Key Words: COVID-19, severe acute respiratory syndrome coronavirus 2, coronavirus infections, systematic review, cell-based therapies, mesenchymal stem cells, coronavirus disease
Abstract
Objective
This living, systematic review aims to provide a timely, rigorous, and continuously updated summary of the available evidence on the role of cell-based therapies in the treatment of patients with COVID-19.
Data sources
We conducted searches in PubMed/Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), grey literature, and in a centralized repository in L·OVE (Living OVerview of Evidence). L·OVE is a platform that maps PICO questions to evidence from the Epistemonikos database. In response to the COVID-19 emergency, L·OVE was adapted to expand the range of evidence it covers and customized to group all COVID-19 evidence in one place. All the searches covered the period until 23 April 2020 (one day before submission).
Eligibility criteria for selecting studies and methods
We adapted an already published standard protocol for multiple parallel systematic reviews to the specificities of this question. We searched for randomized trials evaluating the effectiveness and safety of cell-based therapies versus placebo or no treatment in patients with COVID-19. Anticipating the lack of randomized trials directly addressing this question, we also searched for trials evaluating other coronavirus infections, such as MERS-CoV and SARS-CoV, and nonrandomized studies in COVID-19. Two reviewers independently screened each study for eligibility. A living, web-based version of this review will be openly available during the COVID-19 pandemic. We will resubmit this review to a peer-reviewed journal every time the conclusions change or whenever there are substantial updates.
Results
We screened 1 043 records, but no study was considered eligible. We identified 61 ongoing studies, including 39 randomized trials evaluating different types of cell-based therapies in COVID-19.
Conclusions
We did not find any studies that met our inclusion criteria, and hence there is no evidence to support or refute the use of cell-based therapies for treating patients with COVID-19. A substantial number of ongoing studies should provide valuable evidence to inform researchers and decision-makers in the near future.
PROSPERO Registration number
CRD42020179711
|
Box 1 - Linked resources in this Living Systematic Review Common protocol The standard protocol for the systematic reviews and overviews of systematic reviews conducted by the COVID-19 L·OVE Working Group. Available here Living review The web version of this systematic review, presented in a 'living systematic review format.' This means it is continuously updated as soon as new evidence emerges. Available here Living OVerview of Evidence - L·OVE An open platform that uses artificial intelligence and a broad network of contributors to identify all of the evidence relevant to this and other healthcare questions, including those related to COVID-19. |
Introduction
COVID-19 is an infection caused by the SARS-CoV-2 coronavirus[1]. It was first identified in Wuhan, China, on 31 December 2019[2]. By 23 April 2020, the number of confirmed COVID-19 cases had reached 2 761 121, with 193 671 confirmed deaths[3]. On 11 March 2020, the WHO characterized the COVID-19 outbreak as a pandemic[1].
While the majority of cases result in mild symptoms, some might progress to pneumonia, acute respiratory distress syndrome, and death[4],[5],[6]. The case fatality rate reported across countries, settings, and age groups is highly variable, but it ranges from about 0.5% to 10%[7]. In some studies it has been reported to be higher than 10% in hospitalised patients[8].
Cell-based therapy is a treatment in which viable nucleated cells are injected, grafted, or implanted into a patient to obtain a medicinal effect. It includes different kinds of therapies with different possible effects. For example, stem-cells can be used for regenerative purposes and effector cells, like T-Cell/NK lymphocytes, for targeted immune activity and mesenchymal stem cells as an anti-inflammatory treatment. Of the previously mentioned treatments, Adoptive T Cell Therapy Strategy has been successfully used against cytomegalovirus infection in the transplant setting. However, this treatment presents several difficulties because it requires T-cells with specific activity against the virus from a patient's relative, which means having a recovered patient, and this process usually takes longer than a week. On the other hand, mesenchymal stem cells seem to be more promising because cell processing is speedy and does not require a specific donor.
Mesenchymal stem cells exhibit a capacity for homing to injury sites and inflammation areas where they exert anti-inflammatory and immunomodulatory effects[9]. They can affect the status of T cells and skew them towards a regulatory phenotype, and they also interact with B cells, inhibiting B cell response[10]. Moreover, these cells are the primary type of cell-based therapy proposed in the context of COVID-19. However, in animal experimental models of severe influenza, mesenchymal stem cells, in a prophylactic or therapeutic regimen, failed to decrease pulmonary inflammation and do not improve survival[11]. Their efficacy for SARS-COVS2 infection is unknown.
Preclinical studies in COVID-19 patients suggest that mesenchymal stromal cells would be able to reduce inflammation[12]. Findings from an observational study support this hypothesis in that they express anti-inflammatory and trophic factors and decrease C-reactive protein, tumor necrosis factor-alpha, and cytokine-secreting immune cells[13].
Consequently, it is expected that these cells could attenuate the overactivation of the immune system and support repair by modulating the lung microenvironment after SARS-CoV-2 infection. Some small studies in humans and newspaper reports have sparked global interest in this treatment, but high-quality evidence is still lacking[14].
Using innovative and agile processes, taking advantage of technological tools, and resorting to the collective effort of several research groups, this living, systematic review aims to provide timely,rigorous, and continuously updated summary of the available evidence on the role of cell-based therapies in treating patients with COVID-19.
Methods
This manuscript complies with the 'Preferred Reporting Items for Systematic reviews and Meta-Analyses' (PRISMA) guidelines for reporting systematic reviews and meta-analyses[15] (see Appendix 1 - PRISMA Checklist).
A protocol stating the shared objectives and methodology of multiple evidence syntheses (systematic reviews and overviews of systematic reviews) to be conducted in parallel for different questions relevant to COVID-19 was published elsewhere[16]. The review was registered in PROSPERO with the number CRD42020179711, and a detailed protocol was uploaded to a preprint server[17].
Search strategies
Electronic searches
Our literature search was devised by the team maintaining the L·OVE platform, using the following approach:
- Identification of terms relevant to the population and intervention components of the search strategy, using Word2vec technology[18] to the corpus of documents available in the Epistemonikos Database.
- Discussion of terms with content and methods experts to identify relevant, irrelevant, and missing terms.
- Creation of a sensitive boolean strategy encompassing all the relevant terms
- Iterative analysis of articles missed by the boolean strategy, and refinement of the strategy accordingly.
Our main search source was the Epistemonikos database, a comprehensive database of systematic reviews and other types of evidence[19] that we have supplemented with information coming from 35 sources relevant to COVID-19. The list of sources that have been added to the Epistemonikos Database is continuously expanded. This list of sources regularly screened by Epistemonikos for COVID-19 is updated regularly in our website[20].
We conducted additional searches using highly sensitive searches in PubMed/MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL) and Embase.
The searches in Epistemonikos are continuously updated[20] but were last checked the day of submission of this article (24 April 2020). The additional searches were updated on 23 April 2020 and covered the period from each database's inception date. No study design, publication status, or language restriction were applied to the searches in Epistemonikos or the additional electronic searches.
The following strategy was used to search in the Epistemonikos Database. We adapted it to the syntax of other databases (see Appendix 2 - Search strategies).
((coronavir* OR coronovirus* OR "corona virus" OR "virus corona" OR "corono virus" OR "virus corono" OR hcov* OR "covid-19" OR covid19* OR "covid 19" OR "2019-nCoV" OR cv19* OR "cv-19" OR "cv 19" OR "n-cov" OR ncov* OR "sars-cov-2" OR "sars-cov2" OR "SARS-Coronavirus-2" OR "SARS-Coronavirus2" OR (wuhan* AND (virus OR viruses OR viral)) OR (covid* AND (virus OR viruses OR viral)) OR "sars-cov" OR "sars cov" OR "sars-coronavirus" OR "severe acute respiratory syndrome" OR "mers-cov" OR "mers cov" OR "middle east respiratory syndrome" OR "middle-east respiratory syndrome" OR "covid-19-related" OR "SARS-CoV-2-related" OR "SARS-CoV2-related" OR "2019-nCoV-related" OR "cv-19-related" OR "n-cov-related")) AND ("cell therapy" OR "cell therapies" OR "cell-therapy" OR "cell-therapies" OR "mesenchymal cell" OR "mesenchymal cells" OR MSC OR MSCs OR HMSC* OR stemstromal* OR stromalstem* OR nestcell* OR ((mesenchymal* OR "tissue-derived" OR "derived-mesenchymal") AND (stromal* OR stem OR multipotent* OR progenitor*)) OR (medicinal* AND signalling* AND (cell OR cells)) OR (stromal* AND (stem OR multipotent*)) OR ("tissue-derived" AND mesenchymal*))
Other sources
In order to identify articles that might have been missed in the electronic searches, we proceed, if necessary, as follows:
- We screened the reference lists of other systematic reviews.
- We scanned the reference lists of selected guidelines, narrative reviews, and other documents.
- We reviewed websites specializing in COVID-19.
- We emailed the contact authors of all the included studies to ask for additional publications or data on their studies and other studies on the topic.
- We conducted cross-citation searches in Google Scholar and Microsoft Academic, using each included study as the index reference.
- We reviewed the reference list of each included study.
Eligibility criteria
Types of studies
This living review preferentially includes randomized trials. Nonrandomized studies are included if there is no direct evidence from randomized trials, or the certainty of the evidence for the critical outcomes resulting from the randomized trials is graded as low- or very low, and the certainty provided by the nonrandomized evidence grades higher than the one provided by the randomized evidence[21]. We exclude studies evaluating the effects on animal models or in vitro conditions.
Types of participants
We include trials assessing participants with COVID-19, as defined by the authors of the trials.
Whenever we find substantial clinical heterogeneity on how the condition was defined, we explore it using a sensitivity analysis. If we do not find direct evidence from randomized trials, or if the evidence from randomized trials provides low- or very low-certainty evidence for critical outcomes, we consider eligible randomized trials evaluating cell-based therapy in other coronavirus infections, such as MERS-CoV or SARS-CoV infections[21].
Type of interventions
The interventions of interest are cell-based therapies obtained from any tissue, including mesenchymal stromal cells, hematopoietic stem cells, and any other therapy in which viable nucleated cells are injected, grafted, or implanted into a patient to obtain a medicinal effect. We do not restrict our criteria to any dosage, duration, timing, or route of administration.
The comparison of interest is placebo/sham (cell-based therapy plus optimal treatment versus placebo plus optimal treatment) or no treatment (cell-based therapy plus optimal treatment versus optimal treatment). Trials assessing cell-based therapy plus other interventions are eligible if the cointerventions are identical in both the intervention and the comparison groups. Trials evaluating cell-based therapy in combination with other active interventions versus placebo or no treatment are also eligible.
Type of outcomes
We do not use the outcomes as inclusion criteria during the selection process. Any article meeting all the criteria except for the outcome criterion is preliminarily included and assessed in full text. We used the core outcome set COS-COVID[22], the existing guidelines and reviews, and the judgment of the authors of this review as an input for selecting the primary and secondary outcomes, as well as to decide upon inclusion. The review team regularly revises this list of outcomes to incorporate ongoing efforts to define Core Outcomes Sets (e.g., COVID-19 Core Outcomes)[23].
Primary outcome
- All-cause mortality
Secondary outcomes
- Mechanical ventilation
- Extracorporeal membrane oxygenation
- Length of hospital stay
- Respiratory failure
- Serious adverse events
- Time to SARS-CoV-2 RT-PCR negativity
Other outcomes
- Acute respiratory distress syndrome
- Total adverse events
If we include at least one study, primary and secondary outcomes will be presented in the GRADE 'Summary of Findings' tables, and a table with all the outcomes is presented as an appendix[24].
Selection of studies
The literature search results in the Epistemonikos database are automatically incorporated into the L·OVE platform (automated retrieval). There, they are de-duplicated by an algorithm that compares unique identifiers (database ID, DOI, trial registry ID), and citation details (i.e., author names, journal, year of publication, volume, number, pages, article title, and article abstract). The additional searches are uploaded to the screening software Collaboratron™[25].
In both L·OVE platform and Collaboratron™, two researchers independently screen the titles and abstracts yielded by the search against the inclusion criteria. We obtain the full reports for all titles that appear to meet the inclusion criteria or require further analysis and then decide their inclusion. We record the reasons for excluding trials in any stage of the search and outline the study selection process in a PRISMA flow diagram that we adapted for this project's purpose.
Extraction and management of data
Using standardized forms, two reviewers independently extracted data from each included and ongoing study. We collect the following information: study design, setting, participant characteristics (including disease severity and age) and study eligibility criteria; details about the administered intervention and comparison, including the source of cells, dose, duration, and timing (i.e., time after diagnosis); the outcomes assessed and the time they were measured; the source of funding of the study and the conflicts of interest disclosed by the investigators; the risk of bias assessment for each individual study. We resolve disagreements by discussion, and one arbiter adjudicates unresolved disagreements.
Risk of bias assessment
The risk of bias for each randomized trial is assessed using the 'risk of bias' tool (RoB 2.0: a revised tool to assess risk of bias in randomized trials)[26]. We consider the effect of assignment to the intervention for this review. Two reviewers independently assess five domains of bias for each outcome result of all reported outcomes and time points. These five domains are bias due to (1) the randomization process, (2) deviations from intended interventions (effects of assignment to interventions at baseline), (3) missing outcome data, (4) measurement of the outcome, and (5) selection of reported results. Answers to signaling questions and collectively supporting information lead to a domain‐level judgment in the form of 'Low risk of bias', 'Some concerns', or 'High risk of bias'. These domain‐level judgments inform an overall 'risk of bias' judgment for each result. Discrepancies between review authors are resolved by discussion to reach consensus. If necessary, a third review author is consulted to achieve a decision.
We assess the risk of bias of other study designs with the ROBINS‐I tool (ROBINS-I: Risk Of Bias In Non-randomised Studies of Interventions)[27]. We address the following domains: bias due to confounding, bias in the selection of participants into the study, bias in classification of interventions, bias due to deviations from intended interventions (effect of assignment to intervention), bias due to missing data, bias in the measurement of outcomes, and bias in the selection of the reported result. We judge each domain as low risk, moderate risk, serious risk, critical risk, or no information, and evaluate individual bias items described in ROBINS-I guidance. We do not consider time‐varying confounding, as these confounders are not relevant in this setting[28].
We consider the following factors as potential baseline confounders:
- Age
- Comorbidities (e.g., cardiovascular disease, renal disease, eye disease, liver disease)
- Co-interventions
- Severity, as defined by the authors (i.e., respiratory failure vs. respiratory distress syndrome vs. ICU requirement).
Measures of treatment effect
We express the estimate of the treatment effect of an intervention as risk ratios (RR) or odds ratios (OR) along with 95% confidence intervals for dichotomous outcomes. We use the mean difference and standard deviation for continuous outcomes to summarize the data using a 95% confidence interval. Whenever continuous outcomes are measured using different scales, the treatment effect is expressed as a standardized mean difference with a 95% confidence interval. When possible, we multiply the standardized mean difference by a standard deviation that is representative from the pooled studies, for example, the standard deviation from a well-known scale used by several of the studies included in the analysis on which the result is based. In cases where the minimally important difference is known, we present continuous outcomes as minimally important difference units or inform the results as the difference in the proportion of patients achieving a minimal important effect between intervention and control[28].
These results are then displayed on the 'Summary of Findings Table' as the mean difference[28].
Strategy for data synthesis
If we include more than one trial, we conduct a meta-analysis for clinically homogeneous studies using RevMan 5[29], using the inverse variance method with the random-effects model. For any outcomes where data are insufficient to calculate an effect estimate, a narrative synthesis is presented.
Subgroup and sensitivity analysis
We perform subgroup analysis according to the definition of severe COVID-19 infection (i.e., respiratory failure vs. respiratory distress syndrome vs. ICU requirement). If we identify significant differences between subgroups (test for interaction < 0.05), we report the results of individual subgroups separately.
We perform sensitivity analysis excluding the high risk of bias studies, and if nonrandomized studies are used, excluding studies that do not report adjusted estimates. In cases where the primary analysis effect estimates and the sensitivity analysis effect estimates significantly differ, we present the low risk of bias-adjusted sensitivity analysis estimates or the primary analysis estimates but downgrading the certainty of the evidence because of the risk of bias.
Assessment of certainty of the evidence
The certainty of the evidence for all outcomes is judged using the Grading of Recommendations Assessment, Development and Evaluation working group methodology (GRADE Working Group)[30], across the domains of risk of bias, consistency, directness, precision, and reporting bias. Certainty is adjudicated as high, moderate, low, or very low. For the main comparisons and outcomes, we prepare Summary of Findings tables[28],[24], and also interactive Summary of Findings tables. A Summary of Findings table with all the comparisons and outcomes is presented as an appendix.
Living evidence synthesis
An artificial intelligence algorithm deployed in the Coronavirus/COVID-19 topic of the L·OVE platform provides instant notification of articles with a high likelihood of being eligible. The authors review them, decide upon inclusion, and update the living web version of the review accordingly. We expect to resubmit to a peer-reviewed journal any time there is a change in the direction of the effect on the critical outcomes or a substantial modification to the evidence's certainty. This review is part of a larger project set up to produce multiple parallel systematic reviews relevant to COVID-19[16].
Results
Results of the search
We used a repository that includes searches in 34 trial registries, preprint servers, and websites specialized in COVID-19. We also conducted additional searches in three electronic databases and scanned the references of multiple guidelines, reviews, and other documents. The L·OVE platform search retrieved 272 records, and the additional searches retrieved a further 771 records (total records screened = 1 043). We considered 78 as potentially eligible and retrieved and evaluated their full texts. However, none of the studies were eligible for inclusion. The reasons for exclusion are described in Appendix 3 - List of relevant studies.
The study selection process is summarized in Figure 1 - PRISMA Flowchart.
Description of the studies
No completed study was considered eligible.
Ongoing studies
We identified 61 ongoing studies (39 randomized trials and 22 nonrandomized studies).
See Appendix 3 - List of relevant studies.
Discussion
We performed a comprehensive search of the literature and did not find any randomized trials evaluating the effect of cell-based therapies in patients with COVID-19. Anticipating the lack of randomized trials, we also searched for nonrandomized, comparative studies in COVID-19, and for randomized trials evaluating other coronavirus infections, such as MERS-CoV and SARS-CoV. These additional searches provided no relevant studies either. In sum, we did not find any studies fulfilling the minimum requirements to inform decisions.
Systematic reviews are the gold standard to collect and summarize the available evidence regarding a scientific question. However, the traditional model for conducting reviews has several limitations, including high demand for time and resources[31] and rapid obsolescence[32]. Amid the COVID-19 crisis, researchers should make their best effort to answer health decision-makers' urgent needs without giving up scientific accuracy. Information is being produced at a vertiginous speed[33], so alternative models are needed.
One potential solution to these shortfalls is rapid reviews: reviews that omit some of the traditional systematic review steps to move faster. Unfortunately, in these reviews, speed comes at the cost of quality[34]. Furthermore, they do not solve the issue of obsolescence. Living systematic reviews do address that issue[35]. They are continually updated by incorporating relevant new evidence as it becomes available at a substantial effort. So, an approach combining these two models might prove more successful in providing the scientific community and other interested parties with evidence that is actionable, rapidly and efficiently produced, up to date, and of the highest quality[36].
This review is part of a larger project set up to put such an approach into practice. The project aims to produce multiple parallel living systematic reviews relevant to COVID-19 following the higher quality standards in evidence synthesis production[16]. We believe our methods are well suited to handle the abundance of evidence that is to come, including evidence on the role of cell-based therapies in treating patients with COVID-19. We have identified multiple ongoing studies addressing this question, including 39 randomized trials, which will provide valuable evidence to inform researchers and decision-makers in the near future.
We found two systematic reviews, with a broad scope, addressing this article's question[37],[38]. Their conclusions are similar in terms of the lack of evidence available. So, the main limitation of the existing systematic reviews, including ours, results from the absence of evidence to inform decisions.
Nonetheless, decisions should be made even if the evidence is considered insufficient[39]. While evidence about the potential benefit emerges, decision-makers will have to ponder other relevant aspects[40].
We think the main factors that decision-makers must put in the balance, in addition to the uncertainty of treatment effects (positive and negative), is the fact that until now, no theory could completely explain the mechanism by which cell therapy could have a positive impact on patient outcomes. Mesenchymal stem cells, the theoretically most promising cell therapy in the case of severe steroid-refractory inflammation, have not shown definitive positive results[41]. Researchers in this field should deepen our understanding of how cell-based therapies work and which specific therapy is the better option. We hope that the high number of studies expected to be completed in the next months will shed some light on these issues.
On the other hand, this treatment's high costs should also be considered when examining the equity implications of such a non-evidence-based decision.
During the COVID-19 pandemic, we will maintain a living, web-based, openly available version of this review, and we will resubmit the review to a peer-reviewed journal every time the conclusions change or whenever there are substantial updates. Our systematic review aims to provide a high-quality, up-to-date synthesis of the evidence useful for clinicians and other decision-makers.
NOTES
Differences between protocol and review
Our original protocol intended to include studies evaluating mesenchymal stem cells, the main type of cell-based therapy proposed in the context of COVID-19. In consultation with experts in the field, we expanded our criteria to also ainclude other types of cell-based therapies.
Roles and contributions
GR conceived the standard protocol for all the reviews being conducted by the COVID-19 L·OVE Working Group. GR drafted the manuscript, and all other authors contributed to it. The corresponding author is the guarantor and declares that all authors meet authorship criteria and that no other authors meeting the criteria have been omitted.
Epistemonikos and several expert teams created the COVID-19 L·OVE Working Group to provide decision-makers with the best evidence related to COVID-19. Up-to-date information about the group and its member organizations is available here: epistemonikos.cl/working-group
Acknowledgments
The COVID-19 L·OVE Working Group and Epistemonikos Foundation have made it possible to build the systems and compile the information needed by this project. Epistemonikos is a collaborative effort based on the ongoing volunteer work of over a thousand contributors since 2012.
Competing interests
All authors declare no financial relationships with any organization that might have a real or perceived interest in this work. There are no other relationships or activities that might have influenced the submitted work.
Funding
This project was not commissioned by any organization and did not receive external funding.
Epistemonikos Foundation provides training, support, and tools at no cost for all the members of the COVID-19 L·OVE Working Group.
PROSPERO registration number
CRD42020179711
Ethics
As researchers will not access information that could lead to identifying an individual participant, obtaining ethical approval was waived.
Data sharing
All data related to the project will be available. Epistemonikos Foundation will grant access to data.
Appendix 1
Appendix 1.

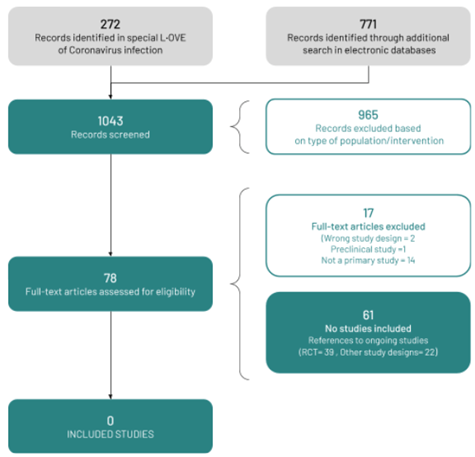 Figure 1 - PRISMA Flowchart.
Figure 1 - PRISMA Flowchart.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Objective
This living, systematic review aims to provide a timely, rigorous, and continuously updated summary of the available evidence on the role of cell-based therapies in the treatment of patients with COVID-19.
Data sources
We conducted searches in PubMed/Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), grey literature, and in a centralized repository in L·OVE (Living OVerview of Evidence). L·OVE is a platform that maps PICO questions to evidence from the Epistemonikos database. In response to the COVID-19 emergency, L·OVE was adapted to expand the range of evidence it covers and customized to group all COVID-19 evidence in one place. All the searches covered the period until 23 April 2020 (one day before submission).
Eligibility criteria for selecting studies and methods
We adapted an already published standard protocol for multiple parallel systematic reviews to the specificities of this question. We searched for randomized trials evaluating the effectiveness and safety of cell-based therapies versus placebo or no treatment in patients with COVID-19. Anticipating the lack of randomized trials directly addressing this question, we also searched for trials evaluating other coronavirus infections, such as MERS-CoV and SARS-CoV, and nonrandomized studies in COVID-19. Two reviewers independently screened each study for eligibility. A living, web-based version of this review will be openly available during the COVID-19 pandemic. We will resubmit this review to a peer-reviewed journal every time the conclusions change or whenever there are substantial updates.
Results
We screened 1 043 records, but no study was considered eligible. We identified 61 ongoing studies, including 39 randomized trials evaluating different types of cell-based therapies in COVID-19.
Conclusions
We did not find any studies that met our inclusion criteria, and hence there is no evidence to support or refute the use of cell-based therapies for treating patients with COVID-19. A substantial number of ongoing studies should provide valuable evidence to inform researchers and decision-makers in the near future.
PROSPERO Registration number
CRD42020179711
 Authors:
Gabriel Rada[1,2,3], Javiera Corbalán[4], Patricio Rojas[5,6], COVID-19 L·OVE Working Group
Authors:
Gabriel Rada[1,2,3], Javiera Corbalán[4], Patricio Rojas[5,6], COVID-19 L·OVE Working Group
Colaboradores:
Affiliation:
[1] Fundación Epistemonikos, Santiago, Chile
[2] UC Evidence Center, Cochrane Chile Associated Center, Pontificia Universidad Católica de Chile, Santiago, Chile
[3] Internal Medicine Department, Faculty of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile
[4] Instituto de Salud Pública, Facultad de Medicina, Universidad Austral de Chile
[5] Hematology and Oncology Department, Faculty of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile
[6] University Hospital, Cleveland Medical Center, Ohio, United States
E-mail: radagabriel@epistemonikos.org
Author address:
[1] Holanda 895, Providencia Santiago Chile

Citation: Rada G, Corbalán J, Rojas P. Cell-based therapies for COVID-19: A living, systematic review . Medwave 2020;20(11):e8078 doi: 10.5867/medwave.2020.11.8078
Submission date: 24/4/2020
Acceptance date: 16/10/2020
Publication date: 17/12/2020
Origin: Not commissioned
Type of review: Externally peer-reviewed by three reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- World Health Organization. Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. 2020. [On line] | Link |
- Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020 Feb;91:264-266. | CrossRef | PubMed |
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533-534. | CrossRef | PubMed |
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed |
- Tavakoli A, Vahdat K, Keshavarz M. Novel Coronavirus Disease 2019 (COVID-19): An Emerging Infectious Disease in the 21st Century. BPUMS. 2020;22(6):432-450. | CrossRef |
- Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 Jun;92(6):577-583. | CrossRef | PubMed |
- Global Covid-19 Case Fatality Rates UK: Centre for Evidence-Based Medicine. [On line] | Link |
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020 Mar-Apr;34:101623. | CrossRef | PubMed |
- Kallmeyer K, Pepper MS. Homing properties of mesenchymal stromal cells. Expert Opin Biol Ther. 2015 Apr;15(4):477-9. | CrossRef | PubMed |
- Elgaz S, Kuçi Z, Kuçi S, Bönig H, Bader P. Clinical Use of Mesenchymal Stromal Cells in the Treatment of Acute Graft-versus-Host Disease. Transfus Med Hemother. 2019 Feb;46(1):27-34. | CrossRef | PubMed |
- Gotts JE, Abbott J, Matthay MA. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol. 2014 Sep 1;307(5):L395-406. | CrossRef | PubMed |
- Shetty AK. Mesenchymal Stem Cell Infusion Shows Promise for Combating Coronavirus (COVID-19)- Induced Pneumonia. Aging Dis. 2020 Mar 9;11(2):462-464. | CrossRef | PubMed |
- Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020 Mar 9;11(2):216-228. | CrossRef | PubMed |
- Atluri S, Manchikanti L, Hirsch JA. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician. 2020 Mar;23(2):E71-E83. | CrossRef | PubMed |
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006-12. | CrossRef | PubMed |
- Rada G, Verdugo-Paiva F, Ávila C, Morel-Marambio M, Bravo-Jeria R, Pesce F, Madrid E, Izcovich A. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020 Apr 1;20(3):e7868. English, Spanish. | CrossRef | PubMed |
- Rada G, Corbalan J, Rojas P. Mesenchymal stromal cells for COVID-19: A living systematic review protocol medRxiv 2020.04.13.20064162. | CrossRef |
- Github repository [Accessed 2020 April 12] [On line] | Link |
- Epistemonikos Database Methods. Santiago: Epistemonikos Foundation [Accessed 2020 12 April]. [On line] | Link |
- Methods for the special L·OVE of Coronavirus infection. Santiago: Epistemonikos Foundation [Accessed 2020 12 April] [On line] | Link |
- Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. 2019 Jul;111:105-114. | CrossRef | PubMed |
- Jin X, Pang B, Zhang J, Liu Q, Yang Z, Feng J, et al. Core Outcome Set for Clinical Trials on Coronavirus Disease 2019 (COS-COVID). Engineering (Beijing). 2020 Mar 18. | CrossRef | PubMed |
- COVID-19 Core Outcomes [On line] | Link |
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013 Feb;66(2):158-72. | CrossRef | PubMed |
- Collaboratron [Software]. Santiago: Epistemonikos Foundation, 2017.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. | CrossRef | PubMed |
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 Oct 12;355:i4919. | CrossRef | PubMed |
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013 Feb;66(2):173-83. | CrossRef | PubMed |
- Review Manager (RevMan) [Software]. Version 5.3.5 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr 26;336(7650):924-6. | CrossRef | PubMed |
- Borah R, Brown AW, Capers PL, Kaiser KA. Analysis of the time and workers needed to conduct systematic reviews of medical interventions using data from the PROSPERO registry. BMJ Open. 2017 Feb 27;7(2):e012545. | CrossRef | PubMed |
- Shojania KG, Sampson M, Ansari MT, Ji J Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007 Aug 21;147(4):224-33. | CrossRef | PubMed |
- Coronavirus and the risks of 'speed science' Reuters; 2020. [On line] | Link |
- Kelly SE, Moher D, Clifford TJ. Quality of conduct and reporting in rapid reviews: an exploration of compliance with PRISMA and AMSTAR guidelines. Syst Rev. 2016 May 10;5:79. | CrossRef | PubMed |
- Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al. Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol. 2017 Nov;91:23-30. | CrossRef | PubMed |
- Akl EA, Haddaway NR, Rada G, Lotfi T. Future of Evidence Ecosystem Series: Evidence synthesis 2.0: when systematic, scoping, rapid, living, and overviews of reviews come together. J Clin Epidemiol. 2020 Jul;123:162-165. | CrossRef | PubMed |
- Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JI, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review. J Clin Med. 2020 Feb 26;9(3):623. | CrossRef | PubMed |
- Tobaiqy M, Qashqary M, Al-Dahery S, Mujallad A , Hershan A, Kamal M, et al. Therapeutic Management of COVID-19 Patients: A systematic review. medRxiv. 2020. | CrossRef |
- Neumann I, Schünemann HJ. Guideline groups should make recommendations even if the evidence is considered insufficient. CMAJ. 2020 Jan 13;192(2):E23-E24. | CrossRef | PubMed |
- Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. | CrossRef | PubMed |
- Kebriaei P, Hayes J, Daly A, Uberti J, Marks DI, Soiffer R, et al. A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2020 May;26(5):835-844. | CrossRef | PubMed |
 World Health Organization. Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. 2020. [On line] | Link |
World Health Organization. Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. 2020. [On line] | Link | Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic
threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020 Feb;91:264-266. | CrossRef | PubMed |
Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic
threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020 Feb;91:264-266. | CrossRef | PubMed | Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533-534. | CrossRef | PubMed |
Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533-534. | CrossRef | PubMed | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed |
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed | Tavakoli A, Vahdat K, Keshavarz M. Novel Coronavirus Disease 2019 (COVID-19): An Emerging Infectious Disease in the 21st Century. BPUMS. 2020;22(6):432-450. | CrossRef |
Tavakoli A, Vahdat K, Keshavarz M. Novel Coronavirus Disease 2019 (COVID-19): An Emerging Infectious Disease in the 21st Century. BPUMS. 2020;22(6):432-450. | CrossRef | Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate
of meta-analysis. J Med Virol. 2020 Jun;92(6):577-583. | CrossRef | PubMed |
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate
of meta-analysis. J Med Virol. 2020 Jun;92(6):577-583. | CrossRef | PubMed | Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020 Mar-Apr;34:101623. | CrossRef | PubMed |
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020 Mar-Apr;34:101623. | CrossRef | PubMed | Kallmeyer K, Pepper MS. Homing properties of mesenchymal stromal cells. Expert Opin Biol Ther. 2015 Apr;15(4):477-9. | CrossRef | PubMed |
Kallmeyer K, Pepper MS. Homing properties of mesenchymal stromal cells. Expert Opin Biol Ther. 2015 Apr;15(4):477-9. | CrossRef | PubMed | Elgaz S, Kuçi Z, Kuçi S, Bönig H, Bader P. Clinical Use of Mesenchymal Stromal Cells in the Treatment of Acute Graft-versus-Host Disease. Transfus Med Hemother. 2019 Feb;46(1):27-34. | CrossRef | PubMed |
Elgaz S, Kuçi Z, Kuçi S, Bönig H, Bader P. Clinical Use of Mesenchymal Stromal Cells in the Treatment of Acute Graft-versus-Host Disease. Transfus Med Hemother. 2019 Feb;46(1):27-34. | CrossRef | PubMed | Gotts JE, Abbott J, Matthay MA. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol. 2014 Sep 1;307(5):L395-406. | CrossRef | PubMed |
Gotts JE, Abbott J, Matthay MA. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol. 2014 Sep 1;307(5):L395-406. | CrossRef | PubMed | Shetty AK. Mesenchymal Stem Cell Infusion Shows Promise for Combating Coronavirus (COVID-19)- Induced Pneumonia. Aging Dis. 2020 Mar 9;11(2):462-464. | CrossRef | PubMed |
Shetty AK. Mesenchymal Stem Cell Infusion Shows Promise for Combating Coronavirus (COVID-19)- Induced Pneumonia. Aging Dis. 2020 Mar 9;11(2):462-464. | CrossRef | PubMed | Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome
of Patients with COVID-19 Pneumonia. Aging Dis. 2020 Mar 9;11(2):216-228. | CrossRef | PubMed |
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome
of Patients with COVID-19 Pneumonia. Aging Dis. 2020 Mar 9;11(2):216-228. | CrossRef | PubMed | Atluri S, Manchikanti L, Hirsch JA. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician. 2020
Mar;23(2):E71-E83. | CrossRef | PubMed |
Atluri S, Manchikanti L, Hirsch JA. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician. 2020
Mar;23(2):E71-E83. | CrossRef | PubMed | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred
reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006-12. | CrossRef | PubMed |
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred
reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006-12. | CrossRef | PubMed | Rada G, Verdugo-Paiva F, Ávila C, Morel-Marambio M, Bravo-Jeria R, Pesce F, Madrid E, Izcovich A. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020 Apr 1;20(3):e7868. English, Spanish. | CrossRef | PubMed |
Rada G, Verdugo-Paiva F, Ávila C, Morel-Marambio M, Bravo-Jeria R, Pesce F, Madrid E, Izcovich A. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020 Apr 1;20(3):e7868. English, Spanish. | CrossRef | PubMed | Rada G, Corbalan J, Rojas P. Mesenchymal stromal cells for COVID-19: A living systematic review protocol medRxiv 2020.04.13.20064162. | CrossRef |
Rada G, Corbalan J, Rojas P. Mesenchymal stromal cells for COVID-19: A living systematic review protocol medRxiv 2020.04.13.20064162. | CrossRef | Epistemonikos Database Methods. Santiago: Epistemonikos Foundation [Accessed 2020 12 April]. [On line]
| Link |
Epistemonikos Database Methods. Santiago: Epistemonikos Foundation [Accessed 2020 12 April]. [On line]
| Link | Methods for the special L·OVE of Coronavirus infection. Santiago: Epistemonikos Foundation [Accessed 2020 12 April] [On line] | Link |
Methods for the special L·OVE of Coronavirus infection. Santiago: Epistemonikos Foundation [Accessed 2020 12 April] [On line] | Link | Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk
of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. 2019 Jul;111:105-114. | CrossRef | PubMed |
Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk
of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. 2019 Jul;111:105-114. | CrossRef | PubMed | Jin X, Pang B, Zhang J, Liu Q, Yang Z, Feng J, et al. Core Outcome Set for Clinical Trials on Coronavirus Disease 2019 (COS-COVID). Engineering (Beijing). 2020 Mar 18. | CrossRef | PubMed |
Jin X, Pang B, Zhang J, Liu Q, Yang Z, Feng J, et al. Core Outcome Set for Clinical Trials on Coronavirus Disease 2019 (COS-COVID). Engineering (Beijing). 2020 Mar 18. | CrossRef | PubMed | Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013 Feb;66(2):158-72. | CrossRef | PubMed |
Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013 Feb;66(2):158-72. | CrossRef | PubMed | Collaboratron [Software]. Santiago: Epistemonikos Foundation, 2017.
Collaboratron [Software]. Santiago: Epistemonikos Foundation, 2017.  Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. | CrossRef | PubMed |
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. | CrossRef | PubMed | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 Oct 12;355:i4919. | CrossRef | PubMed |
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 Oct 12;355:i4919. | CrossRef | PubMed | Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines:
13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013 Feb;66(2):173-83. | CrossRef | PubMed |
Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines:
13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013 Feb;66(2):173-83. | CrossRef | PubMed | Review Manager (RevMan) [Software]. Version 5.3.5 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Review Manager (RevMan) [Software]. Version 5.3.5 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.  Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr
26;336(7650):924-6. | CrossRef | PubMed |
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr
26;336(7650):924-6. | CrossRef | PubMed | Borah R, Brown AW, Capers PL, Kaiser KA. Analysis of the time and workers needed to conduct systematic reviews of medical interventions using data from the PROSPERO registry. BMJ Open. 2017 Feb 27;7(2):e012545. | CrossRef | PubMed |
Borah R, Brown AW, Capers PL, Kaiser KA. Analysis of the time and workers needed to conduct systematic reviews of medical interventions using data from the PROSPERO registry. BMJ Open. 2017 Feb 27;7(2):e012545. | CrossRef | PubMed | Shojania KG, Sampson M, Ansari MT, Ji J Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007 Aug 21;147(4):224-33. | CrossRef | PubMed |
Shojania KG, Sampson M, Ansari MT, Ji J Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007 Aug 21;147(4):224-33. | CrossRef | PubMed | Kelly SE, Moher D, Clifford TJ. Quality of conduct and reporting in rapid reviews: an exploration of compliance with PRISMA and AMSTAR guidelines. Syst Rev. 2016 May 10;5:79. | CrossRef | PubMed |
Kelly SE, Moher D, Clifford TJ. Quality of conduct and reporting in rapid reviews: an exploration of compliance with PRISMA and AMSTAR guidelines. Syst Rev. 2016 May 10;5:79. | CrossRef | PubMed | Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al. Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol. 2017 Nov;91:23-30. | CrossRef | PubMed |
Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al. Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol. 2017 Nov;91:23-30. | CrossRef | PubMed | Akl EA, Haddaway NR, Rada G, Lotfi T. Future of Evidence Ecosystem Series: Evidence synthesis 2.0: when systematic, scoping, rapid, living, and overviews of reviews come together. J Clin Epidemiol. 2020 Jul;123:162-165. | CrossRef | PubMed |
Akl EA, Haddaway NR, Rada G, Lotfi T. Future of Evidence Ecosystem Series: Evidence synthesis 2.0: when systematic, scoping, rapid, living, and overviews of reviews come together. J Clin Epidemiol. 2020 Jul;123:162-165. | CrossRef | PubMed | Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JI, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review. J Clin Med. 2020 Feb 26;9(3):623. | CrossRef | PubMed |
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JI, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review. J Clin Med. 2020 Feb 26;9(3):623. | CrossRef | PubMed | Tobaiqy M, Qashqary M, Al-Dahery S, Mujallad A , Hershan A, Kamal M, et al. Therapeutic Management of COVID-19 Patients: A systematic review. medRxiv. 2020. | CrossRef |
Tobaiqy M, Qashqary M, Al-Dahery S, Mujallad A , Hershan A, Kamal M, et al. Therapeutic Management of COVID-19 Patients: A systematic review. medRxiv. 2020. | CrossRef | Neumann I, Schünemann HJ. Guideline groups should make recommendations even if the evidence is considered insufficient. CMAJ. 2020 Jan 13;192(2):E23-E24. | CrossRef | PubMed |
Neumann I, Schünemann HJ. Guideline groups should make recommendations even if the evidence is considered insufficient. CMAJ. 2020 Jan 13;192(2):E23-E24. | CrossRef | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








