Key Words: COVID-19, severe acute respiratory syndrome coronavirus 2, coronavirus infections, systematic review, macrolides, azithromycin, anti-bacterial agents, coronavirus disease
Abstract
Objective
This living, systematic review aims to provide a timely, rigorous, and continuously updated summary of the evidence available on the role of macrolides for treating patients with COVID-19.
Design
A living, systematic review.
Database
We conducted searches in the centralized repository L·OVE (Living OVerview of Evidence). L·OVE is a platform that maps PICO questions to evidence from the Epistemonikos database. In response to the COVID-19 emergency, L·OVE was adapted to expand the range of evidence it covers and customized to group all COVID-19 evidence in one place. Today it is maintained through regular searches in 39 databases.
Methods
We included randomized trials evaluating the effect of macrolides — as monotherapy or in combination with other drugs — versus placebo or no treatment in patients with COVID-19. Randomized trials evaluating macrolides in infections caused by other coronaviruses, such as MERS-CoV and SARS-CoV, and non-randomized studies in COVID-19 were searched in case we found no direct evidence from randomized trials. Two reviewers independently screened each study for eligibility, extracted data, and assessed the risk of bias. Measures included all-cause mortality; the need for invasive mechanical ventilation; extracorporeal membrane oxygenation, length of hospital stay, respiratory failure, serious adverse events, time to SARS-CoV-2 RT-PCR negativity. We applied the GRADE approach to assess the certainty of the evidence for each outcome. A living, web-based version of this review will be openly available during the COVID-19 pandemic. We will resubmit it every time the conclusions change or whenever there are substantial updates.
Results
The search in the L·OVE platform retrieved 424 references. We considered 260 as potentially eligible and were reviewed in full texts. We included one randomized clinical trial that evaluated the use of azithromycin in combination with hydroxychloroquine compared to hydroxychloroquine alone in hospitalized patients with COVID 19. The estimates for all outcomes evaluated resulted in insufficient power to draw conclusions. The quality of the evidence for the main outcomes was low to very low.
Conclusions
Macrolides in the management of patients with COVID 19 showed no beneficial effects compared to standard of care. The evidence for all outcomes is inconclusive. Larger trials are needed to determine the effects of macrolides on pulmonary and other outcomes in COVID-19 patients.
Systematic review registration
PROSPERO Registration number: CRD42020181032
Protocol preprint DOI: 10.31219/osf.io/rvp59
|
Main messages
|
Introduction
COVID-19 is an infection caused by the SARS-CoV-2 coronavirus[1]. It was first identified in Wuhan, China, on December 31, 2019[2]. On March 11, 2020, the WHO characterized the COVID-19 outbreak as a pandemic[1]. In July 2020, more than fifteen million cases of contagion had been identified worldwide[3].
While the majority of cases result in mild symptoms, some might progress to pneumonia, acute respiratory distress syndrome, and death[4],[5],[6]. The case fatality rate reported across countries, settings, and age groups is highly variable, but it ranges from about 0.8% to 18%[7].
Multiple drugs have been proposed as a possible treatment for patients with moderate to severe COVID-19. Azithromycin, and other macrolides, have been suggested due to their alleged role in preventing bacterial superinfection and their immunomodulatory and anti-inflammatory effects[8],[9]. However, clinical studies evaluating the use of macrolides in the treatment of adult or pediatric patients with different respiratory infections, such as influenza or respiratory syncytial virus, have shown contradictory results[10],[11],[12],[13],[14],[15],[16],[17].
Despite these results, macrolides have been empirically prescribed in patients with pneumonia caused by novel coronaviruses such as SARS and MERS, and, more recently, SARS-CoV-2. Azithromycin attracted attention after the release of a non-randomized study—with considerable methodological limitations—and an observational study, claiming that hydroxychloroquine with azithromycin achieved a higher level of SARS-CoV-2 clearance in respiratory secretions[18],[19].
Using innovative and agile processes, taking advantage of technological tools, and resorting to several research groups' collective effort, this living, systematic review aims to provide a timely, rigorous, and continuously updated summary of the evidence available on patients with COVID-19.
Methods
Protocol and registration
This manuscript complies with the 'Preferred Reporting Items for Systematic reviews and Meta-Analyses' (PRISMA) guidelines[20]. Appendix 1 - PRISMA checklist.
A protocol stating the shared objectives and methodology of multiple evidence syntheses (systematic reviews and overviews of systematic reviews) to be conducted in parallel for different questions relevant to COVID-19 was published elsewhere[21]. This systematic review protocol was adapted to the specificities of the question[22] and submitted to PROSPERO CRD42020181032.
Search strategies
Electronic searches
Our literature search was devised by the team maintaining the L·OVE platform (https://app.iloveevidence.com), using the following approach:
- Identification of terms relevant to the population and intervention components of the search strategy, using Word2vec technology[23] to the corpus of documents available in Epistemonikos Database.
- Discussion of terms with content and methods experts to identify relevant, irrelevant and missing terms.
- Creation of a sensitive boolean strategy encompassing all the relevant terms.
- Iterative analysis of articles missed by the boolean strategy, and refinement of the strategy accordingly.
We conducted searches using the L·OVE (Living OVerview of Evidence) platform (https://app.iloveevidence.com) for COVID-19. This system maps PICO questions to a repository and is maintained through regular searches in 31 databases, preprint servers, trial registries, and websites relevant to COVID-19. The list of sources is regularly updated on our website. All the searches covered the period until August 6, 2020. No date, language, study design, publication status, or language restriction was applied to the searches in the Epistemonikos or the additional searches.
All the platform information comes from a repository developed and maintained by Epistemonikos Foundation through the screening of different sources relevant to COVID-19[24]. At the time of releasing this article, this repository included more than 65 000 articles pertinent to the coronavirus disease, coming from the following databases, trial registries, preprint servers and websites relevant to COVID-19: Epistemonikos database, Pubmed/medline, EMBASE, CINAHL, PsycINFO, ICTRP Search Portal, Clinicaltrials.gov, ISRCTN registry, Chinese Clinical Trial Registry, IRCT - Iranian Registry of Clinical Trials, EU Clinical Trials Register: Clinical trials for covid-19, NIPH Clinical Trials Search (Japan) - Japan Primary Registries Network (JPRN) (JapicCTI, JMACCT CTR, jRCT, UMIN CTR), UMIN-CTR - UMIN Clinical Trials Registry, JRCT - Japan Registry of Clinical Trials, JAPIC Clinical Trials Information, Clinical Research Information Service (CRiS)- Republic of Korea, ANZCTR - Australian New Zealand Clinical Trials Registry , ReBec - Brazilian Clinical Trials Registry, CTRI - Clinical Trials Registry - India, RPCEC - Cuban Public Registry of Clinical Trials, DRKS - German Clinical Trials Register, LBCTR - Lebanese Clinical Trials Registry , TCTR - Thai Clinical Trials Registry, NTR - The Netherlands National Trial Register, PACTR - Pan African Clinical Trial Registry, REPEC - Peruvian Clinical Trial Registry, SLCTR - Sri Lanka, Clinical Trials Registry , medRxiv, bioRxiv and SSRN Preprints
The database[25] acts as a central repository. Only articles fulfilling Epistemonikos criteria are visible by users. The remaining articles are exclusively accessible for members of the COVID-19 L·OVE Working Group.
The following search strategy was used in Epistemonikos Database[25]. We adapted it to the syntax of other databases:
(coronavir* OR coronovirus* OR "corona virus" OR "virus corona" OR "corono virus" OR "virus corono" OR hcov* OR "covid-19" OR covid19* OR "covid 19" OR "2019-nCoV" OR cv19* OR "cv-19" OR "cv 19" OR "n-cov" OR ncov* OR "sars-cov-2" OR "sars-cov2" OR "SARS-Coronavirus-2" OR "SARS-Coronavirus2" OR (wuhan* AND (virus OR viruses OR viral)) OR (covid* AND (virus OR viruses OR viral)) OR "sars-cov" OR "sars cov" OR "sars-coronavirus" OR "severe acute respiratory syndrome" OR "mers-cov" OR "mers cov" OR "middle east respiratory syndrome" OR "middle-east respiratory syndrome" OR "covid-19-related" OR "SARS-CoV-2-related" OR "SARS-CoV2-related" OR "2019-nCoV-related" OR "cv-19-related" OR "n-cov-related") AND ((macrolide*) OR (fidaxomicin* OR clostomicin* OR lipiarm* OR "OPT-80" OR "OPT 80" OR OPT80* OR "PAR-01" OR "PAR 01" OR PAR01* OR "PAR-101" OR "PAR 101" OR PAR101* OR tiacumicin* OR Dificid* OR Dificlir* ) OR (azithromycin* OR Zithromax* OR Azithrocin*) OR (clarithromycin* OR Biaxin* ) OR (erythromycin* OR Eryc* OR Erythrocin* ) OR (josamycin* ) OR (solithromycin* OR "CEM-101" OR "CEM 101" OR CEM101* OR "OP-1068" OR "OP 1068" OR OP1068* OR Solithera*) OR (spiramycin* ) OR (troleandomycin* OR Triocetin* OR Tekmisin* ) OR (roxithromycin* ) OR (telithromycin* OR Ketek* ) OR (cethromycin* OR "ABT-773" OR "ABT 773" OR ABT773* OR Restanza*) OR (carrimycin*))
Eligibility criteria
Types of studies
We included randomized controlled trials. We excluded information from non-randomized studies, post-trial analyses, and studies evaluating animal models' effects or in vitro conditions.
Types of participants
We included trials assessing participants with confirmed COVID-19, as defined by the authors of the trials.
Whenever we found substantial clinical heterogeneity on how the condition was defined, we planned to explore it using a sensitivity analysis.
Type of interventions
The interventions of interest were macrolides (i.e., azithromycin, clarithromycin, erythromycin, carrimycine). We did not restrict our criteria to any dosage, duration, timing, or route of administration.
The comparison of interest was placebo (macrolides plus optimal treatment versus placebo plus optimal treatment) or no treatment (macrolides plus optimal treatment versus optimal treatment).
Trials assessing macrolides plus other drugs were eligible if the cointerventions are identical in both intervention and comparison groups. Trials evaluating macrolides in combination with other active drugs versus placebo or no treatment also were included.
Type of outcomes
We did not use the outcomes as an inclusion criterion during the selection process. Any article meeting all the criteria except for the outcome criterion was preliminarily included and assessed in full text.
We used the core outcome set COS-COVID[26], the existing guidelines and reviews, and the judgment of the authors of this review as an input for selecting the primary and secondary outcomes, as well as to decide upon inclusion. The review team revised this list of outcomes to incorporate ongoing efforts to define Core Outcomes Sets (e.g., COVID-19 Core Outcomes[27]).
The primary outcome was all-cause mortality. The secondary outcomes were mechanical ventilation, extracorporeal membrane oxygenation, length of hospital stay, respiratory failure, serious adverse events, and time to SARS-CoV-2 RT-PCR negativity. Other outcomes were acute respiratory distress syndrome and total adverse events.
We present primary and secondary outcomes in GRADE 'Summary of Findings' tables[28].
Selection of studies
The results of the literature search in all databases were automatically incorporated into the L·OVE platform (automated retrieval), where they were de-duplicated by an algorithm comparing unique identifiers (database ID, DOI, trial registry ID), and citation details (i.e., author names, journal, year of publication, volume, number, pages, article title and article abstract).
Two researchers independently screened the titles and abstracts yielded by the search against the inclusion criteria. We obtained the full reports for all titles that appeared to meet the inclusion criteria or required further analysis to decide their inclusion.
We recorded the reasons for excluding trials in any stage of the search. We outlined the study selection process in a PRISMA flow diagram adapted for this project.
Extraction and management of data
Using standardized forms, two reviewers independently extracted data from each included study. We collected the following information: study design, setting, participant characteristics (including disease severity and age) and study eligibility criteria; details about the administered intervention and comparison, including dose and therapeutic scheme, duration, timing (i.e., time after diagnosis), and route of administration; the outcomes assessed and the time they were measured; the source of funding of the study; the conflicts of interest disclosed by the investigators; and the risk of bias assessment for each study.
We resolved disagreements by discussion, and one referee adjudicated unresolved disagreements.
Risk of bias assessment
The risk of bias for each randomized trial was assessed using the 'risk of bias' tool (RoB 2.0: a revised tool to assess the risk of bias in randomized trials)[29]. Two reviewers independently assessed five domains of bias for each outcome result of all reported outcomes and time points. These five domains were: bias due to (1) the randomization process, (2) deviations from intended interventions (effects of assignment to interventions at baseline), (3) missing outcome data, (4) measurement of the outcome, and (5) selection of reported results. Answers to signaling questions and collectively supporting information were considered to lead to a domain‐level judgment in the form of 'Low risk of bias,' 'Some concerns,' or 'High risk of bias.' These domain‐level judgments informed an overall 'risk of bias' assessment for each result. Discrepancies between review authors were resolved by discussion to reach consensus. If necessary, a third review author was consulted to achieve a decision.
We considered the following factors as potential baseline confounders: age, comorbidities (e.g., cardiovascular disease; renal disease, eye disease, liver disease); co-interventions; and severity, as defined by the authors (i.e., respiratory failure vs. respiratory distress syndrome vs. ICU requirement).
Measures of treatment effect
We expressed the estimate of treatment effect of an intervention as risk ratios or odds ratios along with 95% confidence intervals for dichotomous outcomes. We used mean difference and standard deviation for continuous outcomes to summarize the data using a 95 percent confidence interval.
Strategy for data synthesis
If more than one trial was included, we planned to conduct a meta-analysis for studies clinically homogeneous using RevMan 5[30], using the inverse variance method with the random-effects model. For any outcomes where data were insufficient to calculate an effect estimate, we planned to present a narrative synthesis.
Subgroup and sensitivity analysis
We planned to perform subgroup analysis according to the definition of severe COVID-19 infection (i.e., respiratory failure vs. respiratory distress syndrome vs. ICU requirement). In case we identified significant differences between subgroups (test for interaction < 0.05), we considered reporting the results of individual subgroups separately.
We planned to perform sensitivity analysis, excluding studies with a high risk of bias. In cases where the primary analysis effect estimates and the sensitivity analysis effect estimates significantly differed, we considered presenting either the low risk of bias—adjusted sensitivity analysis estimates—or the primary analysis estimates but downgrading the certainty of the evidence because of risk of bias.
Assessment of certainty of evidence
We judged certainty of the evidence for all outcomes using the Grading of Recommendations Assessment, Development and Evaluation working group methodology (GRADE Working Group)[31], across the domains of risk of bias, consistency, directness, precision, and reporting bias. Certainty was adjudicated as high, moderate, low, or very low. For the main comparisons and outcomes, we prepared a Summary of Findings table[28],[32] and also an interactive Summary of Findings table (http://isof.epistemonikos.org/).
Living evidence synthesis
An artificial intelligence algorithm deployed in the Coronavirus/COVID-19 topic of the L·OVE platform will provide instant notification of articles with a high likelihood of being eligible. The authors will review them, decide upon inclusion, and update the review's living web version accordingly. We will consider resubmission to the journal if the direction of the effect on the critical outcomes changes or a substantial modification to the evidence's certainty.
This review is part of a larger project set up to produce multiple parallel systematic reviews relevant to COVID-19[21].
Results
Results of the search
The search in the L·OVE platform retrieved 424 references. We considered 260 as potentially eligible and retrieved and evaluated their full texts. A total of thirteen records were observational studies that assessed the intervention of interest. Only one study (a randomized clinical trial)[33] was eligible for inclusion. The study selection process is summarized in Figure 1 - PRISMA Flowchart.
Description of the included studies
We included one randomized clinical trial[33], which included a total of 665 patients, but only 438 of them were randomized to the intervention and comparison groups of this systematic review's interest. In this trial, adult patients with suspected or confirmed COVID-19 were randomly assigned to one of three groups: standard care; hydroxychloroquine (400 milligrams twice daily for seven days) plus standard care; or azithromycin (400 milligrams twice daily for seven days) plus hydroxychloroquine (400 milligrams twice daily for seven days) plus standard care. For this systematic review, we extracted data from the latter two groups, which compares the isolated effect of azithromycin. Measured outcomes of our interest were: all-cause mortality, invasive mechanical ventilation, length of hospital stay, respiratory failure, serious adverse events, and total adverse events. Table 1 presents the inclusion criteria of the included study, and table 2 shows the main characteristics and baseline characteristics of the participants. We describe the details of the study in Appendix 2.
Table 1. Inclusion criteria of the study.
Ongoing studies
We identified 78 ongoing studies (71 randomized trials and seven non-randomized studies). See Appendix 2 - List of included, excluded, and ongoing studies.
Excluded studies
We excluded 165 studies that did not fulfill our eligibility criteria. A detailed list of excluded studies with reasons for exclusion is presented in Appendix 2 - List of included, excluded, and ongoing studies.
Risk of bias of the included study
The overall risk of bias was high, mainly due to deviations from the intended interventions. The other four domains (randomization process, missing outcomes, measurement of the outcome, and selection of reported results) were assessed as low risk of bias. Appendix 2 presents the main reasons for this assessment.
Effects of interventions
Of the outcomes of interest for this review, the included study did not report on "Extracorporeal membrane oxygenation" and "Time to SARS-CoV-2 RT-PCR negativity". The results for all other outcomes are presented in Table 3 and our interactive Summary of Findings table.
Table 3. Summary of findings of macrolides for the treatment of COVID-19.
Primary outcome: All-cause mortality
The included study reported the effect of interventions on in-hospital death; we extracted this outcome as "All-cause mortality"[33]. The analysis showed a non-statistically significant difference in the risk of all-cause mortality between intervention groups (risk ratio: 0.57; 95% confidence interval: 0.19 to 1.66; 438 patients; 13 events) (Figure 2).
Secondary outcomes:
1. Mechanical ventilation
The included study reported the effect of interventions on the need for invasive mechanical ventilation within fifteen days. Out of the 217 patients in the intervention group, twenty needed mechanical ventilation within fifteen days compared to the sixteen of 221 patients in the control group. Results did not show statistically significant differences between compared interventions on the need for mechanical ventilation (risk ratio: 1.27; 95% confidence interval: 0.68 to 2.39; 438 patients; 36 events). These results are shown in Figure 2.
2. Extracorporeal membrane oxygenation
The included study did not assess this outcome.
3. Length of hospital stay
The included study reported the length of hospital stay, and these results are shown in Figure 3. The mean duration of hospital stay for the 217 patients in the intervention group was 9.4 days (standard deviation of 7.8). In comparison, the mean duration of hospital stay for the 221 patients in the control group was 8.9 days (standard deviation of 6.2). The mean difference between groups showed very imprecise results from which to draw conclusions (mean difference 0.5; 95% confidence interval: -0.82 to 1.82; 438 patients).
4. Respiratory failure
The included study reported the need for high flow nasal cannula, non-invasive ventilation, and invasive mechanical ventilation within fifteen days. We extracted these outcomes as "Respiratory failure," and the results are shown in Figure 3. Out of 217 patients in the intervention group, 37 had respiratory failure, compared to 35 of 221 patients in the control group. The comparative analysis did not show statistically significant differences between compared interventions in the effect on respiratory failure (risk ratio: 1.08; 95% confidence interval: 0.71 to 1.64; 438 patients; 72 events).
5. Serious adverse events
The per-protocol analysis showed very imprecise results and did not show any trend regarding the risk of adverse events associated with the compared interventions (risk ratio: 2.08; 95% confidence interval: 0.41 to 10.61; 438 patients; 239 in the intervention group; 199 in the control group; seven events). This outcome is presented in Figure 3.
6. Time to SARS-CoV-2 RT-PCR negativity
The included study did not assess this outcome.
7. Total adverse events
The included study reported serious adverse events, and for any other adverse events, we extracted these two outcomes as "Total adverse events." The per-protocol analysis results did not show statistically significant differences between compared interventions in terms of adverse events (risk ratio: 1.19; 95% confidence interval: 0.94 to 1.52; 438 patients; 239 in the intervention group; 199 in the control group; 168 events).
Discussion
This living, systematic review included only one randomized controlled trial that evaluated using azithromycin associated with hydroxychloroquine compared to hydroxychloroquine alone for COVID-19[33]. Its results show that there is not enough evidence to conclude any difference between the intervention and control groups. All assessed outcomes had a wide confidence interval and were evaluated with small sample size, and therefore had a low or very low certainty of evidence.
The severity of some COVID-19 cases[34], added to the lack of a good treatment strategy and the production of vaccines still under development, has led to repurposing different drugs[35]; macrolides are some of them. The use of macrolides was proposed at the beginning of the pandemic due to its immunomodulatory, anti-inflammatory, and anti-viral effects shown in other viral diseases[36],[37],[38]. Gautret. et al.[18] proposed the association of azithromycin and hydroxychloroquine as a possible treatment strategy when they reported a 100% viral clearance in nasopharyngeal swabs in a total of six patients studied. As well as macrolides, hydroxychloroquine has its own immunomodulatory and anti-inflammatory effects[39]. To consider this association as a possible one, we must consider both drugs' adverse effects and their additive toxicity[35]. Both drugs are known for prolonging the QTc interval[40],[41],[42],[43], and it is likely to be suspected that this effect could increase when prescribing the drugs simultaneously[44],[39]. Additionally, we must consider that patients with severe COVID-19 are elderly and have pre-existing comorbidities, so adding a potentially risky combination of drugs may represent a challenge for the patient's health. The results of the included study in this living, systematic review showed a higher frequency of QTc interval increase in the group treated with hydroxychloroquine and azithromycin than the group that received hydroxychloroquine alone[33]. The group treated with azithromycin and hydroxychloroquine also had a higher frequency of any adverse events and elevation of liver enzyme levels[33], suggesting that additive toxicity between these drugs exists.
A still-increasing amount of evidence studying this intervention has been developed. Several observational studies have assessed azithromycin and hydroxychloroquine compared to hydroxychloroquine alone or azithromycin alone compared to standard treatment[45],[46],[47],[48],[49],[50],[51],[52],[53],[54],[55],[56]. The 78 ongoing studies indicated in this review are studying the same intervention as well. Systematic reviews have also been published evaluating the combination of azithromycin with hydroxychloroquine. Turgeon et al. searched the literature evaluating pharmacological properties and toxicity of six different drugs repurposed for COVID-19—including azithromycin—and their results showed that some cases report torsades de pointes after the administration of the macrolide[57]. Yang et al. concluded that the combination of hydroxychloroquine and azithromycin for COVID-19 might be effective because it showed synergic effects[58]. Gbinigie and Frie's rapid review concluded that there is limited evidence to confirm a synergic effect between azithromycin and hydroxychloroquine and that this evidence combined with its high risk of bias does not permit to support the use of azithromycin alone to treat COVID-19 (unless it is used in a trial or to treat a bacterial super-infection)[59]. Recently, a living, systematic review, and network meta-analysis about drug treatments for COVID-19 has been published[60]. However, Siemieniuk et al. did not include the randomized clinical trial included for this review due to the latter's recent publication[33],[60]. The reviews mentioned differ in methodology and conclusions. Our review only assesses one randomized controlled study, so it seems that the question is yet to be answered (by this review's update or other reviews that include the upcoming studies).
What draws our attention—and we cannot look away—is that one single methodologically-poor study initiated a waterfall of studies on the intervention of interest to this review. With "100% of viral clearance in nasopharyngeal swabs", Gautret et al.[18] proposed the intervention as the "heroin of the pandemic," and it was very far from being so. This then led to significant expenditures in different healthcare systems worldwide to treat their patients with the miraculous combination. In a chaotic healthcare system era, where everything is being reinvented, and telemedicine is the safest option to practice medicine[61], it appears peculiar to invest money in therapies that have not yet been proven useful.
This living, systematic review is not exempt from limitations, principally because it only assesses one randomized controlled trial. One of the reported outcomes in this review is "Respiratory failure," which included the patients in mechanical ventilation reported in the study. This must not be understood as a double report of outcome, but as an impossibility to report "Respiratory failure" without them because, obviously, patients in mechanical ventilation are in respiratory failure. The same outcome may be overestimating the number of patients in respiratory failure because the study does not clarify if a patient received more than one ventilatory assistance strategy. And the third limitation is that the results presented here only reflect the ones given by the first randomized clinical trial published assessing this intervention. There are no significant conclusions based on the results provided by one study because we lack a larger sample size. This limitation will be palliated in the future when the ongoing studies shown in this review become published. And this is where this review's strength appears: as it has a living method, it will be updated frequently to find new evidence when it becomes available. This type of review is particularly useful in this type of situation: the clinical question is relevant (pandemic with no clear treatment strategy nor vaccines), existing certainty of the evidence is low or very low (multiple observational studies, only one randomized controlled trial) and the information available will surely increase (over 70 ongoing studies evaluating the intervention presented in this review)[62]. The living method ensures future versions of this review that will include the evidence as it becomes available, so their results and conclusions will be very useful for future research and clinical practice.
This review is part of a larger project set up to put such an approach into practice. This project aims to produce multiple parallel living systematic reviews relevant to COVID-19 following the higher quality standards in evidence synthesis production[21]. We believe that our methods are well suited to handle the abundance of evidence that is to come, including evidence on macrolides' role for COVID-19. We have identified multiple ongoing studies addressing this question, including 71 randomized trials, which will provide valuable evidence to inform researchers and decision-makers soon.
Conclusions
Multiple drugs have been proposed as possible therapies for patients with moderate to severe COVID-19. Azithromycin, and other macrolides, have been suggested as a potential treatment due to their alleged role in preventing bacterial superinfection and their immunomodulatory and anti-inflammatory effects. Macrolides in the management of patients with COVID 19 showed no beneficial effects compared to standard of care. The evidence for all outcomes is inconclusive. Larger trials are needed to determine macrolides' effect on pulmonary and other outcomes in COVID 19 patients.
During the COVID-19 pandemic, we will maintain a living, web-based, openly available version of this review. We will re-submit the review every time the conclusions change or whenever there are substantial updates. Our systematic review aims to provide a high-quality, up-to-date synthesis of the evidence useful for clinicians and other decision-makers.
Notes
Appendix
Appendix 1: PRISMA Checklist.
Appendix 2: Included, excluded and ongoing studies - Macrolides for the treatment of COVID-19: A living systematic review - VERSION 1.0, 6 AUGUST, 2020.
Roles and contributions
GR conceived the standard protocol for all the reviews being conducted by the COVID-19 L·OVE Working Group. CV, LVM, NM, JPB, and MXR drafted the manuscript, and all other authors contributed to it. The corresponding author is the guarantor and declares that all authors meet authorship criteria and that no other authors meeting the criteria have been omitted.
The COVID-19 L·OVE Working Group was formed by Epistemonikos and a number of expert teams to provide decision-makers with the best evidence related to COVID-19. Up-to-date information about the group and its member organizations is available here: https://www.epistemonikos.cl/working-group/
Acknowledgments
The COVID-19 L·OVE Working Group and Epistemonikos Foundation have made it possible to build the systems and compile the information needed by this project. Epistemonikos is a collaborative effort based on the ongoing volunteer work of over a thousand contributors since 2012.
Competing interests
All authors declare no financial relationships with any organization that might have a real or perceived interest in this work. There are no other relationships or activities that might have influenced the submitted work.
Funding
This project was not commissioned by any organization and did not receive external funding.
Epistemonikos Foundation provides training, support, and tools at no cost for all COVID-19 L·OVE Working Group members.
Ethics
As researchers will not access information that could lead to identifying an individual participant, obtaining ethical approval was waived.
Data sharing
All data related to the project will be available. Epistemonikos Foundation will grant access to data.
PROSPERO registration: CRD42020181032.
Differences between protocol and review
In this review, some methods were not implemented. We did not implement either the search in other sources specified in the original protocol[22] or the screening in Collaboratron™ because we considered L·OVE platform as a comprehensive tool that gathers both processes, including diverse databases and registries, as we specified in our methods. We neither needed to express continuous outcomes as a standardized mean difference because we only included one randomized clinical trial in our review. No minimally important difference (MID) was known, so we could not express continuous outcomes as MID units.

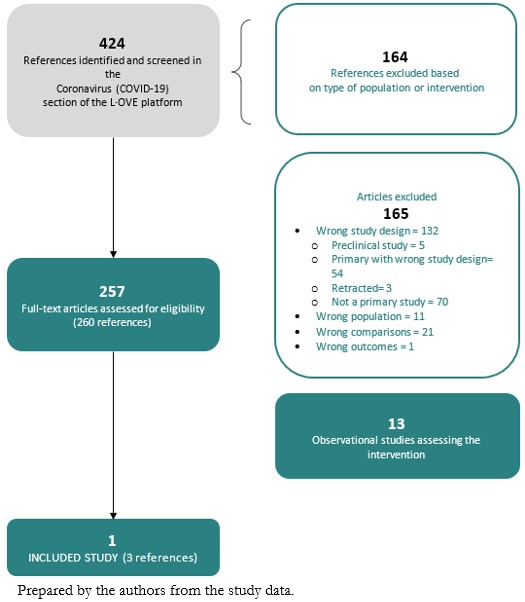 Figure 1. PRISMA Flowchart.
Figure 1. PRISMA Flowchart.

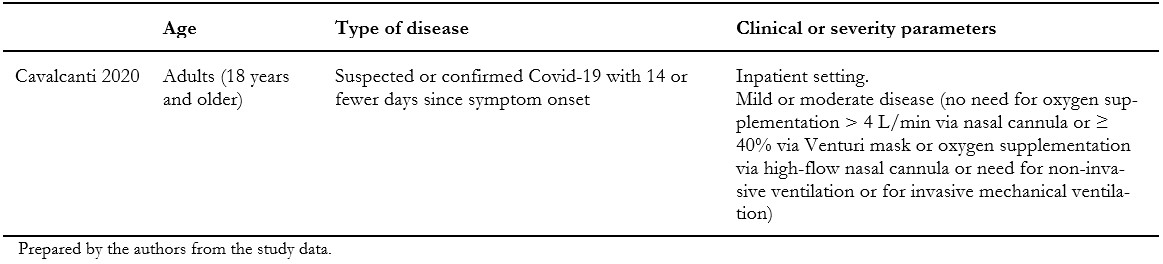 Table 1. Inclusion criteria of the study.
Table 1. Inclusion criteria of the study.

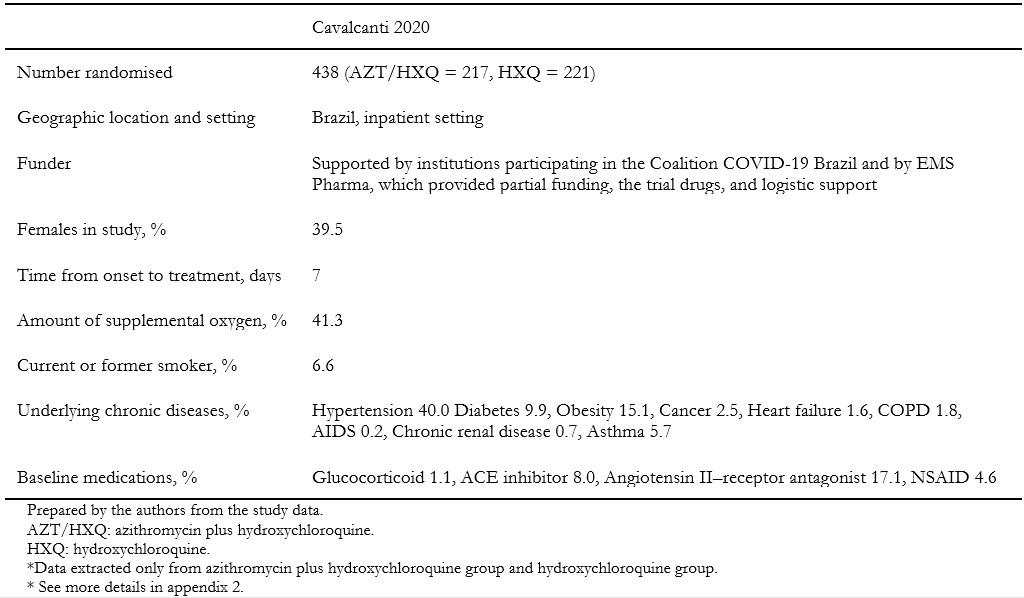 Table 2. Main characteristics of the included study and baseline characteristics of the participants.
Table 2. Main characteristics of the included study and baseline characteristics of the participants.

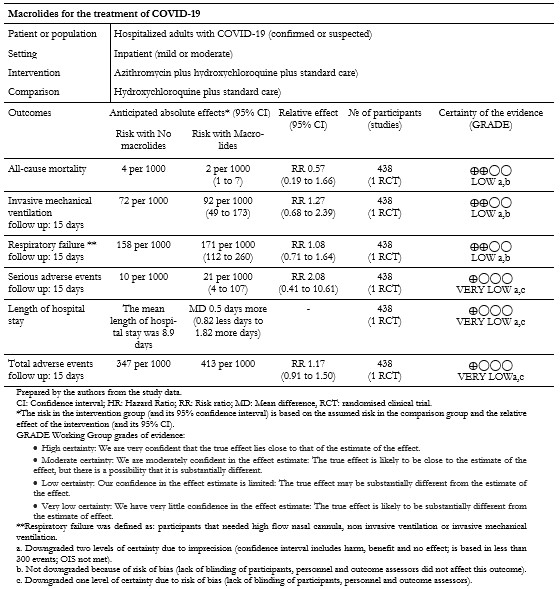 Table 3. Summary of findings of macrolides for the treatment of COVID-19.
Table 3. Summary of findings of macrolides for the treatment of COVID-19.

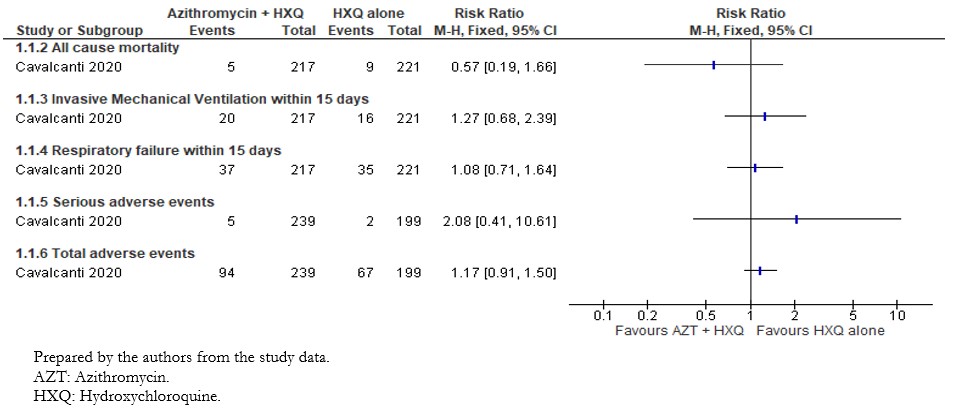 Figure 2. Relative risk for dichotomous outcomes for azithromycin plus hydroxychloroquine versus hydroxychloroquine.
Figure 2. Relative risk for dichotomous outcomes for azithromycin plus hydroxychloroquine versus hydroxychloroquine.

 Figure 3. Mean difference for length of hospital stay for azithromycin plus hydroxychloroquine versus hydroxychloroquine.
Figure 3. Mean difference for length of hospital stay for azithromycin plus hydroxychloroquine versus hydroxychloroquine.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Objetivo
Proporcionar un resumen oportuno, riguroso y continuamente actualizado de la evidencia disponible sobre el papel de los macrólidos para el tratamiento de pacientes con COVID-19.
Diseño
Revisión sistemática viva.
Base de datos
La búsqueda de evidencia se realizó en el repositorio centralizado L·OVE (Living OVerview of Evidence) COVID-19; una plataforma que mapea las preguntas PICO para identificar la evidencia en la base de datos Epistemonikos. En respuesta a la emergencia de COVID-19, L·OVE se adaptó para ampliar el rango de evidencia que cubre y hoy se mantiene a través de búsquedas regulares en 39 bases de datos.
Métodos
Se incluyeron estudios experimentales que evaluaban el efecto de los macrólidos, como monoterapia o en combinación con otros fármacos, versus placebo o ningún tratamiento en pacientes con sospecha o confirmación de COVID-19. Se buscó identificar experimentos clínicos aleatorizados que evaluaran macrólidos en infecciones causadas por otros coronavirus, como MERS-CoV y SARS-CoV. Dos revisores examinaron de forma independiente la elegibilidad de cada estudio, extrajeron los datos y evaluaron el riesgo de sesgo. Se evaluó el efecto de los macrólidos sobre la mortalidad por todas las causas; necesidad de ventilación mecánica invasiva; oxigenación por membrana extracorpórea, duración de la estancia hospitalaria, insuficiencia respiratoria, eventos adversos graves, tiempo hasta la negatividad de la RT-PCR del SARS-CoV-2. La certeza de la evidencia para cada desenlace se evaluó siguiendo la aproximación GRADE. Esta revisión se mantendrá viva y disponible abiertamente durante la pandemia de COVID-19. Se someterán actualizaciones de su publicación cada vez que cambien las conclusiones o cuando haya actualizaciones sustanciales.
Resultados
Se identificó un experimento clínico aleatorio que evaluó el uso de azitromicina en combinación con hidroxicloroquina en comparación con el uso de hidroxicloroquina sola, en pacientes hospitalizados por COVID 19. Las estimaciones para todos los resultados evaluados resultaron en un poder estadístico insuficiente para llegar a conclusiones válidas. La calidad de la evidencia para los resultados principales fue baja a muy baja.
Conclusiones
El uso de macrólidos en el tratamiento de pacientes con COVID 19 no ha mostrado efectos beneficiosos en comparación con el tratamiento estándar. La evidencia para todos los desenlaces no es concluyente. Se necesitan estudios sobre un mayor número de pacientes con COVID 19, para determinar los efectos del uso de macrólidos sobre los desenlaces relacionados con la enfermedad.
Systematic review registration
PROSPERO Registration number: CRD42020181032
Protocol preprint DOI: 10.31219/osf.io/rvp59
 Authors:
Catalina Verdejo[1], Laura Vergara-Merino[2], Nicolás Meza[2], Javier Pérez-Bracchiglione[3], Natalia Carvajal-Juliá[1], Eva Madrid[2], Gabriel Rada[4], María Ximena Rojas Reyes[5]
Authors:
Catalina Verdejo[1], Laura Vergara-Merino[2], Nicolás Meza[2], Javier Pérez-Bracchiglione[3], Natalia Carvajal-Juliá[1], Eva Madrid[2], Gabriel Rada[4], María Ximena Rojas Reyes[5]
Affiliation:
[1] School of Medicine, Cochrane Chile Associated Centre, Universidad de Valparaíso, Chile
[2] Interdisciplinary Centre for Health Studies (CIESAL), Universidad de Valparaíso, Cochrane Chile Associated Centre, Valparaíso, Chile
[3] Interdisciplinary Centre for Health Studies (CIESAL), Universidad de Valparaíso, Cochrane Chile Associated Centre, Viña del Mar, Chile
[4] Fundación Epistemonikos, Santiago, Chile
[5] Department of Research, Fundación Cardioinfantil, Cochrane Colombia Affiliate Centre, Bogotá, Colombia
E-mail: mxrojas@cardioinfantil.org
Author address:
[1] Calle 163A # 13B-60, Bogotá D.C., Colombia

Citation: Verdejo C, Vergara-Merino L, Meza N, Pérez-Bracchiglione J, Carvajal-Juliá N, Madrid E, et al. Macrolides for the treatment of COVID-19: a living, systematic review. Medwave 2020;20(11):e8073 doi: 10.5867/medwave.2020.11.8073
Submission date: 13/8/2020
Acceptance date: 26/10/2020
Publication date: 14/12/2020
Origin: Not commissioned
Type of review: Externally peer-reviewed by three reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- World Health Organization. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. Geneva: WHO; 2020. [On line]. | Link |
- Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020 Feb;91:264-266. | CrossRef | PubMed |
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533-534. | CrossRef | PubMed |
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed |
- Tavakoli A, Vahdat K, Keshavarz M. Novel Coronavirus Disease 2019 (COVID-19): An Emerging Infectious Disease in the 21st Century. ISMJ. 2020;22(6):432-50. | CrossRef |
- Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 Jun;92(6):577-583. | CrossRef | PubMed |
- The Centre for Evidence-Based Medicine. Global Covid-19 Case Fatality Rates. CEBM; 2020. [On line]. | Link |
- Amsden GW. Anti-inflammatory effects of macrolides--an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005 Jan;55(1):10-21. | CrossRef | PubMed |
- Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010 Jul;23(3):590-615. | CrossRef | PubMed |
- Ninomiya K, Fukui T, Imai T, Matsui M, Matsuoka K. Effect of maclorides on duration and resolution of symptoms and complication of pneumonia in children with influenza. J Nippon Med Sch. 2002 Feb;69(1):53-7. | CrossRef | PubMed |
- Kakeya H, Seki M, Izumikawa K, Kosai K, Morinaga Y, Kurihara S, et al. Efficacy of combination therapy with oseltamivir phosphate and azithromycin for influenza: a multicenter, open-label, randomized study. PLoS One. 2014 Mar 14;9(3):e91293. | CrossRef | PubMed |
- Shinahara W, Takahashi E, Sawabuchi T, Arai M, Hirotsu N, Takasaki Y, et al. Immunomodulator clarithromycin enhances mucosal and systemic immune responses and reduces re-infection rate in pediatric patients with influenza treated with antiviral neuraminidase inhibitors: a retrospective analysis. PLoS One. 2013 Jul 17;8(7):e70060. | CrossRef | PubMed |
- Hung IFN, To KKW, Chan JFW, Cheng VCC, Liu KSH, Tam A, et al. Efficacy of Clarithromycin-Naproxen-Oseltamivir Combination in the Treatment of Patients Hospitalized for Influenza A(H3N2) Infection: An Open-label Randomized, Controlled, Phase IIb/III Trial. Chest. 2017 May;151(5):1069-1080. | CrossRef | PubMed |
- Kneyber MC, van Woensel JB, Uijtendaal E, Uiterwaal CS, Kimpen JL; Dutch Antibiotics in RSV Trial (DART) Research Group. Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: a randomized equivalence trial. Pediatr Pulmonol. 2008 Feb;43(2):142-9. | CrossRef | PubMed |
- McCallum GB, Morris PS, Chatfield MD, Maclennan C, White AV, Sloots TP, et al. A single dose of azithromycin does not improve clinical outcomes of children hospitalised with bronchiolitis: a randomised, placebo-controlled trial. PLoS One. 2013 Sep 25;8(9):e74316. | CrossRef | PubMed |
- Pinto LA, Pitrez PM, Luisi F, de Mello PP, Gerhardt M, Ferlini R, et al. Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: a randomized, double-blinded, and placebo-controlled clinical trial. J Pediatr. 2012 Dec;161(6):1104-8. | CrossRef | PubMed |
- Martín-Loeches I, Bermejo-Martin JF, Vallés J, Granada R, Vidaur L, Vergara-Serrano JC, et al. Macrolide-based regimens in absence of bacterial co-infection in critically ill H1N1 patients with primary viral pneumonia. Intensive Care Med. 2013 Apr;39(4):693-702. | CrossRef | PubMed |
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 Jul;56(1):105949. | CrossRef | PubMed |
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020 Mar-Apr;34:101663. | CrossRef | PubMed |
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015 Jan 1;4(1):1. | CrossRef | PubMed |
- Rada G, Verdugo-Paiva F, Ávila C, Morel-Marambio M, Bravo-Jeria R, Pesce F, et al. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020 Apr 1;20(3):e7868. | CrossRef | PubMed |
- Verdejo C, Vergara-Merino L, Carvajal-Juliá N, Meza N, Madrid E, Rada G, et al. Macrolides for the treatment of COVID-19: A living systematic review protocol. OSF Preprints. 2020. | CrossRef |
- GitHub repository. 2020. [On line]. | Link |
- Methods & report of the Special L·OVE of Coronavirus (COVID-19). L·OVE. [On line]. | Link |
- Epistemonikos Foundation. Epistemonikos database methods. Epistemonikos; 2020. [On line]. | Link |
- Jin X, Pang B, Zhang J, Liu Q, Yang Z, Feng J, et al. Core Outcome Set for Clinical Trials on Coronavirus Disease 2019 (COS-COVID). Engineering (Beijing). 2020 Mar 18. | CrossRef | PubMed |
- COVID-19-COS. COVID-19 Core Outcomes. 2020. [On line]. | Link |
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013 Feb;66(2):158-72. | CrossRef | PubMed |
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. | CrossRef | PubMed |
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen. [On line]. | Link |
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr 26;336(7650):924-6. | CrossRef | PubMed |
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013 Feb;66(2):173-83. | CrossRef | PubMed |
- Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020 Nov 19;383(21):2041-2052. | CrossRef | PubMed |
- Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020 May 15. | CrossRef | PubMed |
- Wu R, Wang L, Kuo HD, Shannar A, Peter R, Chou PJ, et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr Pharmacol Rep. 2020 May 11:1-15. | CrossRef | PubMed |
- Ohe M, Shida H, Jodo S, Kusunoki Y, Seki M, Furuya K, et al. Macrolide treatment for COVID-19: Will this be the way forward? Biosci Trends. 2020 May 21;14(2):159-160. | CrossRef | PubMed |
- Min JY, Jang YJ. Macrolide therapy in respiratory viral infections. Mediators Inflamm. 2012;2012:649570. | CrossRef | PubMed |
- Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin Pharmacol Ther. 2020 Aug;108(2):201-211. | CrossRef | PubMed |
- Mégarbane B, Scherrmann JM. Hydroxychloroquine and Azithromycin to Treat Patients With COVID-19: Both Friends and Foes? J Clin Pharmacol. 2020 Jul;60(7):808-814. | CrossRef | PubMed |
- Russo V, Puzio G, Siniscalchi N. Azithromycin-induced QT prolongation in elderly patient. Acta Biomed. 2006 Apr;77(1):30-2. | PubMed |
- Bonaldo G, Andriani LA, D'Annibali O, Motola D, Vaccheri A. Cardiovascular safety of macrolide and fluoroquinolone antibiotics: An analysis of the WHO database of adverse drug reactions. Pharmacoepidemiol Drug Saf. 2019 Nov;28(11):1457-1463. | CrossRef | PubMed |
- Chen CY, Wang FL, Lin CC. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila). 2006;44(2):173-5. | CrossRef | PubMed |
- Stas P, Faes D, Noyens P. Conduction disorder and QT prolongation secondary to long-term treatment with chloroquine. Int J Cardiol. 2008 Jul 4;127(2):e80-2. | CrossRef | PubMed |
- Jean SS, Hsueh PR. Old and re-purposed drugs for the treatment of COVID-19. Expert Rev Anti Infect Ther. 2020 Sep;18(9):843-847. | CrossRef | PubMed |
- Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020 Sep 1;5(9):1036-1041. | CrossRef | PubMed |
- Saleh M, Gabriels J, Chang D, Soo Kim B, Mansoor A, Mahmood E, et al. Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients With SARS-CoV-2 Infection. Circ Arrhythm Electrophysiol. 2020 Jun;13(6):e008662. | CrossRef | PubMed |
- Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv [Preprint]. 2020 Apr 21:2020.04.16.20065920. | CrossRef | PubMed |
- Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA. 2020 Jun 23;323(24):2493-2502. | CrossRef | PubMed |
- Ramireddy A, Chugh H, Reinier K, Ebinger J, Park E, Thompson M, et al. Experience With Hydroxychloroquine and Azithromycin in the Coronavirus Disease 2019 Pandemic: Implications for QT Interval Monitoring. J Am Heart Assoc. 2020 Jun 16;9(12):e017144. | CrossRef | PubMed |
- Sbidian E, Josse J, Lemaitre G, Mayer I, Bernaux M, Gramfort A, et al. Hydroxychloroquine with or without azithromycin and in-hospital mortality or discharge in patients hospitalized for COVID-19 infection: a cohort study of 4,642 in-patients in France. medRxiv. 2020. | CrossRef |
- Bessière F, Roccia H, Delinière A, Charrière R, Chevalier P, Argaud L, et al. Assessment of QT Intervals in a Case Series of Patients With Coronavirus Disease 2019 (COVID-19) Infection Treated With Hydroxychloroquine Alone or in Combination With Azithromycin in an Intensive Care Unit. JAMA Cardiol. 2020 Sep 1;5(9):1067-1069. | CrossRef | PubMed |
- Lagier JC, Million M, Gautret P, Colson P, Cortaredona S, Giraud-Gatineau A, et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med Infect Dis. 2020 Jul-Aug;36:101791. | CrossRef | PubMed |
- Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020 Aug;97:396-403. | CrossRef | PubMed |
- Çalik BaŞaran N, Uyaroğlu OA, Telli Dizman G, Özişik L, Şahin TK, Taş Z, et al. Outcome of Non-Critical COVID-19 Patients with Early Hospitalization and Early Antiviral Treatment Outside the ICU. Turk J Med Sci. 2020 Jul 28. | CrossRef | PubMed |
- Tuncer T, Karaci M, Boga A, Durmaz H, Guven S. QT interval evaluation associated with the use of hydroxychloroquine with combined use of azithromycin among hospitalised children positive for coronavirus disease 2019. Cardiol Young. 2020 Oct;30(10):1482-1485. | CrossRef | PubMed |
- Kelly M, O'Connor R, Townsend L, Coghlan M, Relihan E, Moriarty M, et al. Clinical outcomes and adverse events in patients hospitalised with COVID-19, treated with off-label hydroxychloroquine and azithromycin. Br J Clin Pharmacol. 2020 Jul 20. | CrossRef | PubMed |
- Michaud V, Dow P, Al Rihani SB, Deodhar M, Arwood M, Cicali B, et al. Risk Assessment of Drug-Induced Long QT Syndrome for Some COVID-19 Repurposed Drugs. Clin Transl Sci. 2020 Sep 5. | CrossRef | PubMed |
- Yang TH, Chou CY, Yang YF, Chien CS, Yarmishyn AA, Yang TY, et al. Systematic Review and Meta-analysis of the Effectiveness and Safety of Hydroxychloroquine in Treating COVID-19 Patients. J Chin Med Assoc. 2020 Sep 15. | CrossRef | PubMed |
- Gbinigie K, Frie K. Should azithromycin be used to treat COVID-19? A rapid review. BJGP Open. 2020 Jun 23;4(2):bjgpopen20X101094. | CrossRef | PubMed |
- Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020 Jul 30;370:m2980. | CrossRef | PubMed |
- Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020 Apr;26(4):147-148. | CrossRef | PubMed |
- Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al. Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol. 2017 Nov;91:23-30. | CrossRef | PubMed |
 World Health Organization. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. Geneva: WHO; 2020. [On line]. | Link |
World Health Organization. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. Geneva: WHO; 2020. [On line]. | Link | Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020 Feb;91:264-266. | CrossRef | PubMed |
Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020 Feb;91:264-266. | CrossRef | PubMed | Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533-534. | CrossRef | PubMed |
Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533-534. | CrossRef | PubMed | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed |
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed | Tavakoli A, Vahdat K, Keshavarz M. Novel Coronavirus Disease 2019 (COVID-19): An Emerging Infectious Disease in the 21st Century. ISMJ. 2020;22(6):432-50. | CrossRef |
Tavakoli A, Vahdat K, Keshavarz M. Novel Coronavirus Disease 2019 (COVID-19): An Emerging Infectious Disease in the 21st Century. ISMJ. 2020;22(6):432-50. | CrossRef | Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 Jun;92(6):577-583. | CrossRef | PubMed |
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 Jun;92(6):577-583. | CrossRef | PubMed | The Centre for Evidence-Based Medicine. Global Covid-19 Case Fatality Rates. CEBM; 2020. [On line]. | Link |
The Centre for Evidence-Based Medicine. Global Covid-19 Case Fatality Rates. CEBM; 2020. [On line]. | Link | Amsden GW. Anti-inflammatory effects of macrolides--an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005 Jan;55(1):10-21. | CrossRef | PubMed |
Amsden GW. Anti-inflammatory effects of macrolides--an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005 Jan;55(1):10-21. | CrossRef | PubMed | Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010 Jul;23(3):590-615. | CrossRef | PubMed |
Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010 Jul;23(3):590-615. | CrossRef | PubMed | Ninomiya K, Fukui T, Imai T, Matsui M, Matsuoka K. Effect of maclorides on duration and resolution of symptoms and complication of pneumonia in children with influenza. J Nippon Med Sch. 2002 Feb;69(1):53-7. | CrossRef | PubMed |
Ninomiya K, Fukui T, Imai T, Matsui M, Matsuoka K. Effect of maclorides on duration and resolution of symptoms and complication of pneumonia in children with influenza. J Nippon Med Sch. 2002 Feb;69(1):53-7. | CrossRef | PubMed | Kakeya H, Seki M, Izumikawa K, Kosai K, Morinaga Y, Kurihara S, et al. Efficacy of combination therapy with oseltamivir phosphate and azithromycin for influenza: a multicenter, open-label, randomized study. PLoS One. 2014 Mar 14;9(3):e91293. | CrossRef | PubMed |
Kakeya H, Seki M, Izumikawa K, Kosai K, Morinaga Y, Kurihara S, et al. Efficacy of combination therapy with oseltamivir phosphate and azithromycin for influenza: a multicenter, open-label, randomized study. PLoS One. 2014 Mar 14;9(3):e91293. | CrossRef | PubMed | Shinahara W, Takahashi E, Sawabuchi T, Arai M, Hirotsu N, Takasaki Y, et al. Immunomodulator clarithromycin enhances mucosal and systemic immune responses and reduces re-infection rate in pediatric patients with influenza treated with antiviral neuraminidase inhibitors: a retrospective analysis. PLoS One. 2013 Jul 17;8(7):e70060. | CrossRef | PubMed |
Shinahara W, Takahashi E, Sawabuchi T, Arai M, Hirotsu N, Takasaki Y, et al. Immunomodulator clarithromycin enhances mucosal and systemic immune responses and reduces re-infection rate in pediatric patients with influenza treated with antiviral neuraminidase inhibitors: a retrospective analysis. PLoS One. 2013 Jul 17;8(7):e70060. | CrossRef | PubMed | Hung IFN, To KKW, Chan JFW, Cheng VCC, Liu KSH, Tam A, et al. Efficacy of Clarithromycin-Naproxen-Oseltamivir Combination in the Treatment of Patients Hospitalized for Influenza A(H3N2) Infection: An Open-label Randomized, Controlled, Phase IIb/III Trial. Chest. 2017 May;151(5):1069-1080. | CrossRef | PubMed |
Hung IFN, To KKW, Chan JFW, Cheng VCC, Liu KSH, Tam A, et al. Efficacy of Clarithromycin-Naproxen-Oseltamivir Combination in the Treatment of Patients Hospitalized for Influenza A(H3N2) Infection: An Open-label Randomized, Controlled, Phase IIb/III Trial. Chest. 2017 May;151(5):1069-1080. | CrossRef | PubMed | Kneyber MC, van Woensel JB, Uijtendaal E, Uiterwaal CS, Kimpen JL; Dutch Antibiotics in RSV Trial (DART) Research Group. Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: a randomized equivalence trial. Pediatr Pulmonol. 2008 Feb;43(2):142-9. | CrossRef | PubMed |
Kneyber MC, van Woensel JB, Uijtendaal E, Uiterwaal CS, Kimpen JL; Dutch Antibiotics in RSV Trial (DART) Research Group. Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: a randomized equivalence trial. Pediatr Pulmonol. 2008 Feb;43(2):142-9. | CrossRef | PubMed | McCallum GB, Morris PS, Chatfield MD, Maclennan C, White AV, Sloots TP, et al. A single dose of azithromycin does not improve clinical outcomes of children hospitalised with bronchiolitis: a randomised, placebo-controlled trial. PLoS One. 2013 Sep 25;8(9):e74316. | CrossRef | PubMed |
McCallum GB, Morris PS, Chatfield MD, Maclennan C, White AV, Sloots TP, et al. A single dose of azithromycin does not improve clinical outcomes of children hospitalised with bronchiolitis: a randomised, placebo-controlled trial. PLoS One. 2013 Sep 25;8(9):e74316. | CrossRef | PubMed | Pinto LA, Pitrez PM, Luisi F, de Mello PP, Gerhardt M, Ferlini R, et al. Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: a randomized, double-blinded, and placebo-controlled clinical trial. J Pediatr. 2012 Dec;161(6):1104-8. | CrossRef | PubMed |
Pinto LA, Pitrez PM, Luisi F, de Mello PP, Gerhardt M, Ferlini R, et al. Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: a randomized, double-blinded, and placebo-controlled clinical trial. J Pediatr. 2012 Dec;161(6):1104-8. | CrossRef | PubMed | Martín-Loeches I, Bermejo-Martin JF, Vallés J, Granada R, Vidaur L, Vergara-Serrano JC, et al. Macrolide-based regimens in absence of bacterial co-infection in critically ill H1N1 patients with primary viral pneumonia. Intensive Care Med. 2013 Apr;39(4):693-702. | CrossRef | PubMed |
Martín-Loeches I, Bermejo-Martin JF, Vallés J, Granada R, Vidaur L, Vergara-Serrano JC, et al. Macrolide-based regimens in absence of bacterial co-infection in critically ill H1N1 patients with primary viral pneumonia. Intensive Care Med. 2013 Apr;39(4):693-702. | CrossRef | PubMed | Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 Jul;56(1):105949. | CrossRef | PubMed |
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 Jul;56(1):105949. | CrossRef | PubMed | Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020 Mar-Apr;34:101663. | CrossRef | PubMed |
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020 Mar-Apr;34:101663. | CrossRef | PubMed | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015 Jan 1;4(1):1. | CrossRef | PubMed |
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015 Jan 1;4(1):1. | CrossRef | PubMed | Rada G, Verdugo-Paiva F, Ávila C, Morel-Marambio M, Bravo-Jeria R, Pesce F, et al. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020 Apr 1;20(3):e7868. | CrossRef | PubMed |
Rada G, Verdugo-Paiva F, Ávila C, Morel-Marambio M, Bravo-Jeria R, Pesce F, et al. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020 Apr 1;20(3):e7868. | CrossRef | PubMed | Verdejo C, Vergara-Merino L, Carvajal-Juliá N, Meza N, Madrid E, Rada G, et al. Macrolides for the treatment of COVID-19: A living systematic review protocol. OSF Preprints. 2020. | CrossRef |
Verdejo C, Vergara-Merino L, Carvajal-Juliá N, Meza N, Madrid E, Rada G, et al. Macrolides for the treatment of COVID-19: A living systematic review protocol. OSF Preprints. 2020. | CrossRef | Jin X, Pang B, Zhang J, Liu Q, Yang Z, Feng J, et al. Core Outcome Set for Clinical Trials on Coronavirus Disease 2019 (COS-COVID). Engineering (Beijing). 2020 Mar 18. | CrossRef | PubMed |
Jin X, Pang B, Zhang J, Liu Q, Yang Z, Feng J, et al. Core Outcome Set for Clinical Trials on Coronavirus Disease 2019 (COS-COVID). Engineering (Beijing). 2020 Mar 18. | CrossRef | PubMed | Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013 Feb;66(2):158-72. | CrossRef | PubMed |
Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013 Feb;66(2):158-72. | CrossRef | PubMed | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. | CrossRef | PubMed |
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. | CrossRef | PubMed | The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen. [On line]. | Link |
The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen. [On line]. | Link | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr 26;336(7650):924-6. | CrossRef | PubMed |
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr 26;336(7650):924-6. | CrossRef | PubMed | Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013 Feb;66(2):173-83. | CrossRef | PubMed |
Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013 Feb;66(2):173-83. | CrossRef | PubMed | Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020 Nov 19;383(21):2041-2052. | CrossRef | PubMed |
Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020 Nov 19;383(21):2041-2052. | CrossRef | PubMed | Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020 May 15. | CrossRef | PubMed |
Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020 May 15. | CrossRef | PubMed | Wu R, Wang L, Kuo HD, Shannar A, Peter R, Chou PJ, et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr Pharmacol Rep. 2020 May 11:1-15. | CrossRef | PubMed |
Wu R, Wang L, Kuo HD, Shannar A, Peter R, Chou PJ, et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr Pharmacol Rep. 2020 May 11:1-15. | CrossRef | PubMed | Ohe M, Shida H, Jodo S, Kusunoki Y, Seki M, Furuya K, et al. Macrolide treatment for COVID-19: Will this be the way forward? Biosci Trends. 2020 May 21;14(2):159-160. | CrossRef | PubMed |
Ohe M, Shida H, Jodo S, Kusunoki Y, Seki M, Furuya K, et al. Macrolide treatment for COVID-19: Will this be the way forward? Biosci Trends. 2020 May 21;14(2):159-160. | CrossRef | PubMed | Min JY, Jang YJ. Macrolide therapy in respiratory viral infections. Mediators Inflamm. 2012;2012:649570. | CrossRef | PubMed |
Min JY, Jang YJ. Macrolide therapy in respiratory viral infections. Mediators Inflamm. 2012;2012:649570. | CrossRef | PubMed | Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin Pharmacol Ther. 2020 Aug;108(2):201-211. | CrossRef | PubMed |
Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin Pharmacol Ther. 2020 Aug;108(2):201-211. | CrossRef | PubMed | Mégarbane B, Scherrmann JM. Hydroxychloroquine and Azithromycin to Treat Patients With COVID-19: Both Friends and Foes? J Clin Pharmacol. 2020 Jul;60(7):808-814. | CrossRef | PubMed |
Mégarbane B, Scherrmann JM. Hydroxychloroquine and Azithromycin to Treat Patients With COVID-19: Both Friends and Foes? J Clin Pharmacol. 2020 Jul;60(7):808-814. | CrossRef | PubMed | Russo V, Puzio G, Siniscalchi N. Azithromycin-induced QT prolongation in elderly patient. Acta Biomed. 2006 Apr;77(1):30-2. | PubMed |
Russo V, Puzio G, Siniscalchi N. Azithromycin-induced QT prolongation in elderly patient. Acta Biomed. 2006 Apr;77(1):30-2. | PubMed | Bonaldo G, Andriani LA, D'Annibali O, Motola D, Vaccheri A. Cardiovascular safety of macrolide and fluoroquinolone antibiotics: An analysis of the WHO database of adverse drug reactions. Pharmacoepidemiol Drug Saf. 2019 Nov;28(11):1457-1463. | CrossRef | PubMed |
Bonaldo G, Andriani LA, D'Annibali O, Motola D, Vaccheri A. Cardiovascular safety of macrolide and fluoroquinolone antibiotics: An analysis of the WHO database of adverse drug reactions. Pharmacoepidemiol Drug Saf. 2019 Nov;28(11):1457-1463. | CrossRef | PubMed | Chen CY, Wang FL, Lin CC. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila). 2006;44(2):173-5. | CrossRef | PubMed |
Chen CY, Wang FL, Lin CC. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila). 2006;44(2):173-5. | CrossRef | PubMed | Stas P, Faes D, Noyens P. Conduction disorder and QT prolongation secondary to long-term treatment with chloroquine. Int J Cardiol. 2008 Jul 4;127(2):e80-2. | CrossRef | PubMed |
Stas P, Faes D, Noyens P. Conduction disorder and QT prolongation secondary to long-term treatment with chloroquine. Int J Cardiol. 2008 Jul 4;127(2):e80-2. | CrossRef | PubMed | Jean SS, Hsueh PR. Old and re-purposed drugs for the treatment of COVID-19. Expert Rev Anti Infect Ther. 2020 Sep;18(9):843-847. | CrossRef | PubMed |
Jean SS, Hsueh PR. Old and re-purposed drugs for the treatment of COVID-19. Expert Rev Anti Infect Ther. 2020 Sep;18(9):843-847. | CrossRef | PubMed | Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020 Sep 1;5(9):1036-1041. | CrossRef | PubMed |
Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020 Sep 1;5(9):1036-1041. | CrossRef | PubMed | Saleh M, Gabriels J, Chang D, Soo Kim B, Mansoor A, Mahmood E, et al. Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients With SARS-CoV-2 Infection. Circ Arrhythm Electrophysiol. 2020 Jun;13(6):e008662. | CrossRef | PubMed |
Saleh M, Gabriels J, Chang D, Soo Kim B, Mansoor A, Mahmood E, et al. Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients With SARS-CoV-2 Infection. Circ Arrhythm Electrophysiol. 2020 Jun;13(6):e008662. | CrossRef | PubMed | Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv [Preprint]. 2020 Apr 21:2020.04.16.20065920. | CrossRef | PubMed |
Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv [Preprint]. 2020 Apr 21:2020.04.16.20065920. | CrossRef | PubMed | Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA. 2020 Jun 23;323(24):2493-2502. | CrossRef | PubMed |
Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA. 2020 Jun 23;323(24):2493-2502. | CrossRef | PubMed | Ramireddy A, Chugh H, Reinier K, Ebinger J, Park E, Thompson M, et al. Experience With Hydroxychloroquine and Azithromycin in the Coronavirus Disease 2019 Pandemic: Implications for QT Interval Monitoring. J Am Heart Assoc. 2020 Jun 16;9(12):e017144. | CrossRef | PubMed |
Ramireddy A, Chugh H, Reinier K, Ebinger J, Park E, Thompson M, et al. Experience With Hydroxychloroquine and Azithromycin in the Coronavirus Disease 2019 Pandemic: Implications for QT Interval Monitoring. J Am Heart Assoc. 2020 Jun 16;9(12):e017144. | CrossRef | PubMed | Sbidian E, Josse J, Lemaitre G, Mayer I, Bernaux M, Gramfort A, et al. Hydroxychloroquine with or without azithromycin and in-hospital mortality or discharge in patients hospitalized for COVID-19 infection: a cohort study of 4,642 in-patients in France. medRxiv. 2020. | CrossRef |
Sbidian E, Josse J, Lemaitre G, Mayer I, Bernaux M, Gramfort A, et al. Hydroxychloroquine with or without azithromycin and in-hospital mortality or discharge in patients hospitalized for COVID-19 infection: a cohort study of 4,642 in-patients in France. medRxiv. 2020. | CrossRef | Bessière F, Roccia H, Delinière A, Charrière R, Chevalier P, Argaud L, et al. Assessment of QT Intervals in a Case Series of Patients With Coronavirus Disease 2019 (COVID-19) Infection Treated With Hydroxychloroquine Alone or in Combination With Azithromycin in an Intensive Care Unit. JAMA Cardiol. 2020 Sep 1;5(9):1067-1069. | CrossRef | PubMed |
Bessière F, Roccia H, Delinière A, Charrière R, Chevalier P, Argaud L, et al. Assessment of QT Intervals in a Case Series of Patients With Coronavirus Disease 2019 (COVID-19) Infection Treated With Hydroxychloroquine Alone or in Combination With Azithromycin in an Intensive Care Unit. JAMA Cardiol. 2020 Sep 1;5(9):1067-1069. | CrossRef | PubMed | Lagier JC, Million M, Gautret P, Colson P, Cortaredona S, Giraud-Gatineau A, et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med Infect Dis. 2020 Jul-Aug;36:101791. | CrossRef | PubMed |
Lagier JC, Million M, Gautret P, Colson P, Cortaredona S, Giraud-Gatineau A, et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med Infect Dis. 2020 Jul-Aug;36:101791. | CrossRef | PubMed | Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020 Aug;97:396-403. | CrossRef | PubMed |
Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020 Aug;97:396-403. | CrossRef | PubMed | Çalik BaŞaran N, Uyaroğlu OA, Telli Dizman G, Özişik L, Şahin TK, Taş Z, et al. Outcome of Non-Critical COVID-19 Patients with Early Hospitalization and Early Antiviral Treatment Outside the ICU. Turk J Med Sci. 2020 Jul 28. | CrossRef | PubMed |
Çalik BaŞaran N, Uyaroğlu OA, Telli Dizman G, Özişik L, Şahin TK, Taş Z, et al. Outcome of Non-Critical COVID-19 Patients with Early Hospitalization and Early Antiviral Treatment Outside the ICU. Turk J Med Sci. 2020 Jul 28. | CrossRef | PubMed | Tuncer T, Karaci M, Boga A, Durmaz H, Guven S. QT interval evaluation associated with the use of hydroxychloroquine with combined use of azithromycin among hospitalised children positive for coronavirus disease 2019. Cardiol Young. 2020 Oct;30(10):1482-1485. | CrossRef | PubMed |
Tuncer T, Karaci M, Boga A, Durmaz H, Guven S. QT interval evaluation associated with the use of hydroxychloroquine with combined use of azithromycin among hospitalised children positive for coronavirus disease 2019. Cardiol Young. 2020 Oct;30(10):1482-1485. | CrossRef | PubMed | Kelly M, O'Connor R, Townsend L, Coghlan M, Relihan E, Moriarty M, et al. Clinical outcomes and adverse events in patients hospitalised with COVID-19, treated with off-label hydroxychloroquine and azithromycin. Br J Clin Pharmacol. 2020 Jul 20. | CrossRef | PubMed |
Kelly M, O'Connor R, Townsend L, Coghlan M, Relihan E, Moriarty M, et al. Clinical outcomes and adverse events in patients hospitalised with COVID-19, treated with off-label hydroxychloroquine and azithromycin. Br J Clin Pharmacol. 2020 Jul 20. | CrossRef | PubMed | Michaud V, Dow P, Al Rihani SB, Deodhar M, Arwood M, Cicali B, et al. Risk Assessment of Drug-Induced Long QT Syndrome for Some COVID-19 Repurposed Drugs. Clin Transl Sci. 2020 Sep 5. | CrossRef | PubMed |
Michaud V, Dow P, Al Rihani SB, Deodhar M, Arwood M, Cicali B, et al. Risk Assessment of Drug-Induced Long QT Syndrome for Some COVID-19 Repurposed Drugs. Clin Transl Sci. 2020 Sep 5. | CrossRef | PubMed | Yang TH, Chou CY, Yang YF, Chien CS, Yarmishyn AA, Yang TY, et al. Systematic Review and Meta-analysis of the Effectiveness and Safety of Hydroxychloroquine in Treating COVID-19 Patients. J Chin Med Assoc. 2020 Sep 15. | CrossRef | PubMed |
Yang TH, Chou CY, Yang YF, Chien CS, Yarmishyn AA, Yang TY, et al. Systematic Review and Meta-analysis of the Effectiveness and Safety of Hydroxychloroquine in Treating COVID-19 Patients. J Chin Med Assoc. 2020 Sep 15. | CrossRef | PubMed | Gbinigie K, Frie K. Should azithromycin be used to treat COVID-19? A rapid review. BJGP Open. 2020 Jun 23;4(2):bjgpopen20X101094. | CrossRef | PubMed |
Gbinigie K, Frie K. Should azithromycin be used to treat COVID-19? A rapid review. BJGP Open. 2020 Jun 23;4(2):bjgpopen20X101094. | CrossRef | PubMed | Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020 Jul 30;370:m2980. | CrossRef | PubMed |
Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020 Jul 30;370:m2980. | CrossRef | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








