Key Words: deep endometriosis, surgical management, endometriosis unit, surgical outcomes,
Abstract
Background
Laparoscopy has become the standard of care in the surgical management of deep infiltrating endometriosis (DIE). However, it is a challenging procedure with a high complication rate. Despite the benefits of the minimally invasive approach, DIE resection is often performed by surgeons without adequate training, especially in developing countries like Chile.
Objective
To assess our experience in the diagnosis and laparoscopic management of DIE for seven years.
Methods
We conducted a retrospective cohort study of data for 137 patients with pathology-proven DIE. We evaluated surgical and fertility outcomes.
Results
All procedures were performed laparoscopically without conversion. Dysmenorrhea and dyspareunia were the most common symptoms in 85.4% and 56.9%, respectively. Uterosacral ligaments were the most common DIE location. Endometrioma was present in 48.9% of cases. Median operative time was 140 minutes; however, it was longer in cases requiring bowel surgery (p < 0.0001). The complica-tion rate was 10.9%. Median follow-up was 24.5 months. The pregnancy rate was 58.1%, and 90% of patients reported significant symptom relief after surgery.
Conclusion
Laparoscopic surgical management of DIE is effective and safe, but it must be performed in tertiary centers with the availability of mul-tidisciplinary teams.
|
Main messages
|
Introduction
Endometriosis is the presence of functional endometrial tissue (glands and stroma) outside the uterine cavity[1] and is considered “deep infiltrating” when it affects ≥ 5 mm of retroperitoneal space[2]. While clinical manifestations can vary, DIE should be suspected in patients with severe refractory dysmenorrhea, deep dyspareunia, and catamenial dyschezia[3]. Medical treatment, while effective in some cases[4], has only a transient effect, and aggressive surgical treatment is often required to obtain long-term symptom relief[5]. However, due to the extensive anatomic distortion and fibrosis characteristic of the disease, surgical resection of endometriosis is complex and associated with a high rate of complications, even when performed by experts[6]. This study aimed to report our experiences with diagnosis and surgical management of patients with deep endometriosis from 2010 to 2017.
Methods
We retrospectively reviewed information prospectively collected in our database on patients undergoing surgery for diagnosed cases of DIE between 2010 and 2017. Our health facility has become a reference center for patients with endometriosis, especially for cases of DIE. Of all patients receiving consultations for pelvic pain or suspicion of endometriosis, approximately 30% received diagnostic confirmation of the disease, and one-third of the cases were DIE.
Of all patients diagnosed with DIE, approximately 50% end up having surgery, which results in a total of 50 to 80 patients being treated surgically for DIE each year. In this study, only patients with histological confirmation of DIE were included. We collected data on patient demographics, time to diagnosis, previous surgeries for endometriosis, surgical indication, preoperative workup, intraoperative findings, operating time, and complications. Our clinical approach to patients with suspected DIE is shown in Figure 1.
In patients that wanted to become pregnant, we performed a comprehensive evaluation of symptoms, personal goals, and ovarian reserve before deciding between primary surgery and the use of assisted reproduction techniques. In patients with intestinal involvement, the surgical strategy was discussed with the patient and coloproctologist. In patients with suspicion or diagnosis of endometriosis of the urinary tract, a preoperative evaluation was performed with the urologist.
Figure 1. Clinical management of endometriosis at Clínica Santa María.
In symptomatic patients with a history of previous surgeries due to endometriosis, completed parity, and/or age of > 40 years who had concomitant adenomyosis, in addition to resection of the infiltrating disease, hysterectomy was proposed. History of laparotomies and/or previous surgery was not considered a contraindication for the laparoscopic approach. Routine bowel preparation was performed in all patients, and a signed informed consent form explaining the risks and potential complications/sequelae associated with the procedure was obtained prior to surgery. Before any data collection, authorization for the study was obtained from the institutional ethics committee.
All patients were operated under general anesthesia and examined under anesthesia by two operators to confirm preoperative findings. The patients were placed in a gynecological position (to allow for intraoperative vaginal access) with the arms alongside the body. Foley catheter placement and uterine manipulation were performed systematically. The latter procedure is essential for adequate exposure of the posterior fornix. The laparoscopic procedure was carried out according to basic principles for safety and ergonomics in gynecologic laparoscopy, using a 10 mm optical umbilical trocar (for the zero-degree laparoscope) and three 5 mm auxiliary trocars, installed under direct vision[7].
The surgical technique used in the management of DIE at our institution has been described previously[8]. In general, after adherenciolysis, DIE lesions (nodules) are identified and isolated through blunt dissection of the avascular spaces of the pelvis. In cases where the uterosacral ligaments were compromised, identification with lateralization of the uterus (ureterolysis) are performed systematically. In the case of vaginal involvement, vaginal resection, and dissection of the rectovaginal septum are performed.
In patients with bowel endometriosis who had a single infiltrating lesion < 30 mm in diameter and involvement of < 40% of the bowel circumference, conservative surgery was used (“rectal shaving”—separation of the nodule from the rectal wall—or discoid excision). In patients with multiple intestinal lesions, stenosis, or involvement of 40% or more of the bowel circumference, segmental bowel resection was performed[9]. In the case of ultra-low rectal anastomosis (< 6 cm from the anal margin), a protective ileostomy was performed. Starting in 2012, the reverse surgical technique[8] was used, if preferred by the surgeon. All patients were staged according to the revised American Fertility Society (rAFS) classification of endometriosis[10]. Complications were described according to Clavien–Dindo criteria[11].
Postoperative follow-up was done by telephone. Patients were asked to select one of the following choices to describe their level of pain post-surgery versus pre-surgery: “no more pain,” “significant improvement,” “slight improvement,” “no improvement,” and “worse than before.” Patients who had previously expressed the desire to be pregnant were asked if they got pregnant.
Categorical variables were expressed in frequencies (percentages). The Shapiro–Wilk test was used to test for the presence of normal distribution in quantitative variables. Normal distribution variables were expressed as means (± standard deviation). Nonparametric variables were expressed as medians (ranges) and compared with the Wilcoxon–Mann–Whitney test. The chances of recurrence were estimated with the Kaplan–Meier method. Statistical analysis was performed using SPSS statistical software, version 20.0 (IBM Corp., Armonk, NY, USA). Statistical significance was established as p < 0.05.
Results
During the study period (January 2010 to December 2017), a total of 137 patients with histological confirmation of deep endometriosis were operated in our institution. All surgeries were completed by laparoscopy, without conversion. The mean age was 34 ± 5.8 years and 90 patients (65.7%) were nulliparous. Median time from the beginning of symptoms to surgery was 24 months (range: 2 to 210). Thirty-one patients (22.6%) had a history of at least one previous surgery for endometriosis, and three of them had had a hysterectomy. Symptoms and surgical indications and preoperative workups are summarized in Tables 1 and 2 respectively. The median follow-up was 24.5 months (range: 0.3 to 99.4). Forty-three women were able to get pregnant during follow-up, which is equivalent to 58.1% of the patients who had expressed a desire for pregnancy before surgery. Of these, 22 (51.2%) did so spontaneously, 10 (23.3%) underwent intrauterine insemination, and 11 (23.3%) underwent in vitro fertilization. The median time to achieve pregnancy was 9.9 months (range: 2.2 to 31.6).
Table 1. Symptoms and surgical indications (n = 137)*.
The most frequent locations of deep endometriosis were the uterosacral ligaments and the uterine torus, and there was at least one concurrent ovarian endometrioma in 48.9% of cases (Table 3).
The surgical procedures are shown in Table 4. The median operating time was 140 minutes (range: 50 to 463). The operating times for those needing bowel procedures were significantly longer (187 minutes; range: 68 to 463) compared to those who did not (125 minutes; range: 50 to 357) (p < 0.0001)).
Table 3. Anatomic location of DIEa.
Table 4. Surgical data and proceduresa.
Fifteen patients (10.9%) presented perioperative complications (Table 5). Surgical outcomes in terms of pelvic pain, dysmenorrhea, dyspareunia, and dyschezia are shown in Table 4. The probability of recurrence one, two, and three years post-surgery is shown in Figure 2.
Figure 2. Probability of recurrence at 1, 2, and 3 years.
Table 5. Complications by Clavien–Dindo grade
Discussion
The clinical approach to patients with deep endometriosis is complex. Diagnosis requires a high degree of suspicion, and there is usually a delay of several years in reaching a definitive diagnosis[12]. Therefore, initial surgical management is frequently performed by gynecologists without adequate training in advanced laparoscopy, leading to partial resections, symptom persistence, and reoperations. In our study, time to diagnosis was considerably shorter than what has been reported in other studies[12]. We believe that is due to the creation of a specialized endometriosis unit at our institution and the use of a clinical management protocol that allows for timely referral to a specialist (Figure 1).
In this study, 23% of the patients had had at least one previous surgery for endometriosis, and three had undergone a hysterectomy. Given that endometriosis is currently considered a non-recurring and non-progressive disease, this level of “recurrence” is mostly due to previous partial resections and is, therefore, more accurately described as persistence; also, this disease tends to recur in the same place[2],[13]; we also question the belief that hysterectomy is always the definitive treatment for this disease[14]. Therefore, per Fedele et al.[15], we recommend the resection of all deep lesions at the time of surgery, even if a hysterectomy will be performed (Figure 3).
To date, there is consensus that the only indication for surgery in deep endometriosis is the presence of symptoms, and that risky surgery for an asymptomatic patient makes no sense, regardless of the extent of the disease. In this study, as in other studies[16], dysmenorrheal was the most frequent symptom, regardless of the location of the endometriosis. However, it is remarkable that most of the patients presented more than one symptom. Although 30% of our patients were infertile, it should be noted that the role of surgery in treatment for infertility in patients with deep endometriosis is controversial[17],[18]; at our center, infertility alone is not considered an indication for surgery.
Figure 3. Surgical specimen from a radical hysterectomy in a patient with deep endometriosis.
In a patient who is a candidate for surgery, a comprehensive preoperative workup is essential, as it reveals the extent of the disease and thus allows for more informed planning/decision-making, as well as the opportunity to inform the patient about the implications and potential complications of the surgery. At the time of this study, imaging to assess the extent of deep endometriosis (extended transvaginal ultrasound or magnetic resonance imaging) was only carried out in 50% of the patients. However, currently, this type of imaging is carried out in 80% of patients, probably due to a higher index of suspicion by the gynecologists of our institution and the timely referral of patients to the endometriosis unit.
In the cases covered in our study, the most frequent locations for deep endometriosis were the uterosacral ligaments and the uterine torus, matching what has been reported in the literature[19]. It should be noted that in 48.9% of our cases, there was an associated endometrioma. This finding is important because it confirms that endometriomas are reliable “markers” of disease severity, with infiltrating lesions reported in up to 90% of cases[20]. In addition, given the multiple methods for surgical management of them (e.g., cystectomy, drainage, and fulguration), their presence leads to surgery decisions based on patient goals, determined according to various factors (e.g., patient age, desire for future parity, risk of recurrence, and ovarian reserve).
Although cystectomy is currently the recommended treatment for ovarian endometriosis, because it minimizes recurrence, the impact of this procedure on the ovarian reserve is significantly higher than drainage and fulguration[21]. At our institution, since 2014, for every patient with endometriomas and a desire for future fertility, the ovarian reserve was estimated presurgery, using Anti-Müllerian hormone (AMH), due to the potential for damage to it during surgery, which can be even worse in the case of reinterventions[22]. In these cases, we believe the risk of reintervention (in the case of symptomatic recurrence) is preferable to the risk of postoperative ovarian failure in patients with reproductive desire.
Regarding surgical procedures, we believe it is essential to insist on proper presurgery planning and coordination of a multidisciplinary team with laparoscopic training (gynecologist, urologist, coloproctologist) in order to achieve proper management and follow-up in these patients. Due to the deep location of the endometriotic nodules in the pelvis, careful identification and dissection of the ureter (ureterolysis) are often necessary, especially in nodules that involve the uterosacral ligaments[23]. Therefore, the gynecologist who operates on endometriosis should be familiar with this procedure laparoscopically[23],[24].
If intrinsic ureteral involvement is detected, the team must be prepared for ureteral reimplantation[24],[25]. Preplanning for this outcome is important because the diagnosis of intrinsic ureteral endometriosis often occurs during surgery when it is not possible to release the ureter from the uterosacral–cardinal complex with the initial ureterolysis. Although this situation is infrequent, at our center, it is considered an indication for intraoperative examination by a urologist. In the same way, in patients in whom extensive ureterolysis has been necessary, or in cases of bladder endometriosis, we ask the urologist for the prophylactic installation of ureteral stents.
In those cases with symptomatic intestinal involvement, resection of the intestinal lesions should be attempted. This can be done using rectal shaving, discoid resection, or segmental bowel resection[26]. In terms of symptomatic relief and functional sequelae, there is no consensus on the best procedure for treating intestinal endometriosis, so this decision should be made on a case-by-case, intraoperative basis. Only at that time can the severity and degree of intestinal involvement (multifocality, compromised segment, size of the lesion, stenosis, etc.) be determined with certainty. In our group, dissection and delimitation of the intestinal lesion are performed by the gynecologist. The goal is to leave the lesion(s) on the intestinal wall and, intraoperatively, decide on the procedure. We always try to use “shaving” (carried out by the gynecologist) first. If that is not sufficient, additional intestinal procedures are performed by the colorectal surgeon.
The complexity of these procedures makes infiltrating endometriosis surgery a high-risk treatment in terms of ureteral and digestive complications[6]. This is especially true in cases of reinterventions, in which the fibrosis produced by previous surgery adds to the anatomic distortion caused by the disease. In our study, 14 patients (10.2%) had complications, a rate similar to what has been reported in other studies conducted by specialized centers[6]. It should be noted that complications can occur even if all available measures are taken to prevent them. For example, in one case, a patient presented a uroperitoneum eight weeks after surgery—two weeks after the double-J stents that had been installed to prevent a probable leak were removed after extensive ureterolysis.
Other types of complications related to the surgical treatment of endometriosis are the urinary and digestive functional sequelae, which can be very limiting, and prolonged, and can significantly affect the quality of life[27],[28]. This type of dysfunction is probably due to surgical trauma on the plexus of the autonomic nervous system[28],[29],[30]. However, there is evidence of preoperative bladder and digestive dysfunctions in patients with deep endometriosis[31],[32],[33],[34], probably due to endometriotic infiltration of the nerves themselves[35].
Recently, results from several studies that evaluated postoperative urinary and digestive dysfunctions in patients with deep endometriosis indicate that these conditions are concentrated in patients who needed resection of uterosacral ligaments, parametrectomy, and/or segmental bowel resection[28],[29],[30],[36]. In our study, most of these dysfunctions were transient, or adequately responded to with physical therapy; however, three of our patients (2%) had prolonged bladder or digestive dysfunction. One of them was a case in which resection of a 4-cm rectovaginal nodule with parametrial involvement and segmental bowel resection was carried out; urinary retention was prolonged for three months after surgery, requiring self-catheterism. This type of prolonged urinary retention is due to surgical trauma to the lower hypogastric plexus in the vicinity of the ischial spine[27], and it may last for years, or even be irreversible[27],[29]. The other case was a patient who had both a hysterectomy and resection of a rectovaginal endometriotic nodule with multifocal involvement of the rectum that required segmental resection. This patient presented alternate constipation with intermittent diarrhea for 20 postoperative months.
To reduce these disorders and achieve radicality without compromising functionality, nerve-sparing surgical techniques have begun to be applied to endometriosis surgery[37]. However, the retraction and infiltrative nature of the disease in the retroperitoneal space sometimes results in an intimate relationship between the endometriotic nodules and the nerves, making the use of these new techniques difficult[27],[29],[38]. Unlike some other specialty groups, we do not perform systematic dissection of the pelvic nerves[37]. At this level, the nerve plexuses are thin, easily traumatized by the dissection itself, and at risk of damage from the lateral thermal diffusion of the coagulation[28].
For the reasons above, to minimize trauma to the pelvic plexuses during surgery, we try to stay close to the lesion; minimize bleeding (to optimize operators’ view of the nerve fibers); lateralize nerve fibers; and avoid deep bilateral dissections in the pararectal spaces, especially at the level of the uterosacral ligaments and posterior parametrium[27],[30],[36].
Although complete surgical resection is currently considered the best long-term treatment in patients with deep endometriosis[5], various studies conducted in specialized centers report up to 30% of recurrence of symptoms[39]. Although in this study, 30% of patients continued to experience some symptoms post-surgery, 90% of them reported significant improvement. The persistence of pelvic pain could be due to a multifactorial mechanism and would not be directly related to the appearance of new lesions[40],[41], in fact, similar to what was reported by Abbott et al.[42], we have not been able to demonstrate the presence of endometriotic lesions in the majority of our patients with persistent symptomatology. Therefore, we make it clear to patients presurgery that the primary objective of the procedure is to make their symptoms more tolerable, and that elimination of them can be challenging. This is especially true in the case of dysmenorrhea, since it begins in the uterus, due to its contractility[43] and / or the concomitant presence of adenomyosis[44]. This is important for patients to know because most of them undergo surgery with uterine preservation.
In this study, 58.1% of patients who wanted to become pregnant were able to after surgery. However, this study could not determine the impact of surgery on the pregnancy rate in patients with deep endometriosis because it only included patients who were undergoing surgery; we did not control for women with deep endometriosis who became pregnant without having had surgery. The lack of a control group without surgery and because infiltrating lesions coexist with other forms of endometriosis, the effect of deep endometriosis surgery on the fertility rate is difficult to determine[17].
This study has some limitations. First, we used a verbal scale to evaluate the results of the surgery in terms of symptoms. This makes it difficult to compare results with other studies because different terms are used in the literature for each category of pain[45]. However, the same grading system has been used successfully in similar studies[46]. Second, given the nature and various anatomic distributions of deep endometriosis, surgical resection requires different procedures for each patient. Therefore, the surgery we performed was not the same for all patients but was adapted to each case, making it impossible to assess the therapeutic efficacy of a particular procedure. One of the strengths of the study was the fact that the surgical team remained relatively stable over time, so the criteria used to determine the best course of action for different types of infiltrating lesions were the same for all cases included in the study.
Conclusion
Based on our experience with surgical management of deep endometriosis, the treatment should be individualized, and planned, based on presurgery knowledge about the location of the infiltrating lesions, in order to provide adequate information to the patient about the implications of their diagnosis and the potential consequences of their treatment.
In addition, we believe it is essential that this type of surgery be restricted to specialized units with trained surgeons—the only context in which proper, multidisciplinary, laparoscopic management can be carried out—in order to maximize long-term therapeutic success and minimize the risk of recurrences due to partial treatments, which increase associated costs and increase morbidity.
Notes
Authorship contributions
DL, HB, JP, IR: Conceptualization, methodology, formal analysis, research, writing—review and editing, supervision, manuscript preparation, project management.
Conflicts of interests
The authors declare that there are no conflicts of interest involving this work.
Funding statement
The authors declare that there were no external sources of funding.
Informed consent
The Santa María Clinical Scientific Committee approved this study.

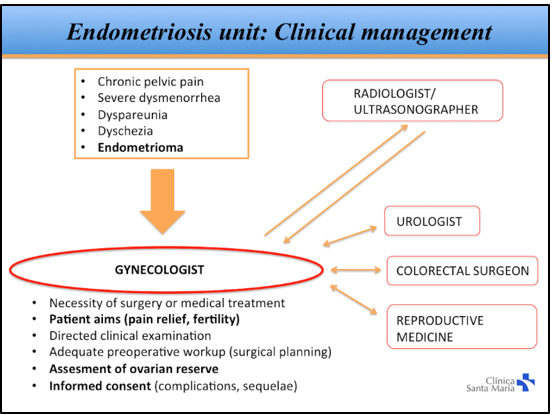 Figure 1. Clinical management of endometriosis at Clínica Santa María.
Figure 1. Clinical management of endometriosis at Clínica Santa María.

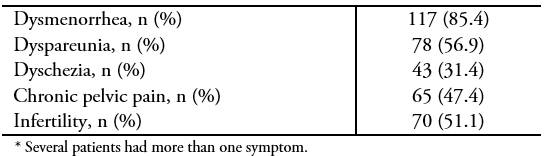 Table 1. Symptoms and surgical indications (n = 137)*.
Table 1. Symptoms and surgical indications (n = 137)*.

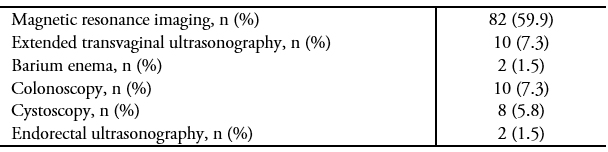 Table 2. Preoperative workup.
Table 2. Preoperative workup.

 Table 3. Anatomic location of DIEa.
Table 3. Anatomic location of DIEa.

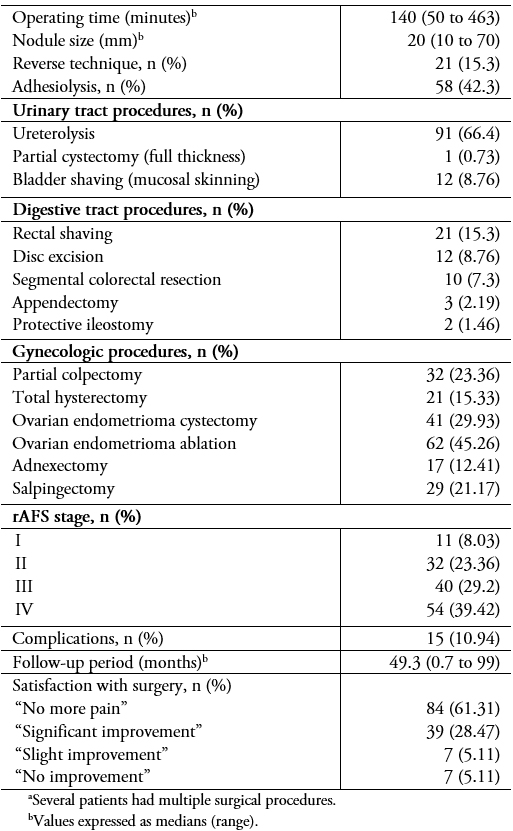 Table 4. Surgical data and proceduresa.
Table 4. Surgical data and proceduresa.

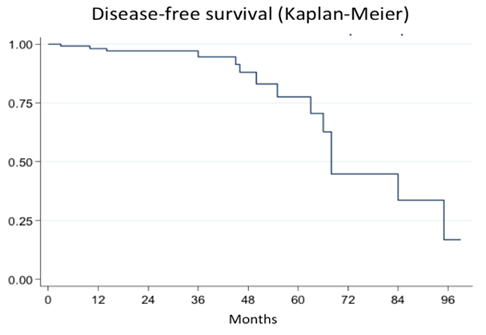 Figure 2. Probability of recurrence at 1, 2, and 3 years.
Figure 2. Probability of recurrence at 1, 2, and 3 years.

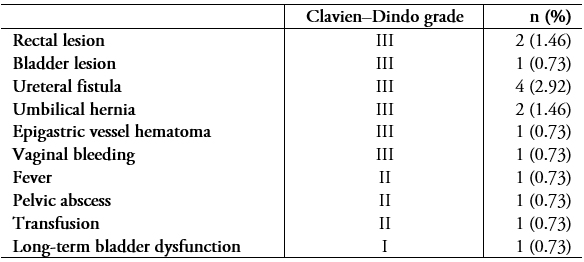 Table 5. Complications by Clavien–Dindo grade
Table 5. Complications by Clavien–Dindo grade

 Figure 3. Surgical specimen from a radical hysterectomy in a patient with deep endometriosis.
Figure 3. Surgical specimen from a radical hysterectomy in a patient with deep endometriosis.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Introducción
La laparoscopía es actualmente el estándar en el manejo de la endometriosis profunda. Sin embargo, requiere de un entrenamiento específico e involucra la realización de procedimientos complejos y asociados a una alta tasa de complicaciones. Por lo anterior en Chile y Latinoamérica, la endometriosis profunda es frecuentemente manejada de manera inadecuada.
Objetivo
Describir nuestra experiencia en el enfrentamiento clínico y manejo quirúrgico laparoscópico de la endometriosis profunda, durante los últimos siete años.
Métodos
Estudio de cohorte retrospectivo de 137 pacientes consecutivas operadas y con confirmación histológica de endometriosis profunda. Se recolectaron los datos demográficos, datos quirúrgicos, complicaciones, resultados reproductivos y seguimiento.
Resultados
Todas las cirugías fueron completadas por laparoscopía, sin conversión. La dismenorrea y la dispareunia fueron los síntomas más frecuentes en 85,4 y 56,9%, respectivamente. La localización más frecuente de endometriosis profunda fueron los ligamentos úterosacros, coexistiendo un endometrioma en 48,9% de los casos. La mediana de tiempo operatorio fue de 140 minutos, siendo significativamente más prolongado en casos con compromiso intestinal (p < 0,0001). Quince pacientes (10,9%) presentaron complicaciones. El seguimiento medio fue de 24,5 meses. La tasa de embarazo fue de 58,1% y 90% de las pacientes reportó una mejoría significativa de su sintomatología.
Conclusiones
El manejo laparoscópico de la endometriosis profunda es efectivo y seguro, pero debe reservarse a centros especializados y con disponibilidad de equipo multidisciplinario.
 Authors:
Demetrio Larraín [1], Hans Buckel[1], Jaime Prado[1], Mario Abedrapo[2], Iván Rojas[1]
Authors:
Demetrio Larraín [1], Hans Buckel[1], Jaime Prado[1], Mario Abedrapo[2], Iván Rojas[1]
Affiliation:
[1] Unidad de Endometriosis, Servicio de Obstetricia y Ginecología, Clínica Santa María, Santiago, Chile
[2] Servicio de Cirugía, Clínica Santa María, Santiago, Chile
E-mail: dlarraind@gmail.com
Author address:
[1] Santa María 0500
Providencia, RM
Chile
Código Postal 7500000

Citation: Larraín D, Buckel H, Prado J, Abedrapo M, Rojas I. Multidisciplinary laparoscopic management of deep infiltrating endometriosis from 2010 to 2017: A retrospective cohort study. Medwave 2019;19(11):e7750 doi: 10.5867/medwave.2019.11.7750
Submission date: 29/6/2019
Acceptance date: 25/10/2019
Publication date: 23/12/2019
Origin: not commissioned
Type of review: reviewed by four external peer reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








