Key Words: risk factors, vancomycin-resistant enterococcus, pediatric intensive care unit
Abstract
Introduction
Vancomycin-resistant enterococci (VRE) infections have become widespread and a challenge in hospitalized patients. The threat of infection by intractable enterococci and the possibility that vancomycin resistance could involve pneumococci or staphylococci advocate for careful surveillance of resistant strains.
Objective
To determine the risk factors associated with VRE colonization in pediatric patients admitted to the Pediatric Intensive Care Unit (PICU) in the period between January 2012 and June 2013.
Methods
We conducted a cross-sectional study analyzing the clinical histories of 140 patients admitted to the PICU (children from 1 month to 18 years) who underwent rectal swab cultures within 48 hours of admission. We calculated the odds ratios and confidence intervals of the risk factors for VRE colonization in the PICU, and then we used multiple logistic regression for the statistically significant variables.
Results
VRE colonization was present in 18.6% of patients. The following variables were identified as risk factors associated with VRE colonization: prior hospitalization in the past year (odds ratio: 10.8; 95% confidence interval: 2.43 to 47.8; p = 0.001); prior use of one broad-spectrum antibiotic (odds ratio: 5.05; 95% confidence interval: 2.04 to 12.5; p = 0.000); use of two or more broad-spectrum antibiotics in past year (odds ratio: 5.4; 95% confidence interval: 1.5 to 18.4; p = 0.009); prior hospitalization in a high-risk area (odds ratio: 4.91; 95% confidence interval: 1.83 to 13.2; p = 0.000); hospitalization for more than five days in a high-risk area (odds ratio: 5.64; 95% confidence interval: 2.18 to 14.6; p = 0.000); and use of immunosuppressant drugs (odds ratio: 4.84; 95% confidence interval: 1.92 to 11.9; p = 0.001). In a logistic multiple regression the use of two or more broad-spectrum antibiotics (odds ratio: 4.81; 95% confidence interval: 1.01 to 22.8; p = 0.047) and prior hospitalization in past year (odds ratio: 7.84; 95% confidence interval: 1.24 to 49.32; p = 0.028) were identified as independent factors statistically associated with VRE colonization.
Conclusion
Pediatric patients admitted for intensive care with a history of prior hospitalization in the past year and exposure to two or more broad-spectrum antibiotics have a greater risk of colonization by vancomycin-resistant enterococci.
Introduction
The evolution of antibiotic resistance evolution has become problematic worldwide[1]. First identified in the mid-1980s, vancomycin-resistant enterococci (VRE) have intrinsic resistance to most common antibiotics, can develop resistance to most other current antibiotics, and can transfer vancomycin resistance genes to other gram-positive organisms[2],[3],[4].
The largest reservoir of VRE is the gastrointestinal tract, and the human being can be a carrier. This contributes to the dissemination of VRE in hospitals[5]. Several previous studies on VRE risk factors have found that the following variables are associated with VRE colonization: exposure to previous antibiotics; previous and prolonged hospitalizations; use of antibiotics in the year prior to admission; surgical conditions; proximity of carrier patients; and exposure to equipment contaminated with VRE[6],[7],[8],[9],[10],[11],[12]. Children colonized with VRE in the gastrointestinal tract can transmit it by fecal-oral dissemination as well as by contaminated fomites[13].
The threat of infection by intractable enterococci and the possibility that vancomycin resistance can spread from enterococci to pneumococci or staphylococci underscores the importance of improved control measures and prevention of hospital infections; careful analysis of empirical antimicrobial schemes; reductions in the length of hospital stays; and follow-up surveillance evaluating the effectiveness of these types of steps and procedures[14],[15],[16].
At the time of the study, there were no data on the prevalence of VRE infection in pediatric intensive care units (PICUs) in our setting, nor regarding the factors associated with VRE colonization. Therefore, the objective of this study is to determine the risk factors associated with the colonization of VRE in pediatric patients admitted to an intensive care unit.
Methods
Study design and setting
This cross-sectional study was carried out in patients admitted to the pediatric intensive care unit (PICU) of Clinicas Hospital at the National University of Asunción, Paraguay, between January 2012 and June 2013. Clinicas Hospital PICU is a multipurpose referral unit with eight beds that serves hospitals in the Greater Asunción area (approximately 2.5 million inhabitants). The study protocol was approved by the Universidad Nacional de Asunción (UNA) Institutional Ethics Committee. Informed consent was not required because the study consisted of a review of medical records. Confidentiality of personal data was guaranteed with the use of encrypted files exclusively managed by researchers.
Convenience sampling was carried out using the medical records for all consecutive patients admitted to the PICU between January 2012 and June 2013. A total of 173 patients were admitted during the study period, of which 33 were excluded (15 for incomplete clinical data, 5 due to readmission during the study period, and 13 for lack of a rectal swab), resulting in a final sample of 140 patients (Figure 1).
Study sample
A total of 140 pediatric patients, ranging in age from 1 month to 18 years, were included in the study. Inclusion criteria were: 1) had a rectal swab within 48 hours of admission to the PICU to detect Enterococcus sp., with results available in the medical record; had complete medical records; and 3) admitted to the PICU during the study period. Exclusion criteria were: incomplete clinical data and not undergoing a rectal swab (which in most cases was due to lack of laboratory supplies). If a study subject was admitted to the PICU more than once, only the first admission was considered in the study.
At the time of the study, according to protocol, a rectal swab was supposed to be carried out for all PICU patients at or within 48 hours of admission. All patients were considered positive and subject to isolation until the return of the culture. Cases in which a swab was not taken had two main causes: administrative factors such as lack of supplies, or death of the patient within the first few hours of admission.
Figure 1. Study sample selection.
Laboratory testing
Rectal samples were collected with a wooden swab at or within 48 hours of admission to the PICU. The samples were placed in Stuart transport medium and sent to the PICU’s microbiology laboratory for cultivation in bile esculin azide agar with vancomycin (6 µg / ml).
Data analysis
Demographic and clinical variables, including age, sex, and primary or underlying diagnosis on the day of admission, were compiled from the clinical history of each patient. For the data analysis, patients were divided into two groups: positive rectal swab culture (VRE+) and negative rectal swab culture (VRE-).
Variables studied in the two groups included patients’ baseline characteristics; prior hospitalization(s) in the past year; and prior hospitalization in a high-risk area (hemato-oncology or intensive care) or a low-risk area (any other reason for pediatric hospitalization). The low- and high-risk area groups were divided into subgroups by length of hospital stay (≤ 10 days versus ≥ 11 days for low-risk areas and ≤ 5 days versus ≥ 6 days for high-risk areas).
Other variables that were analyzed included use of antibiotics in the past year and range of the antimicrobial spectrum (narrow versus broad, with the latter category including anti-infective agents with activity against both gram-positive and gram-negative bacteria). The antibiotics studied were those used in hospital settings, such as vancomycin and third generation cephalosporins (e.g., ceftriaxone or ceftazidime, as well as clindamycin, imipenem, or meropenem). Other variables that were studied included duration of exposure to or use of antibiotics (≤ 7 days versus ≥ 8 days); number of broad-spectrum antibiotics used (one versus two or more); use of invasive devices (defined as a central venous line, a bladder catheter, dialysis catheters, or mechanical ventilation); and use of immunosuppressants (defined as administration of antineoplastic therapy within 6 months prior to sampling, or prior bone marrow transplantation).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, assuming that the variables had normal distribution. Categorical variables were expressed as numbers and percentages. The t test was used to compare continuous variables and the chi-square test was used to compare categorical variables, with p < 0.05 considered significant. In the data analysis, the odds ratio and 95% confidence interval were determined for each variable. Multiple logistic regression analysis was performed on variables that were statistically significant during the bivariate relationship analysis. All statistical analysis was carried out using the EpiInfo 3.4 statistical package.
Results
A total of 140 patients met the study inclusion criteria, including admission to the PICU during the study period January 2012 to June 2013. Of these, based on the rectal swab results, 26 patients (18.6%) were positive for VRE and 114 (81.4%) were negative for VRE. No patient with a culture positive for VRE developed any disease associated with the bacteria.
The average age for the patient sample was 6 ± 5.7 years and distribution by sex was 52.8% boys and 47.1% girls (p = 0.75) (Table 1). Patients with VRE colonization were significantly older than those without VRE colonization (8 ± 5.3 years versus 5.5 ± 5.6 years, p = 0.03) (Table 1). The frequency distribution by age group is shown in Table 1.
According to the data on comorbidities, 48 patients (34.3%) presented hematological pathologies (leukemia or lymphomas) and 21 (20%) had some type of tumor. A total of 36 patients (25.7%) did not present comorbidities. Hematological pathology (odds ratio: 5.4; 95% confidence interval: 2.2 to 13; p = 0.00) and lupus or immunodeficiencies (odds ratio: 18; 95% confidence interval: 1.8 to 18.1; p = 0.01) significantly increased the risk of VRE colonization (Table 2).
The main diagnoses for the patient sample upon admission to the PICU were shock (44 or 31.4%), postoperative conditions (17 or 12.1%), dengue (16 or 11%), and sepsis (10 or 7%) (Table 3). The frequency of the diagnoses was similar for both VRE+ and VRE- patients.
In the bivariate analysis, a statistically significant difference was observed for the following variables: previous hospitalization in past year (odds ratio: 10.8; 95% confidence interval: 2.43 to 47.8; p = 0.001); exposure to antibiotics for 8 or more days (odds ratio: 5.54; 95% confidence interval: 2.18 to 14.8; p = 0.05); exposure to two or more broad-spectrum antibiotics in the past year (odds ratio: 5.4; 95% confidence interval: 1.5 to 18.4; p = 0.009); prior hospitalization in a high-risk area (odds ratio: 4.9; 95% confidence interval: 1.83 to 11.9; p = 0.00); use of immunosuppressants (odds ratio: 4.84; 95% confidence interval: 1.95 to 11.9; p = 0.000); and hospitalization in a high-risk area for 6 or more days (odds ratio: 5.64; 95% confidence interval: 2, 18 to 14.6; p = 0.00). There were no significant differences for the following variables: antibiotic exposure for less than 7 days; no exposure to broad-spectrum antibiotics; prior hospitalization in a low-risk area in the past year; invasive procedures in the past year; and hospitalization in a low-risk area for ≤ 10 days (Table 4).
Table 1. Profile of study sample by VRE carrier status.
Table 2. Comorbidities prior to admission.
Table 3. Causes of admission to intensive care.
Table 4. Factors related to VRE colonization.
The multiple logistic regression analysis for the variables that showed statistical significance is shown in Table 5. It showed that the factors associated with VRE colonization were use of two or more broad-spectrum antibiotics in the past year (odds ratio: 4.8; 95% confidence interval: 1.016 to 22.83; p = 0.04) and previous hospitalizations in past year (odds ratio: 7.84; 95% confidence interval: 1.24 to 49.32; p = 0.02).
Table 5. Results of logistic regression analysis.
Discussion
The main findings of this study were:
1) the prevalence of PICU VRE colonization was 18.6%,
2) the factors significantly associated with VRE colonization in PICU patients were prior hospitalization in past year and exposure to two or more broad-spectrum antibiotics.
In this study, 18.6% of the patients studied had VRE colonization. This frequency of colonization is similar to what was found in another study conducted in a Chilean reference hospital, outside the scope of Intensive care, in which 13% of the patients were VRE+[17].
In one study conducted in Brazil on VRE by Tresoldi et al.[4], a colonization rate of 8.0% was found. However, another study in Chile by Loyola et al. reported a VRE colonization rate of 23.1%[8]. In Argentina, Reale et al. reported a prevalence of VRE colonization of 17.9%[7]. In one study in Turkey by Sutcu et al., a 9.5% VRE colonization rate was found[18]. Another case-control study in Turkey by Ulu-Kilic et al.[19] retrospectively analyzed VRE surveillance data from a pediatric hospital for the period 2010 to 2014, and found a 3.75% rate of positive cultures among all patients examined.
In Pakistan, a study by Yameen et al. evaluating the local epidemiology of nasal and rectal VRE colonization in children admitted to the PICU found a rate of 2.7%[15].
Research results on VRE colonization in developed countries have been highly variable. In New Zealand, Briggs et al. found a colonization rate of 0.3%[20]. In the United Kingdom, Gray et al. found that gastrointestinal VRE colonization was 38.3% in hematology/oncology patients, decreasing to 11% in patients admitted to hepatology/gastroenterology units, and to only 2.3% in children in the PICU[21]. In Korea, Yoon et al. found a VRE colonization rate of 30.3%[5].
The overall frequency of VRE colonization can be considered high if the results obtained in a systematic meta-analysis review by Flokas et al.[22] are taken into account. The review included 19 studies covering a total of 20,234 children hospitalized in general ICUs and PICUs where VRE colonization was 5% overall (3% to 8% in general ICUs and 2% to 9% in PICUs).
Variations in prevalence could be attributed to several different factors, including type of hospital, patient characteristics, and sample collection techniques, as well as the effective use of recommended measures to control bacteria transmission[12].
Based on the results of this study, one factor associated with VRE colonization is prior hospitalization in the past year. According to the results of the study by Reale et al. in Argentina, another factor associated with VRE colonization is prolonged hospitalization (p = 0.0001)[7].
In Korea, Kim et al. found that hospitalization and its duration (odds ratio: 7.24; 95% confidence interval: 3.22 to 16.26; p = < 0.001) were both risk factors for VRE colonization[16]. The results from other studies concur with the findings from this study. For example, in Peru, Elizaga et al. found that patients with a prior hospitalization had a 3.6 times greater risk of VRE colonization (odds ratio: 3.6; 95% confidence interval: 1.6 to 8.4; p = < 0.004)[10]. Previous hospitalization can increase the risk of VRE colonization because there is a greater possibility of receiving broad-spectrum antibiotics, especially when the admission is due to a high-risk condition. A previous hospital stay could also lead to exposure to microorganisms intrinsically resistant to vancomycin and, therefore, the acquisition of the enterococcus through contact with another carrier or through contaminated fomites[5],[6].
Patients who had used two or more broad-spectrum antibiotics also had a higher risk of VRE colonization. According to Reale et al.[7], in Argentina, the use of vancomycin increased the risk of colonization with the bacteria (odds ratio: 5.15; 95% confidence interval: 1.22 to 21.76; p = 0.02). Other studies concur with the findings of this study. For example, Singh et al.[1] found that the use of ceftazidime combined with vancomycin increased the risk of VRE colonization 1.7 times (p = 0.004) and the use of vancomycin or ceftazidime alone represented an even greater risk (odds ratio: 50.39; 95% confidence interval: 3.77 to 672; p = 0.003; and odds ratio: 95.62; 95% confidence interval: 7.41 to 999; p = 0.001, respectively). LIkewise, Henning et al. observed that vancomycin and ceftazidime were the antibiotics most associated with VRE colonization (p = 0.002 and p < 0.001, respectively)[13]. In Chicago (USA), Elizaga et al. found that the previous use of any antibiotic increased the risk of VRE colonization sevenfold (odds ratio: 7; 95% confidence interval: 2.7 to 18.4; p = < 0.001), which increased to 13.5 when vancomycin was used (odds ratio: 13.5; 95% confidence interval: 1.6 to 111; p = 0.005)[10]. In Peru, Flores-Paredes found that use of antibiotics led to a sixfold greater risk (odds ratio: 6; 95% confidence interval: 1.13 to 31.7; p = 0.03)[12].
In Turkey, Sutcu et al. found an association between VRE colonization and previous use of glucopeptides (odds ratio: 12.8; 95% confidence interval: 1.9 to 26.6; p = 0.012)[18], and Özkaya-Parlakay et al. found that antibiotic exposure in the previous three months was significantly related to VRE colonization time (p = 0.004)[23].
Finally, a systematic meta-analysis review carried out by Flokas et al. found that prior exposure to vancomycin and ceftazidime was a significant risk factor for VRE colonization in hospitalized children (odds ratio = 4.34; 95% confidence interval: 2.77 to 6.82; and odds ratio = 4.15; 95% confidence interval: 2.69 to 6.40, respectively)[22].
Use of two or more broad-spectrum antibiotics is related to VRE colonization because these drugs generate high antibiotic pressure by altering normal intestinal flora, leading to selective growth of VRE. Colonization can persist for weeks to even years[24].
This study had several limitations, including its retrospective nature (as it was based on medical records) and small sample size as well as the non-determination of the broad-spectrum antibiotic(s) that had the greatest association with VRE colonization (since the analysis was limited to two main groups of antibiotics categorized according to their spectrum of action).
Studies with prospective and multicenter designs that could help validate the findings reported here are required.
Conclusion
Patients admitted to the PICU with a history of prior hospitalization within the past year and exposure to two or more broad-spectrum antibiotics have a greater risk of VRE colonization.
Knowledge of the prevalence of VRE colonization and associated risk factors allows for monitoring for the correct and timely use of infection prevention measures such as contact isolation and hand hygiene by health personnel and members of the family that help with child care, which should be applied in the care of all patients. Continuing education, the adoption of policies for rational administration of antibiotics, and adequate surveillance of cases could help decrease the rates of VRE colonization so should also be emphasized.
Notes
Roles and authorship contributions
LD, AA: conceptualization, data management, formal analysis, research, methodology, project management, data presentation, manuscript preparation (development of the original draft), writing (revisions and editions).
MG: formal analysis, research, methodology, supervision, data presentation, manuscript preparation (development of the original draft), writing (revisions and editions).
IR, LD, HJJ: conceptualization, data management, formal analysis, research, methodology, project administration, supervision, data presentation, manuscript preparation (development of the original draft), writing (revisions and editions).
Competing interests
The authors have completed the ICMJE conflict of interest declaration form and declare that they have not received funding for the completion of this report; have no financial relationships with organizations that might have an interest in the published article, in the last three years; and have no other relationships or activities that could influence the published article. The forms can be requested from the lead author or the Journal editor.
Funding
The authors declare that there were no external sources of funding.
Ethics
This study was reviewed and approved by the ethics committee of the Faculty of Medical Sciences of the National University of Asunción (Paraguay), according to note UNA_FCM_DIN 279/2018, dated December 11, 2018.

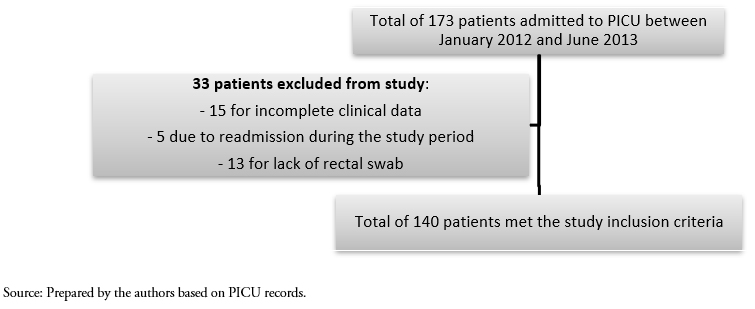 Figure 1. Study sample selection.
Figure 1. Study sample selection.

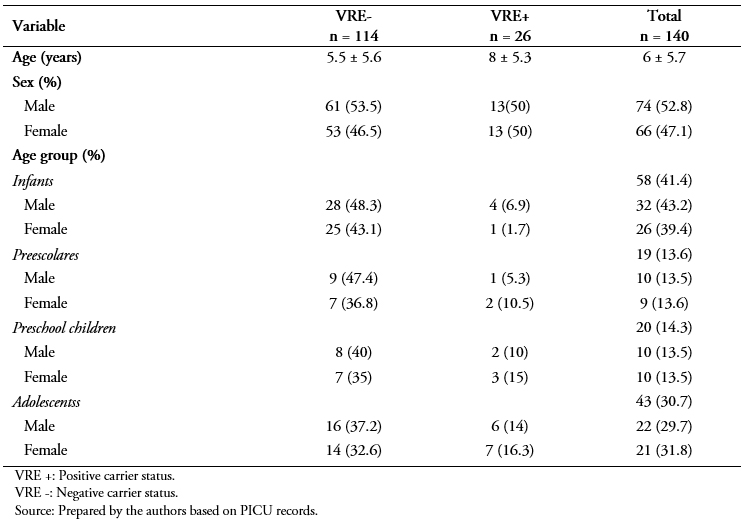 Table 1. Profile of study sample by VRE carrier status.
Table 1. Profile of study sample by VRE carrier status.

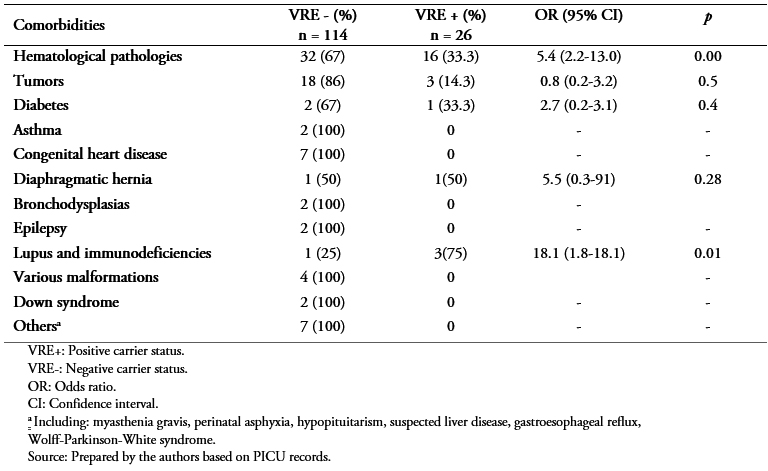 Table 2. Comorbidities prior to admission.
Table 2. Comorbidities prior to admission.

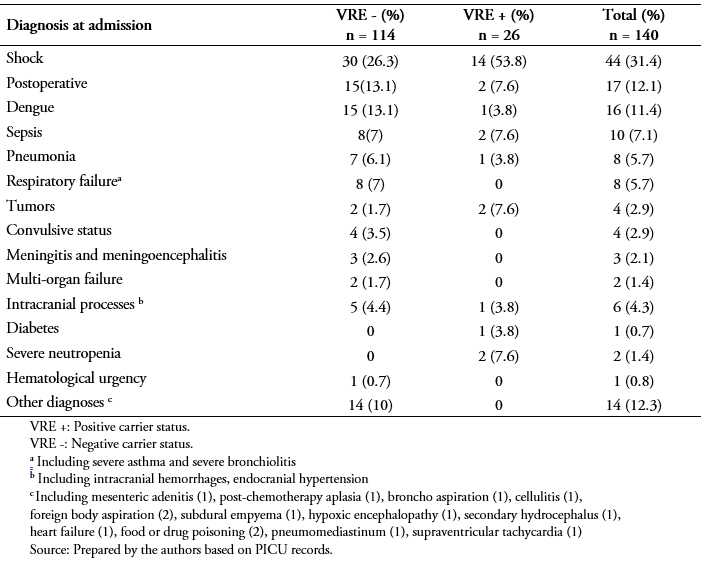 Table 3. Causes of admission to intensive care.
Table 3. Causes of admission to intensive care.

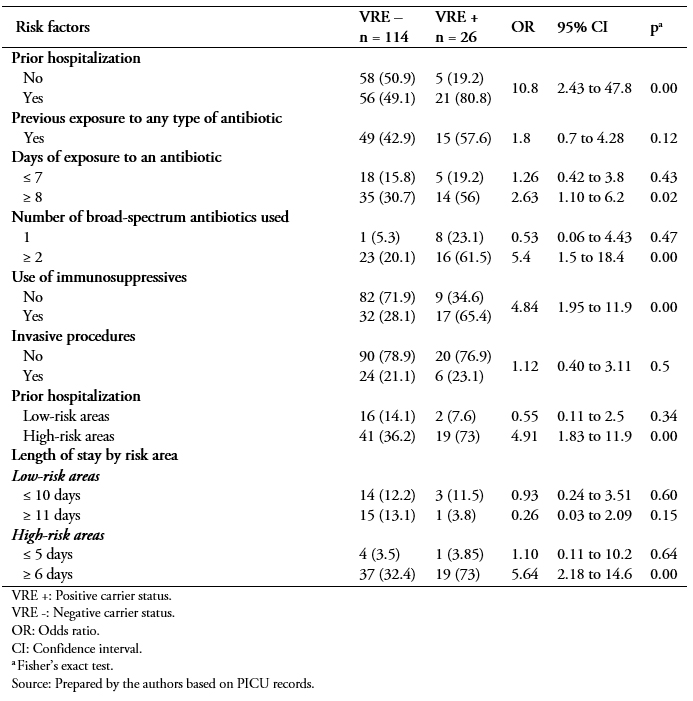 Table 4. Factors related to VRE colonization.
Table 4. Factors related to VRE colonization.

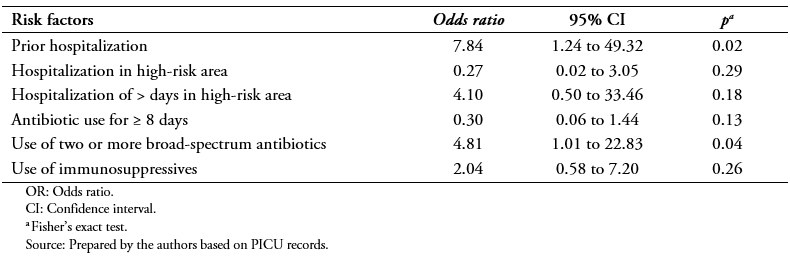 Table 5. Results of logistic regression analysis.
Table 5. Results of logistic regression analysis.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Introducción
Las infecciones por Enterococcus sp resistente a la vancomicina se han diseminado y generan un desafío clínico-terapéutico en los pacientes hospitalizados. La amenaza de que la infección por enterococos intratables y la posibilidad que la resistencia a la vancomicina pueda propagarse a neumococos o estafilococos, abogan por la vigilancia atenta de las cepas resistentes.
Objetivo
Determinar los factores de riesgos asociados a la portación de Enterococcus sp resistente a la vancomicina en pacientes pediátricos ingresados en una unidad de cuidados intensivos pediátricos del Paraguay en el periodo entre enero de 2012 y junio de 2013.
Métodos
Estudio transversal. Se analizaron las historias clínicas previas de 140 pacientes ingresados a terapia intensiva (niños de un mes a 18 años), a quienes se realizaron cultivos de hisopado rectal dentro de las 48 horas del ingreso, para determinar los factores asociados a la portación de Enterococcus sp resistente a la vancomicina en unidad de cuidados intensivos pediátricos. Se calculó el Odd ratio con sus intervalos de confianza y p < 0,05 para las variables de estudio. Posteriormente, se realizó regresión logística múltiple para las variables estadísticamente significativas.
Resultados
La portación de Enterococcus sp resistente a la vancomicina se observó en 18,6% de los pacientes. Se identificaron como factores asociados: la hospitalización previa durante el último año (Odds ratio: 10,8; intervalo de confianza 95%: 2,43 a 47,8; p = 0,001), uso previo de antibióticos de amplio espectro (Odds ratio: 5,05; intervalo de confianza 95%: 2,04 a 12,5; p = 0,000), uso de dos o más antibióticos de amplio espectro en el último año (Odds ratio: 5,4; intervalo de confianza 95%: 1,5 a 18,4; p = 0,009), internación previa en área de alto riesgo (Odds ratio: 4,91; intervalo de confianza 95%: 1,83 a 13,2; p = 0,000), internación por igual o mayor a seis días en área de alto riesgo (Odds ratio: 5,64; intervalo de confianza 95%: 2,18 a 14,6; p = 0,000) y uso de inmunosupresores (Odds ratio: 4,84; intervalo de confianza 95%: 1,92 a 11,9; p = 0,001). La regresión múltiple señala a la utilización de dos o más antibióticos de amplio espectro (Odds ratio: 4,81; intervalo de confianza 95%: 1,01 a 22,8; p = 0,047) y a la historia de hospitalización previa dentro del año (Odds ratio: 7,84; intervalo de confianza 95%: 1,24 a 49,32; p = 0,028) como factores independientes asociados estadísticamente con la portación de Enterococcus sp resistente a la vancomicina.
Conclusión
Los pacientes pediátricos ingresados en la unidad de cuidados intensivos con historia de internación previa dentro del año y la exposición a dos o más antibióticos de amplio espectro, tienen mayor riesgo de colonización por el enterococo resistente a vancomicina.
 Authors:
Laura Duarte[1], Antonio Arbo[2], Mirna Gallardo[1], Irma Riquelme[1], Lorena Delgadillo[1], Hassel Jimmy Jiménez[1]
Authors:
Laura Duarte[1], Antonio Arbo[2], Mirna Gallardo[1], Irma Riquelme[1], Lorena Delgadillo[1], Hassel Jimmy Jiménez[1]
Affiliation:
[1] Unidad de Cuidados Intensivos Pediátricos, Facultad de Ciencias Médicas, Universidad Nacional de Asunción, Asunción, Paraguay
[2] Departamento de Pediatría, Instituto de Medicina Tropical, Ministerio de Salud Pública y Bienestar Social, Asunción, Paraguay
E-mail: hasseljimenez@gmail.com
Author address:
[1] Sargento Clemente Rojas 497
Asunción
Paraguay

Citation: Duarte L, Arbo A, Gallardo M, Riquelme I, Delgadillo L, Jiménez HJ. Factors associated with vancomycin-resistant enterococci colonization in a pediatric intensive care unit of Paraguay: A cross-sectional study on hospital charts. Medwave 2019;19(8):e7694 doi: 10.5867/medwave.2019.08.7694
Submission date: 22/4/2019
Acceptance date: 2/9/2019
Publication date: 12/9/2019
Origin: not commissioned
Type of review: reviewed by four external peer reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Singh-Naz N, Sleemi A, Pikis A, Patel KM, Campos JM. Vancomycin-resistant Enterococcus faecium colonization in children. J Clin Microbiol. 1999 Feb;37(2):413-6. | PubMed |
- Amberpet R, Sistla S, Parija SC, Rameshkumar R. Risk factors for intestinal colonization with vancomycin resistant enterococci' A prospective study in a level III pediatric intensive care unit. J Lab Physicians. 2018 Jan-Mar;10(1):89-94. | CrossRef | PubMed |
- Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clin Microbiol Rev. 2000 Oct;13(4):686-707. | PubMed |
- Tresoldi AT, Cardoso LGO, Castilho G V, Dantas SRPE, von Nowakonski A, Pereira RM, et al. Low prevalence of vancomycin resistant enterococci colonization in intensive care patients in a Brazilian teaching hospital. Braz J Infect Dis. 2006; 10(4):239-41. | CrossRef |
- Yoon YK, Lee SE, Lee J, Kim HJ, Kim JY, Park DW, et al. Risk factors for prolonged carriage of vancomycin-resistant Enterococcus faecium among patients in intensive care units: a case-control study. J Antimicrob Chemother. 2011 Aug;66(8):1831-8. | CrossRef | PubMed |
- Moemen D, Tawfeek D, Badawy W. Healthcare-associated vancomycin resistant Enterococcus faecium infections in the Mansoura University Hospitals intensive care units, Egypt. Braz J Microbiol. 2015 Jul 1;46(3):777-83. | CrossRef | PubMed |
- Reale AL, Depetri ML, Culasso C, Paviolo M, Cheguirián ML, Enrico MC, et al. [Vancomycin-resistant enterococci: prevalence and factors associated with intestinal colonization in oncology patients from Hospital de Niños de Córdoba]. Rev Argent Microbiol. 2009 Apr-Jun;41(2):92-6. | PubMed | Link |
- Loyola P, Tordecilla J, Benadof D, Yohannessen K, Acuña M. [Risk factor of intestinal colonization with vancomycin resistant Enterococcus spp in hospitalized pediatric patients with oncological disease]. Rev Chilena Infectol. 2015 Aug;32(4):393-8. | CrossRef | PubMed |
- Furuno JP, McGregor JC, Harris AD, Johnson JA, Johnson JK, Langenberg P, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med. 2006 Mar 13;166(5):580-5. | PubMed |
- Elizaga ML, Weinstein RA, Hayden MK. Patients in long-term care facilities: a reservoir for vancomycin-resistant enterococci. Clin Infect Dis. 2002 Feb 15;34(4):441-6. | PubMed |
- Yiş R, Aslan S, Cıtak C, Değirmenci S. [Evaluation of vancomycin-resistant enterococcus colonization at Gaziantep Children's Hospital, Turkey]. Mikrobiyol Bul. 2011 Oct;45(4):646-54. Turkish. | PubMed |
- Flores-Paredes W. Epidemiología de la colonización intestinal con enterococo resistente a vancomicina en pacientes de alto riesgo del Hospital Nacional Edgardo Rebagliati Martins. Lima, Perú. Rev Medica Hered. 2010; 21(3):128-39. | Link |
- Henning KJ, Delencastre H, Eagan J, Boone N, Brown A, Chung M, et al. Vancomycin-resistant Enterococcus faecium on a pediatric oncology ward: duration of stool shedding and incidence of clinical infection. Pediatr Infect Dis J. 1996 Oct;15(10):848-54. | PubMed |
- Bonten MJ, Slaughter S, Ambergen AW, Hayden MK, van Voorhis J, Nathan C, et al. The role of "colonization pressure" in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch Intern Med. 1998 May 25;158(10):1127-32. | PubMed |
- Yameen MA, Iram S, Mannan A, Khan SA, Akhtar N. Nasal and perirectal colonization of vancomycin sensitive and resistant enterococci in patients of paediatrics ICU (PICU) of tertiary health care facilities. BMC Infect Dis. 2013 Mar 27;13:156. | CrossRef | PubMed |
- Kim HS, Kim DH, Yoon HJ, Lee WJ, Woo SH, Choi SP. Factors Associated with Vancomycin-Resistant Enterococcus Colonization in Patients Transferred to Emergency Departments in Korea. J Korean Med Sci. 2018 Oct 25;33(48):e295. | CrossRef | PubMed |
- Talavera G, Rodriguez M, Castro H, Melgarejo N, Noguera J, Portillo S BW. Colonización intestinal por enterococo resistente a la vancomicina en pacientes oncológicos con factores de riesgo. Pediatr (Asunción). 2011;38(2):123-5. | Link |
- Sutcu M, Akturk H, Acar M, Salman N, Aydın D, Akgun Karapınar B, et al. Impact of vancomycin-resistant enterococci colonization in critically ill pediatric patients. Am J Infect Control. 2016 May 1;44(5):515-9. | CrossRef | PubMed |
- Ulu-Kilic A, Özhan E, Altun D, Perçin D, Güneş T, Alp E. Is it worth screening for vancomycin-resistant Enterococcus faecium colonization?: Financial burden of screening in a developing country. Am J Infect Control. 2016 Apr 1;44(4):e45-9. | CrossRef | PubMed |
- Briggs S, Upton A, Bilkey M, Taylor S, Roberts S, Holland D. Vancomycin-resistant enterococcal colonisation of hospitalised patients in Auckland. N Z Med J. 2002 Aug 23;115(1160):U145. | PubMed |
- Gray JW, George RH. Experience of vancomycin-resistant enterococci in a children's hospital. J Hosp Infect. 2000 May;45(1):11-8. | CrossRef | PubMed |
- Flokas ME, Karageorgos SA, Detsis M, Alevizakos M, Mylonakis E. Vancomycin-resistant enterococci colonisation, risk factors and risk for infection among hospitalised paediatric patients: a systematic review and meta-analysis. Int J Antimicrob Agents. 2017 May;49(5):565-572. | CrossRef | PubMed |
- Özkaya-Parlakay A, Cengiz AB, Ceyhan M, Bağdat A, Barın-Kurtoğlu Ç, Gürbüz V, et al. Vancomycin-resistant enterococcus colonization and infection in children: six-year follow-up. Turk J Pediatr. 2014 Nov-Dec;56(6):618-25. | PubMed |
- Rice LB. Emergence of vancomycin-resistant enterococci. Emerg Infect Dis. 2001 Mar-Apr;7(2):183-7. | PubMed |
 Singh-Naz N, Sleemi A, Pikis A, Patel KM, Campos JM. Vancomycin-resistant Enterococcus faecium colonization in children. J Clin Microbiol. 1999 Feb;37(2):413-6. | PubMed |
Singh-Naz N, Sleemi A, Pikis A, Patel KM, Campos JM. Vancomycin-resistant Enterococcus faecium colonization in children. J Clin Microbiol. 1999 Feb;37(2):413-6. | PubMed | Amberpet R, Sistla S, Parija SC, Rameshkumar R. Risk factors for intestinal colonization with vancomycin resistant enterococci' A prospective study in a level III pediatric intensive care unit. J Lab Physicians. 2018 Jan-Mar;10(1):89-94. | CrossRef | PubMed |
Amberpet R, Sistla S, Parija SC, Rameshkumar R. Risk factors for intestinal colonization with vancomycin resistant enterococci' A prospective study in a level III pediatric intensive care unit. J Lab Physicians. 2018 Jan-Mar;10(1):89-94. | CrossRef | PubMed | Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clin Microbiol Rev. 2000 Oct;13(4):686-707. | PubMed |
Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clin Microbiol Rev. 2000 Oct;13(4):686-707. | PubMed | Tresoldi AT, Cardoso LGO, Castilho G V, Dantas SRPE, von Nowakonski A, Pereira RM, et al. Low prevalence of vancomycin resistant enterococci colonization in intensive care patients in a Brazilian teaching hospital. Braz J Infect Dis. 2006; 10(4):239-41. | CrossRef |
Tresoldi AT, Cardoso LGO, Castilho G V, Dantas SRPE, von Nowakonski A, Pereira RM, et al. Low prevalence of vancomycin resistant enterococci colonization in intensive care patients in a Brazilian teaching hospital. Braz J Infect Dis. 2006; 10(4):239-41. | CrossRef | Yoon YK, Lee SE, Lee J, Kim HJ, Kim JY, Park DW, et al. Risk factors for prolonged carriage of vancomycin-resistant Enterococcus faecium among patients in intensive care units: a case-control study. J Antimicrob Chemother. 2011 Aug;66(8):1831-8. | CrossRef | PubMed |
Yoon YK, Lee SE, Lee J, Kim HJ, Kim JY, Park DW, et al. Risk factors for prolonged carriage of vancomycin-resistant Enterococcus faecium among patients in intensive care units: a case-control study. J Antimicrob Chemother. 2011 Aug;66(8):1831-8. | CrossRef | PubMed | Moemen D, Tawfeek D, Badawy W. Healthcare-associated vancomycin resistant Enterococcus faecium infections in the Mansoura University Hospitals intensive care units, Egypt. Braz J Microbiol. 2015 Jul 1;46(3):777-83. | CrossRef | PubMed |
Moemen D, Tawfeek D, Badawy W. Healthcare-associated vancomycin resistant Enterococcus faecium infections in the Mansoura University Hospitals intensive care units, Egypt. Braz J Microbiol. 2015 Jul 1;46(3):777-83. | CrossRef | PubMed | Reale AL, Depetri ML, Culasso C, Paviolo M, Cheguirián ML, Enrico MC, et al. [Vancomycin-resistant enterococci: prevalence and factors associated with intestinal colonization in oncology patients from Hospital de Niños de Córdoba]. Rev Argent Microbiol. 2009 Apr-Jun;41(2):92-6. | PubMed | Link |
Reale AL, Depetri ML, Culasso C, Paviolo M, Cheguirián ML, Enrico MC, et al. [Vancomycin-resistant enterococci: prevalence and factors associated with intestinal colonization in oncology patients from Hospital de Niños de Córdoba]. Rev Argent Microbiol. 2009 Apr-Jun;41(2):92-6. | PubMed | Link | Loyola P, Tordecilla J, Benadof D, Yohannessen K, Acuña M. [Risk factor of intestinal colonization with vancomycin resistant Enterococcus spp in hospitalized pediatric patients with oncological disease]. Rev Chilena Infectol. 2015 Aug;32(4):393-8. | CrossRef | PubMed |
Loyola P, Tordecilla J, Benadof D, Yohannessen K, Acuña M. [Risk factor of intestinal colonization with vancomycin resistant Enterococcus spp in hospitalized pediatric patients with oncological disease]. Rev Chilena Infectol. 2015 Aug;32(4):393-8. | CrossRef | PubMed | Furuno JP, McGregor JC, Harris AD, Johnson JA, Johnson JK, Langenberg P, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med. 2006 Mar 13;166(5):580-5. | PubMed |
Furuno JP, McGregor JC, Harris AD, Johnson JA, Johnson JK, Langenberg P, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med. 2006 Mar 13;166(5):580-5. | PubMed | Elizaga ML, Weinstein RA, Hayden MK. Patients in long-term care facilities: a reservoir for vancomycin-resistant enterococci. Clin Infect Dis. 2002 Feb 15;34(4):441-6. | PubMed |
Elizaga ML, Weinstein RA, Hayden MK. Patients in long-term care facilities: a reservoir for vancomycin-resistant enterococci. Clin Infect Dis. 2002 Feb 15;34(4):441-6. | PubMed | Yiş R, Aslan S, Cıtak C, Değirmenci S. [Evaluation of vancomycin-resistant enterococcus colonization at Gaziantep Children's Hospital, Turkey]. Mikrobiyol Bul. 2011 Oct;45(4):646-54. Turkish. | PubMed |
Yiş R, Aslan S, Cıtak C, Değirmenci S. [Evaluation of vancomycin-resistant enterococcus colonization at Gaziantep Children's Hospital, Turkey]. Mikrobiyol Bul. 2011 Oct;45(4):646-54. Turkish. | PubMed | Flores-Paredes W. Epidemiología de la colonización intestinal con enterococo resistente a vancomicina en pacientes de alto riesgo del Hospital Nacional Edgardo Rebagliati Martins. Lima, Perú. Rev Medica Hered. 2010; 21(3):128-39. | Link |
Flores-Paredes W. Epidemiología de la colonización intestinal con enterococo resistente a vancomicina en pacientes de alto riesgo del Hospital Nacional Edgardo Rebagliati Martins. Lima, Perú. Rev Medica Hered. 2010; 21(3):128-39. | Link | Henning KJ, Delencastre H, Eagan J, Boone N, Brown A, Chung M, et al. Vancomycin-resistant Enterococcus faecium on a pediatric oncology ward: duration of stool shedding and incidence of clinical infection. Pediatr Infect Dis J. 1996 Oct;15(10):848-54. | PubMed |
Henning KJ, Delencastre H, Eagan J, Boone N, Brown A, Chung M, et al. Vancomycin-resistant Enterococcus faecium on a pediatric oncology ward: duration of stool shedding and incidence of clinical infection. Pediatr Infect Dis J. 1996 Oct;15(10):848-54. | PubMed | Bonten MJ, Slaughter S, Ambergen AW, Hayden MK, van Voorhis J, Nathan C, et al. The role of "colonization pressure" in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch Intern Med. 1998 May 25;158(10):1127-32. | PubMed |
Bonten MJ, Slaughter S, Ambergen AW, Hayden MK, van Voorhis J, Nathan C, et al. The role of "colonization pressure" in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch Intern Med. 1998 May 25;158(10):1127-32. | PubMed | Yameen MA, Iram S, Mannan A, Khan SA, Akhtar N. Nasal and perirectal colonization of vancomycin sensitive and resistant enterococci in patients of paediatrics ICU (PICU) of tertiary health care facilities. BMC Infect Dis. 2013 Mar 27;13:156. | CrossRef | PubMed |
Yameen MA, Iram S, Mannan A, Khan SA, Akhtar N. Nasal and perirectal colonization of vancomycin sensitive and resistant enterococci in patients of paediatrics ICU (PICU) of tertiary health care facilities. BMC Infect Dis. 2013 Mar 27;13:156. | CrossRef | PubMed | Kim HS, Kim DH, Yoon HJ, Lee WJ, Woo SH, Choi SP. Factors Associated with Vancomycin-Resistant Enterococcus Colonization in Patients Transferred to Emergency Departments in Korea. J Korean Med Sci. 2018 Oct 25;33(48):e295. | CrossRef | PubMed |
Kim HS, Kim DH, Yoon HJ, Lee WJ, Woo SH, Choi SP. Factors Associated with Vancomycin-Resistant Enterococcus Colonization in Patients Transferred to Emergency Departments in Korea. J Korean Med Sci. 2018 Oct 25;33(48):e295. | CrossRef | PubMed | Talavera G, Rodriguez M, Castro H, Melgarejo N, Noguera J, Portillo S BW. Colonización intestinal por enterococo resistente a la vancomicina en pacientes oncológicos con factores de riesgo. Pediatr (Asunción). 2011;38(2):123-5. | Link |
Talavera G, Rodriguez M, Castro H, Melgarejo N, Noguera J, Portillo S BW. Colonización intestinal por enterococo resistente a la vancomicina en pacientes oncológicos con factores de riesgo. Pediatr (Asunción). 2011;38(2):123-5. | Link | Sutcu M, Akturk H, Acar M, Salman N, Aydın D, Akgun Karapınar B, et al. Impact of vancomycin-resistant enterococci colonization in critically ill pediatric patients. Am J Infect Control. 2016 May 1;44(5):515-9. | CrossRef | PubMed |
Sutcu M, Akturk H, Acar M, Salman N, Aydın D, Akgun Karapınar B, et al. Impact of vancomycin-resistant enterococci colonization in critically ill pediatric patients. Am J Infect Control. 2016 May 1;44(5):515-9. | CrossRef | PubMed | Ulu-Kilic A, Özhan E, Altun D, Perçin D, Güneş T, Alp E. Is it worth screening for vancomycin-resistant Enterococcus faecium colonization?: Financial burden of screening in a developing country. Am J Infect Control. 2016 Apr 1;44(4):e45-9. | CrossRef | PubMed |
Ulu-Kilic A, Özhan E, Altun D, Perçin D, Güneş T, Alp E. Is it worth screening for vancomycin-resistant Enterococcus faecium colonization?: Financial burden of screening in a developing country. Am J Infect Control. 2016 Apr 1;44(4):e45-9. | CrossRef | PubMed | Briggs S, Upton A, Bilkey M, Taylor S, Roberts S, Holland D. Vancomycin-resistant enterococcal colonisation of hospitalised patients in Auckland. N Z Med J. 2002 Aug 23;115(1160):U145. | PubMed |
Briggs S, Upton A, Bilkey M, Taylor S, Roberts S, Holland D. Vancomycin-resistant enterococcal colonisation of hospitalised patients in Auckland. N Z Med J. 2002 Aug 23;115(1160):U145. | PubMed | Gray JW, George RH. Experience of vancomycin-resistant enterococci in a children's hospital. J Hosp Infect. 2000 May;45(1):11-8. | CrossRef | PubMed |
Gray JW, George RH. Experience of vancomycin-resistant enterococci in a children's hospital. J Hosp Infect. 2000 May;45(1):11-8. | CrossRef | PubMed | Flokas ME, Karageorgos SA, Detsis M, Alevizakos M, Mylonakis E. Vancomycin-resistant enterococci colonisation, risk factors and risk for infection among hospitalised paediatric patients: a systematic review and meta-analysis. Int J Antimicrob Agents. 2017 May;49(5):565-572. | CrossRef | PubMed |
Flokas ME, Karageorgos SA, Detsis M, Alevizakos M, Mylonakis E. Vancomycin-resistant enterococci colonisation, risk factors and risk for infection among hospitalised paediatric patients: a systematic review and meta-analysis. Int J Antimicrob Agents. 2017 May;49(5):565-572. | CrossRef | PubMed | Özkaya-Parlakay A, Cengiz AB, Ceyhan M, Bağdat A, Barın-Kurtoğlu Ç, Gürbüz V, et al. Vancomycin-resistant enterococcus colonization and infection in children: six-year follow-up. Turk J Pediatr. 2014 Nov-Dec;56(6):618-25. | PubMed |
Özkaya-Parlakay A, Cengiz AB, Ceyhan M, Bağdat A, Barın-Kurtoğlu Ç, Gürbüz V, et al. Vancomycin-resistant enterococcus colonization and infection in children: six-year follow-up. Turk J Pediatr. 2014 Nov-Dec;56(6):618-25. | PubMed | Rice LB. Emergence of vancomycin-resistant enterococci. Emerg Infect Dis. 2001 Mar-Apr;7(2):183-7. | PubMed |
Rice LB. Emergence of vancomycin-resistant enterococci. Emerg Infect Dis. 2001 Mar-Apr;7(2):183-7. | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








