, et al. Renin-angiotensin-aldosterone system inhibitors and mortality in patients with hypertension hospitalized for COVID-19: a systematic review and meta-analysis. medRxiv. 2020. | CrossRef | Aref A. Bin Abdulhak, Tarek Kashour, Anas Noman, Haytham Tlayjeh, Ala Mohsen, Mouaz H. Al-Mallah, et al. Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers and Outcome of COVID-19 : A Systematic Review and Meta-analysis. medRxiv. 2020. | CrossRef |
Aref A. Bin Abdulhak, Tarek Kashour, Anas Noman, Haytham Tlayjeh, Ala Mohsen, Mouaz H. Al-Mallah, et al. Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers and Outcome of COVID-19 : A Systematic Review and Meta-analysis. medRxiv. 2020. | CrossRef | Carlos Diaz-Arocutipa, Jose Saucedo-Chinchay, Adrian V. Hernandez. Association Between ACEIs or ARBs Use and Clinical Outcomes in COVID-19 Patients: A Systematic Review and Meta-analysis. medRxiv 2020. | CrossRef |
Carlos Diaz-Arocutipa, Jose Saucedo-Chinchay, Adrian V. Hernandez. Association Between ACEIs or ARBs Use and Clinical Outcomes in COVID-19 Patients: A Systematic Review and Meta-analysis. medRxiv 2020. | CrossRef | Dambha-Miller H, Albasri A, Hodgson S, Wilcox C, Islam N, Khan S, et al. Drug treatments affecting ACE2 in COVID-19 infection: a systematic review protocol. BJGP Open. 2020 Aug 25;4(3):bjgpopen20X101115. | CrossRef | PubMed |
Dambha-Miller H, Albasri A, Hodgson S, Wilcox C, Islam N, Khan S, et al. Drug treatments affecting ACE2 in COVID-19 infection: a systematic review protocol. BJGP Open. 2020 Aug 25;4(3):bjgpopen20X101115. | CrossRef | PubMed | Flacco ME, Acuti Martellucci C, Bravi F, Parruti G, Cappadona R, Mascitelli A, et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: a meta-analysis. Heart. 2020 Oct;106(19):1519-1524. | CrossRef | PubMed |
Flacco ME, Acuti Martellucci C, Bravi F, Parruti G, Cappadona R, Mascitelli A, et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: a meta-analysis. Heart. 2020 Oct;106(19):1519-1524. | CrossRef | PubMed | Greco A, Buccheri S, D'Arrigo P, Calderone D, Agnello F, Monte M, et al. Outcomes of renin-angiotensin- aldosterone system blockers in patients with COVID-19: a systematic review and
meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2020 Sep 1;6(5):335-337. | CrossRef | PubMed |
Greco A, Buccheri S, D'Arrigo P, Calderone D, Agnello F, Monte M, et al. Outcomes of renin-angiotensin- aldosterone system blockers in patients with COVID-19: a systematic review and
meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2020 Sep 1;6(5):335-337. | CrossRef | PubMed | Grover A, Oberoi M. A systematic review and meta-analysis to evaluate the clinical outcomes in COVID-19 patients on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Eur Heart J Cardiovasc Pharmacother. 2020 Jun 15:pvaa064. | CrossRef | PubMed |
Grover A, Oberoi M. A systematic review and meta-analysis to evaluate the clinical outcomes in COVID-19 patients on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Eur Heart J Cardiovasc Pharmacother. 2020 Jun 15:pvaa064. | CrossRef | PubMed | Guangbo Qu, Liqin Shu, Evelyn J Song, Dhiran Verghese, John Patrick Uy, Ce Cheng, et al. Association between angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers use and the risk of infection and clinical outcome of COVID-19: a comprehensive systematic review and meta-analysis. medRxiv. 2020. | CrossRef |
Guangbo Qu, Liqin Shu, Evelyn J Song, Dhiran Verghese, John Patrick Uy, Ce Cheng, et al. Association between angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers use and the risk of infection and clinical outcome of COVID-19: a comprehensive systematic review and meta-analysis. medRxiv. 2020. | CrossRef | Guo X, Zhu Y, Hong Y. Decreased Mortality of COVID-19 With Renin-Angiotensin-Aldosterone System Inhibitors Therapy in Patients With Hypertension: A Meta-Analysis. Hypertension. 2020 Aug;76(2):e13-e14. | CrossRef | PubMed |
Guo X, Zhu Y, Hong Y. Decreased Mortality of COVID-19 With Renin-Angiotensin-Aldosterone System Inhibitors Therapy in Patients With Hypertension: A Meta-Analysis. Hypertension. 2020 Aug;76(2):e13-e14. | CrossRef | PubMed | Barochiner J, Martinez R. Use of inhibitors of the renin angiotensin system and COVID-19 prognosis: a systematic review and meta-analysis. medRxiv. 2020. | CrossRef |
Barochiner J, Martinez R. Use of inhibitors of the renin angiotensin system and COVID-19 prognosis: a systematic review and meta-analysis. medRxiv. 2020. | CrossRef | Nunes JPL. Mortality and use of angiotensin converting enzyme inhibitors in Covid 19 disease - a systematic review. medRxiv. 2020. | CrossRef |
Nunes JPL. Mortality and use of angiotensin converting enzyme inhibitors in Covid 19 disease - a systematic review. medRxiv. 2020. | CrossRef | Mackey K, King VJ, Gurley S, Kiefer M, Liederbauer E, Vela K, et al. Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or
Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults: A Living Systematic Review. Ann Intern Med. 2020 Aug 4;173(3):195-203. | CrossRef | PubMed |
Mackey K, King VJ, Gurley S, Kiefer M, Liederbauer E, Vela K, et al. Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or
Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults: A Living Systematic Review. Ann Intern Med. 2020 Aug 4;173(3):195-203. | CrossRef | PubMed | Mohitosh Biswas. Effects of ACEIs and ARBs on Clinical Outcomes in COVID-19 Patients: A Meta-Analysis. SSRN. 2020. | CrossRef |
Mohitosh Biswas. Effects of ACEIs and ARBs on Clinical Outcomes in COVID-19 Patients: A Meta-Analysis. SSRN. 2020. | CrossRef | Pirola CJ, Sookoian S. Estimation of Renin Angiotensin-Aldosterone-System (RAAS)-Inhibitor effect on COVID-19 outcome: A Meta-analysis. J Infect. 2020 Aug;81(2):276-281. | CrossRef | PubMed |
Pirola CJ, Sookoian S. Estimation of Renin Angiotensin-Aldosterone-System (RAAS)-Inhibitor effect on COVID-19 outcome: A Meta-analysis. J Infect. 2020 Aug;81(2):276-281. | CrossRef | PubMed | Pranata R, Permana H, Huang I, Lim MA, Soetedjo NNM, Supriyadi R, et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Diabetes Metab Syndr. 2020 Sep-Oct;14(5):983 990. | CrossRef | PubMed |
Pranata R, Permana H, Huang I, Lim MA, Soetedjo NNM, Supriyadi R, et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Diabetes Metab Syndr. 2020 Sep-Oct;14(5):983 990. | CrossRef | PubMed | Baral R, White M, Vassilios S. Impact of hospitalised patients with COVID-19 taking Renin-Angiotensin-Aldosterone System inhibitors: a systematic review and meta-analysis. medRxiv. 2020. | CrossRef |
Baral R, White M, Vassilios S. Impact of hospitalised patients with COVID-19 taking Renin-Angiotensin-Aldosterone System inhibitors: a systematic review and meta-analysis. medRxiv. 2020. | CrossRef | SAMIT GHOSAL, Jagat Jyoti Mukherjee, Binayak Sinha, Kalyan Kumar Gangopadhyay. The effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on death and severity of disease in patients with coronavirus disease 2019 (COVID-19): A meta-analysis. medRxiv. 2020. | CrossRef |
SAMIT GHOSAL, Jagat Jyoti Mukherjee, Binayak Sinha, Kalyan Kumar Gangopadhyay. The effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on death and severity of disease in patients with coronavirus disease 2019 (COVID-19): A meta-analysis. medRxiv. 2020. | CrossRef | Usman MS, Siddiqi TJ, Khan MS, Ahmed A, Ali SS, Michos ED, et al. A Meta-analysis of the Relationship Between Renin-Angiotensin-Aldosterone System Inhibitors and COVID-19. Am J Cardiol. 2020 Sep 1;130:159-161. | CrossRef | PubMed |
Usman MS, Siddiqi TJ, Khan MS, Ahmed A, Ali SS, Michos ED, et al. A Meta-analysis of the Relationship Between Renin-Angiotensin-Aldosterone System Inhibitors and COVID-19. Am J Cardiol. 2020 Sep 1;130:159-161. | CrossRef | PubMed | Yihienew Mequanint Bezabih, Alemayehu Bezabih, Endalkachew Alamneh, Gregory M. Peterson, Woldesellassie M. Bezabhe. Comparison of renin-angiotensin-aldosterone system inhibitors with other antihypertensives in association with coronavirus disease-19 clinical outcomes: systematic review and meta-analysis. medRxiv. 2020. | CrossRef |
Yihienew Mequanint Bezabih, Alemayehu Bezabih, Endalkachew Alamneh, Gregory M. Peterson, Woldesellassie M. Bezabhe. Comparison of renin-angiotensin-aldosterone system inhibitors with other antihypertensives in association with coronavirus disease-19 clinical outcomes: systematic review and meta-analysis. medRxiv. 2020. | CrossRef | Zhang X, Yu J, Pan LY, Jiang HY. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: A systematic review and meta-analysis. Pharmacol Res. 2020 Aug;158:104927. | CrossRef | PubMed |
Zhang X, Yu J, Pan LY, Jiang HY. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: A systematic review and meta-analysis. Pharmacol Res. 2020 Aug;158:104927. | CrossRef | PubMed | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 Oct 12;355:i4919. | CrossRef | PubMed |
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 Oct 12;355:i4919. | CrossRef | PubMed | Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al. Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol. 2017 Nov;91:23-30. | CrossRef | PubMed |
Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al. Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol. 2017 Nov;91:23-30. | CrossRef | PubMed | Akl EA, Haddaway NR, Rada G, Lotfi T. Future of Evidence Ecosystem Series: Evidence synthesis 2.0: when systematic, scoping, rapid, living, and overviews of reviews come together. J Clin Epidemiol. 2020 Jul;123:162-165. | CrossRef | PubMed |
Akl EA, Haddaway NR, Rada G, Lotfi T. Future of Evidence Ecosystem Series: Evidence synthesis 2.0: when systematic, scoping, rapid, living, and overviews of reviews come together. J Clin Epidemiol. 2020 Jul;123:162-165. | CrossRef | PubMed | Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020 Jul 30;370:m2980. | CrossRef | PubMed |
Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020 Jul 30;370:m2980. | CrossRef | PubMed | Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk
of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. 2019 Jul;111:105-114. | CrossRef | PubMed |
Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk
of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. 2019 Jul;111:105-114. | CrossRef | PubMed | Collaboratron [Software]. Santiago: Epistemonikos Foundation, 2017.
Collaboratron [Software]. Santiago: Epistemonikos Foundation, 2017. Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








