Key Words: risk factors, hepatitis C, blood transfusion
Abstract
INTRODUCTION
Most blood transfusions occur in female patients. The introduction of serologic screening practices by blood banks reduced the transfusion-related rate of infection with hepatitis C virus (HCV). In Mexico patients with pre-1994 transfusion history are at high risk of being detected with HCV infection. We aimed at establishing an interrelationship between two variables: pre-1994 transfusion history and rate of infection in women treated in the Guadalajara Metropolitan Area hospitals, in Mexico.
METHODS
Analytical observational case-control study which included both non-infected women and patients diagnosed with hepatitis C virus infection, in whom the pre-1994 transfusion history was determined. The cases were 150 women with confirmed hepatitis C virus serologic diagnosis. The controls were 150 women whose hepatitis C virus-detection serologic tests had yielded negative results.
RESULTS
An odds ratio of 9.07 (95% CI: 5.37 – 15.3; p< 0.001) was found where the rate of infection for the case group was 0.72 while the control group had a ratio of 0.22; population attributable risk (PAR) was 0.64 (95% CI: 0.53 – 0.73), while etiologic fraction was 0.88 (95% CI: 0.81 – 0.93).
CONCLUSIONS
Among women, having been exposed to pre-1994 blood transfusion means a risk 9.07 times higher than not being exposed to blood transfusion in the same time frame.
Introduction
Blood transfusion, a life-saving option for patients who have lost blood or suffer from anemia, carries risks for the patient. Regarding Hepatitis C, in Mexico, before 1994 blood screening tests to guarantee safe blood transfusions were not available. Nowadays, nucleic acid technology (NAT) is available for early viral detection. Early viral detection has allowed for a reduction of the so-called window period, during which viremia occurs making hepatitis C virus detection possible [1].
Since the nineteen seventies, descriptions of a new type of hepatitis proliferated, which was quite frequently found in patients who had received blood transfusions, and which was not related to any other type of hepatotropic virus. Originally, the newfound virus was named HNANB (Hepatitis Not A, Not B); and by 1975, the literature blames the new virus for 90% of transfusion-related cases of hepatitis [2].
The real ratio of transfusion-related hepatitis C virus (HCV) infection cases is currently unknown, although transfusions are the most widely acknowledged hepatitis C virus single source of infection, accounting for 7 to 10% of patients with various transfusions [3]. At present, blood transfusions can be considered safe, but that has not always been the case. Advances in transfusion medicine during the first half of the twentieth century allowed for the widespread clinical use of blood and blood derivatives, which contributed to the spread of transfusion-related hepatitis C virus infections [4].
Hepatitis C virus was discovered in 1989. However, in 1993, the Mexican Official Norm NOM-003-SSA2-1993, required blood banks to screen for hepatitis C virus antibodies of all blood donors, but it was not until the following year (July 18th, 1994) that the norm was published in its final form [5].
The Central Blood Bank of the Western National Medical Center in Guadalajara, which belongs to the Mexican Social Security Institute (IMSS) found, after a 10-year study that compared the different hepatitis C virus screening techniques, that ELISA (Enzyme Linked Immunosorbent Assay) returned 1% positive serologic results, whereas the percentage was 0.96 for EIA (enzyme immunoassay1: microparticles) and 0.91 for chemoluminescence [6].
A paper published in Spain [2] comprehensively reports on the legal requirement for all donors in that country to be screened for hepatitis C virus, which was effective during 1990, four years before Mexico, and two years before the introduction of similar measures in Hawaii, which introduced screening tests in 1992 [7].
The potential risk of transfusion-related transmission of infection can be estimated by analyzing blood donors’ data, screening procedures that were followed and the prevalence of markers in the target populations. The estimation of such risks is useful in keeping track of blood transfusion safety and providing data for an informed medical decision in considering to the suitability of allogeneic transfusion over other therapeutic measures [8].
Risk per unit of exposure in the case of hepatitis C virus is the same regardless of blood component type used in transfusion (red blood cells, platelets, plasma or cryoprecipitates). Screening of blood donors is of paramount importance in Latin America, where first-time donors, who are more likely to be carriers of viral infections, provide most of the blood supply. The supply from repeated altruistic blood donors who are followed periodically and thus less likely infection carriers are a rarity in the region.
Hepatitis C virus infection is more frequently found in patients between the ages of 40 and 50 and it is the most common cause of hepatitis and cirrhosis of the liver worldwide [9]. At present, there is no vaccination available to prevent hepatitis C virus infection. Therefore, the planning of prevention and treatment strategies requires full understanding of the risk factors involved. Based on this study, knowing the risk of hepatitis C virus transmission due to pre 1994 blood transfusions is vital to implement blood screening that must impact in the early detection of this disease. Our main focus is: requiring serologic screening practices to detect hepatitis C virus in female patients who have received a pre-1994 blood transfusion, due to the higher risk that has been detected to acquire the infection through these conditions.
Methods
Case-control study design with the aim of measuring the link between pre-1994 blood transfusion history and hepatitis C virus infection risk in female patient. The study followed women who were being treated by the Mexican Social Security Institute in one of the Guadalajara Metropolitan Area, in Mexico. The group of controls, all under treatment, between January and December 2014 were treated at the Gineco-Obstetrics Unit of the High-end Clinic located in the Western National Medical Center (CMNO in Spanish) of Guadalajara, were female patients who had transfusion history, but negative hepatitis C virus screening test results.
The cases were women who between January 2007 and December 2014 had been diagnosed with hepatitis C virus infection and informed by the Information and Strategic Analysis Unit of the CMNO, who are sent their test results. All patients were resident in the Guadalajara Metropolitan Area. The control patients have the same characteristics as the case patient (transfused and attended women in a second level hospital of the metropolitan area of Guadalajara, only they did not present the hepatitis C virus infection which corresponds to our independent variable). Case patients were also transfused and cared for in the second level hospitals in the metropolitan area of Guadalajara, except that they presented a positive diagnosis for hepatitis C virus infection, in both groups the transfusion history was sought before 1994 (our variable dependent). Both the confirmation of hepatitis C virus infection in the patients as well as the disposal of the infection in the control patients were performed by positive antibodies and diagnosis included the identification of hepatitis C virus antibodies, and diagnosis confirmation by the Blood Bank at the CMNO which used nucleic acid amplification tests, both probing and alternative nucleic acid technology were positive: COBAS Amplicor HCV, Version 2.0, lower limit: ARN 50 UI / ml in plasma and 60 UI / ml in serum with two-pool coordinated positivity which represent our only target variable.
We are aware that neither controls nor cases were deemed exposed to a high risk of bias, which were taken into account as risk factors such as intravenous drug use, sexual promiscuity, tattoos or use of hemodialysis, so it was necessary to have a control over them. And the decision was not to allow women who present these risk factors.
Sample size was 150 cases and 150 controls, useful to determine the association of hepatitis C in women with a history of transfusion (considering an odds ratio of 2 or more), when exposure (prior to 1994) in (95% confidence level and 80% power). A methodology used by Contreras et al., 2011 to ensure a reliable sample size in studies where surgical procedures represent a risk factor [10]. The software used in this work was Epi Info 7.
Results
The results showed that the mean age in the controls was 45.6 years with a standard deviation of 16.1, mean age in the cases was 52.8 years with a standard deviation of 12.8, and both groups had no associated confounding variables (Table 1). One single transfusion is the most common number (mode) in the clinical history of patients within the case group, accounting for 96 patients, or 64% of the sample (table 2). There was a high frequency of the risk factor (pre-1994 transfusion); for these patients was 72.7% of the sample (109/150 table 2). Although the control group’s mode was still one transfusion for most patients, the proportion concerning the sample was higher, at 119/150, or 79.3% of the patients; notably, the frequency of the risk factor (pre-1994 transfusion) for these patients was lower, at 34 cases or 22.7% of the sample (table 3).
Table 1. Description of sociodemographic variables of both groups.
Table 2. Number of transfusions as antecedent in both groups.
Table 3. Contingency table for cases and controls.
Additionally, subjects in both samples were re-grouped according to risk factor exposure or lack thereof (Table 3). The resulting odds ratio was 9.07 (Confidence Interval 95% from 5.37 to 15.3. Significance level was tested by performing a chi-squared test (73.12 p< 0.001). Moreover, exposure rates were calculated (Table 4) to determine attributable fraction values (Table 5).
Table 4. Exposure proportions in both groups.
Table 5. Attributable fractions.
The measures of association in cases and controls, based on the comparison between the frequencies of the damage in different groups, can be made through odds ratios or their differences (attributable risk and attributable fraction). In this case the fraction attributable or preventable for the population is an impact measure, this assuming that the exposure to an etiological agent is causal. Having obtained a p less than 0.05 indicates that it is possible to discard chance as an explanation of the observed association with a reduced probability of committing the error of the first type (to make an improper rejection) [11].
Discussion
The main contribution of this study is that it made it possible for us to establish the odds ratio pertaining to the variable “pre-1994 transfusion in female patients” as a risk factor for hepatitis C virus infection in the Guadalajara Metropolitan Area, in Mexico, which had not been previously reported in the literature. As we compare our findings with those of similar studies, we find that a study done in Hawaii reveals that hepatitis C virus infection is frequently found in patients between the ages of 45 and 65 (prevalence of 8.4%) more than other age groups [12]. The average age of our subjects was similar to theirs at (mean 52.6 ± 12.7 SD).
Within this research, it was found that the odds ratio of pre-1992 transfusion was 1.67 (confidence interval 95%: 1.24 – 2.24, p<0.001); multivariate analysis (logistic regression) including four more variables (tattoos or other piercings in precarious sanitary conditions, exposure to blood-contaminated needles, HCV+ sexual partner, use of injected drugs) returned an odds ratio of 1.91 (95% confidence Interval: 1.35 – 2.70, p<0.001). Paradoxically, only males kept statistical significance when stratified by sex: 1.92 (95% confidence Interval: 1.28 – 2.89, p=0.002); females 1.85 (95% confidence Interval: 0.94 – 3.65, p=0.074); [12], the difference observed when compared to our findings can be explained taking into account the fact that in Hawaii, blood transfusion in women does not have so long a tradition as is the case of Mexico, where transfusion is used as therapy for labor hemorrhage, which accounts for 25% of complications. [13].
A similar study, which intended to measure the prevalence of hepatitis C virus infection in two IMSS hospitals in Puebla [14] by studying the same risk factors heretofore mentioned, found that hepatitis C virus infection rate in Mexico is 30% to 35% in patients with active hepatitis [15], [16],[17]. Unlike the present study, they included one more year as risk factor, looking at patients with blood transfusion history previous to 1995; the data identified that from a total 6,561 patients in one of the hospitals (Clinic 6), 1,608 had been exposed to the above risk factor (24.5%), and in the other hospital (Clinic 55), 2,018 were identified with the risk factor from a total of 5,954 patients (33.9%); Multinomial logistic regression returned the finding that only pre-1995 transfusion was statistically significant for viral contagion (p=0.004) [14]. Besides, patients were stratified by age groups: 362 of them were younger than 30 years of age (16.9%), 1,758 (41.8%) were aged between 31 and 50, whereas 1,491 (56.7%) were older than 50, which coincides with the findings reported elsewhere in pointing at mature and senior patients as more likely to be affected by the virus [14].
Yet, another similar study in Catanduva a province of Sao Paulo, Brazil, found that transfusion-related hepatitis C virus transmission has been described comprehensively. Prevalence of infection in Brazil is similar to Mexico, which is between 1% and 2% of the population [18]. Furthermore, a recent case-control study that sought to measure a number of risk factors (intravenous drugs, blood transfusion, tattoos, among others), with a prevalence of male patients (68.9%) in the sample [19] giving a difference from our research that shows this data in both genres.
The risk factor “blood transfusion history” is key to our findings. They found 55 exposed cases and 135 non-exposed cases, compared to 20 exposed controls and 170 non-exposed controls; the above returns a raw odds ratio of 3.92 (95% confidence interval: 2.07 – 7.38); multivariate logistic regression returned an odds ratio of 7.33 (95% confidence interval: 2.44 – 22.0), [19].
The above study found that 28.9% of subjects had blood (or blood derivatives) transfusion history, of which 70.9% (39/55) occurred before 1993. There was no differentiation concerning the number of transfusion events. Multivariate analysis returned a strong association between transfusion history and rate of infection with hepatitis C virus [19]. The above results are similar to ours, except for two variables, which were different: sex and date of transfusions. The above study included both males and females, whereas ours included only females; furthermore, date of exposure to the risk factor was not reported in the above study, stating only that 39 patients had been exposed to it.
The conclusion of our study is that females with a history of blood transfusion previous to 1994 in the Guadalajara Metropolitan Area have a risk 9.07 times higher when compared to females without transfusion history previous to 1994.
With regards to the exposure rates (Table 4), recalling the exposed-case rate of 72% and the exposed-control rate of 22%, we can deduce that the higher exposure rate of cases points at a risk factor which can be attributed to the presence of blood transfusion, which can be verified by the odds ratio.
Concerning the attributable fractions (Table 5), the population attributable fraction represents the percentage of all cases within the population, which can be attributed to pre-1994 transfusion or else. In this study, the percentage was 64% of cases which could have potentially been prevented had the pre-1994 blood transfusion not occurred. Similarly, the attributable proportion among the exposed (the proportion of hepatitis C virus infection which can be attributed to the risk factor) was 88%.
Now that we have been able to measure the size of the risk factor, we can implement screening procedures and watch both asymptomatic patients as well as those with icteric syndrome (jaundice) with pre-1994 transfusion history in order to be able to start prompt treatment after early diagnosis.
Notes
From the editor
The authors originally submitted this article in Spanish and subsequently translated it into English. The Journal has not copyedited this version.
Ethical aspects
The Journal has evidence that the Local Research and Research Ethics Committee of the Hospital of Specialties of the National Medical Center of the West, Guadalajara, Mexico; was informed about this study and its possible publication in a biomedical dissemination journal.
Conflicts of Interest
The authors completed the ICMJE conflict of interest declaration form, and declare not having received funding for the preparation of this report, not having any financial relationships with organizations that could have interests in the published article in the last three years, and not having other relations or activities that might influence the article´s content. Forms can be requested to the responsible author or the editorial direction of the Journal.
Financing
The authors state that there were no external sources of funding.

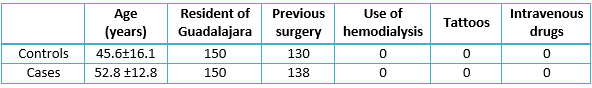 Table 1. Description of sociodemographic variables of both groups.
Table 1. Description of sociodemographic variables of both groups.

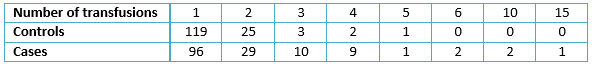 Table 2. Number of transfusions as antecedent in both groups.
Table 2. Number of transfusions as antecedent in both groups.

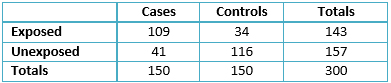 Table 3. Contingency table for cases and controls.
Table 3. Contingency table for cases and controls.

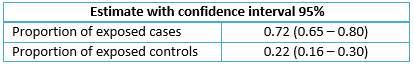 Table 4. Exposure proportions in both groups.
Table 4. Exposure proportions in both groups.

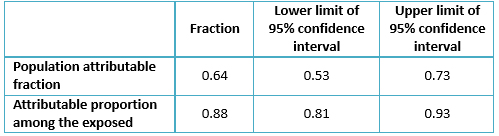 Table 5. Attributable fractions.
Table 5. Attributable fractions.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
La mayor parte de las transfusiones se llevan a cabo en mujeres. La introducción en los bancos de sangre de las técnicas serológicas disminuyó la incidencia de infección por virus de hepatitis C después de una transfusión. En México, las pacientes que se transfundieron antes de 1994 están en riesgo de presentar una infección por virus de hepatitis C. El objetivo de este estudio fue medir la asociación entre el antecedente transfusional antes de 1994 e infección por virus de hepatitis C en mujeres atendidas en la zona metropolitana de Guadalajara, México.
MÉTODOS
Estudio observacional, analítico, de casos y controles, en el que se incluyeron mujeres sanas y mujeres con infección por hepatitis vírica tipo C, en las cuales se determinó el antecedente transfusional antes y después de 1994. El grupo de casos lo conforman 150 mujeres con diagnóstico serológico y confirmatorio de hepatitis C, en tanto el grupo control son 150 mujeres sanas con serología negativa.
RESULTADOS
Se encontró un odds ratio de 9,07 (intervalo de confianza 95% 5,37 – 15,3; p<0,001), una proporción de casos expuestos de 0,72, de controles expuestos de 0,22, una fracción atribuible poblacional de 0,64 (intervalo de confianza 0,53 – 0,73) y una fracción atribuible en expuestos de 0,88 (intervalo de confianza 0,81 – 0,93).
CONCLUSIONES
En las mujeres, el haber tenido una transfusión antes de 1994 en la zona metropolitana de Guadalajara, representa un riesgo 9,07 veces mayor de infección por virus de la hepatitis C que no tener antecedente transfusional en esa fecha.
 Authors:
Christian Ramos Flores [1 ], Ernesto Echeagaray [2 ], Guadalupe Castañeda [3 ], Maria de Lourdes Vargas [4 ], Raúl Montes-González [5 ], Susana Luna [6 ], Laura Díaz [7 ], Oscar Torres [8 ]
Authors:
Christian Ramos Flores [1 ], Ernesto Echeagaray [2 ], Guadalupe Castañeda [3 ], Maria de Lourdes Vargas [4 ], Raúl Montes-González [5 ], Susana Luna [6 ], Laura Díaz [7 ], Oscar Torres [8 ]
Affiliation:
[1] Departamento de Epidemiología, Unidad de Medicina Familiar 168 Instituto Mexicano del Seguro Social, Tepatitlán, Jalisco, México
[2] Departamento de Infectología, Centro Médico Nacional de Occidente, Hospital de Especialidades Instituto Mexicano del Seguro Social, Guadalajara Jalisco, México
[3] Coordinación de Información y Análisis Estratégico, Jefatura de Servicios de Prestaciones Médicas, IMSS, Guadalajara Jalisco, México
[4] Banco de Sangre Central, Centro Médico Nacional de Occidente, Guadalajara Jalisco, México
[5] Banco de Sangre Central, Centro Médico Nacional de Occidente, Guadalajara Jalisco, México
[6] Hospital de Ginecoobstetricia, Centro Médico Nacional de Occidente, Guadalajara Jalisco, México
[7] Hospital de Ginecoobstetricia, Centro Médico Nacional de Occidente, Guadalajara Jalisco, México
[8] Banco de Sangre Central, Centro Médico Nacional de Occidente, Guadalajara Jalisco, México
E-mail: hugo_ramos@ucol.mx
Author address:
[1] Avenida Patria 3000
Edificio 23
Departamento 6
Colonia Lagos del Country
Zapopan
Jalisco
México

Citation: Ramos Flores C , Echeagaray E , Castañeda G , Vargas ML , Montes-González R , Luna S , et al. Linking hepatitis C virus infection to pre-1994 blood transfusions in female patients. Medwave 2017 Mar;17(2):e6886 doi: 10.5867/medwave.2016.02.6886
Submission date: 16/9/2016
Acceptance date: 26/1/2017
Publication date: 16/3/2017
Origin: not requested
Type of review: reviewed by three external peer reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Rodríguez-Moyado H. Enfermedades infecciosas por transfusión en México. RevMexMedTran. 2011; 4 (2):78-90. | Link |
- Blas OC. Contagio transfusional de VHC. Reflexiones sobre la previsibilidad del daño. DS 2008; 16(2):179-216. | Link |
- Manual de procedimientos estandarizados para la vigilancia epidemiológica de las hepatitis virales. SSA Mex. DGEPI. 2012.
- Engle RE, Bukh J, Alter HJ, Emerson SU, Trenbeath JL, Nguyen HT, Brockington A, Mitra T, Purcell RH. Transfusion-associated hepatitis before the screening of blood for hepatitis risk factors. Transfusion. 2014 Nov;54(11):2833-41. | CrossRef | PubMed |
- Conde González C, Torres-Poveda K, Madrid-Marina V. [The viral hepatitides].Salud Publica Mex. 2011;53 Suppl 1:S4-6. | PubMed |
- Hernández Lugo MI, Contreras NAM. Hepatitis C en el contexto de la donación sanguínea. Rev Med Inst Mex SeguroSoc 2006; 44 (2): 3-6. | Link |
- Moyer VA; U.S. Preventive Services Task Force.. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013 Sep 3;159(5):349-57. | CrossRef | PubMed |
- Blejer JL, Carreras Vescio LA, Salamone HJ. [Risk of transfusional-transmitted infections]. Medicina (B Aires). 2002;62(3):259-78. | PubMed |
- Panduro A, Roman S, Khan A, Tanaka Y, Kurbanov F, Martinez-Lopez E,et al. Molecular epidemiology of hepatitis C virus genotypes in west Mexico. Virus Res. 2010 Jul;151(1):19-25. | CrossRef | PubMed |
- Contreras AM, Sotelo M, Celis A, Villalobos DB, Ancona-Piste O, Ochoa-Jiménez RJ, López-Ramírez K. [Nosocomial transmission of hepatitis C associated with anesthesia procedures: a case-control study]. Salud Publica Mex. 2011;53 Suppl1:S19-25. | PubMed |
- Coughlin SS, Benichou J, Weed DL. Estimación del riesgo atribuible en los estudios de casos y controles. Bol Oficina Sanit Panam. 1996;121(2):143-58. | Link |
- Takeuchi LC, Pham TK, Katz AR. Hepatitis C virus antibody prevalence, demographics and associated factors among persons screened at Hawai'i community-based health settings, 2010-2013. Hawaii J Med Public Health. 2015 Jan;74(1):9-15. | PubMed |
- Zamorano, F. Donación Voluntaria de Sangre: Análisis de Estrategias de Articulación entre los Servicios de Salud y la Sociedad. Documento Técnico Área Temática IV: Políticas de Salud Pública y Control de Riesgos. EUROPEAID 2007.
- Lopez-Colombo A, Melendez-Mena A, Sedeño-Monge V, Camacho-Hernandez JR, Vazquez-Cruz E, Morales-Hernandez ER, et al. Hepatitis C virus infection in patients and family members attending two primary care clinics in Puebla, Mexico. Annals of hepatology, 2014: 13(6): 746-752. | Link |
- Santos-López G, Sosa-Jurado F, Vallejo-Ruiz V, Meléndez-Mena D, Reyes-Leyva J. Prevalence of hepatitis C virus in the Mexican population: a systematic review. J Infect. 2008 Apr;56(4):281-90. | CrossRef | PubMed |
- Valdespino JL, Conde-González CJ, Olaiz-Fernández G, Oswaldo-Palma O, Kershenobich D, Sepúlveda J. Seroprevalencia de la hepatitis C en adultos de México: ¿un problema de salud pública emergente? Salud Publica Mex. 2007;49:(suppl 3):S395-S-403. | Link |
- Chiquete E, Panduro A. Low prevalence of anti-hepatitis C virus antibodies in Mexico: A systematic review. Intervirology. 2007;50(1):1-8. Review. | PubMed |
- Ministério da Saúde. Estudo de prevalência de base populacional das infecções pelos vírus das hepatites A, B e C nascapitais do Brasil.Boletim Epidemiológico HepatitesVirais. Brasília: Ministério da Saúde; 2010:11-15.
- Rosa RS, Martinelli Ade L, Passos AD. Risk factors for hepatitis C virus transmission in the municipality of Catanduva, State of São Paulo: a case-control study. Rev Soc Bras Med Trop. 2014 May-Jun;47(3):295-301. | PubMed |
 Rodríguez-Moyado H. Enfermedades infecciosas por transfusión en México. RevMexMedTran. 2011; 4 (2):78-90. | Link |
Rodríguez-Moyado H. Enfermedades infecciosas por transfusión en México. RevMexMedTran. 2011; 4 (2):78-90. | Link | Blas OC. Contagio transfusional de VHC. Reflexiones sobre la previsibilidad del daño. DS 2008; 16(2):179-216. | Link |
Blas OC. Contagio transfusional de VHC. Reflexiones sobre la previsibilidad del daño. DS 2008; 16(2):179-216. | Link | Manual de procedimientos estandarizados para la vigilancia epidemiológica de las hepatitis virales. SSA Mex. DGEPI. 2012.
Manual de procedimientos estandarizados para la vigilancia epidemiológica de las hepatitis virales. SSA Mex. DGEPI. 2012.  Engle RE, Bukh J, Alter HJ, Emerson SU, Trenbeath JL, Nguyen HT, Brockington A, Mitra T, Purcell RH. Transfusion-associated hepatitis before the screening of blood for hepatitis risk factors. Transfusion. 2014 Nov;54(11):2833-41. | CrossRef | PubMed |
Engle RE, Bukh J, Alter HJ, Emerson SU, Trenbeath JL, Nguyen HT, Brockington A, Mitra T, Purcell RH. Transfusion-associated hepatitis before the screening of blood for hepatitis risk factors. Transfusion. 2014 Nov;54(11):2833-41. | CrossRef | PubMed | Conde González C, Torres-Poveda K, Madrid-Marina V. [The viral hepatitides].Salud Publica Mex. 2011;53 Suppl 1:S4-6. | PubMed |
Conde González C, Torres-Poveda K, Madrid-Marina V. [The viral hepatitides].Salud Publica Mex. 2011;53 Suppl 1:S4-6. | PubMed | Hernández Lugo MI, Contreras NAM. Hepatitis C en el contexto de la donación sanguínea. Rev Med Inst Mex SeguroSoc 2006; 44 (2): 3-6. | Link |
Hernández Lugo MI, Contreras NAM. Hepatitis C en el contexto de la donación sanguínea. Rev Med Inst Mex SeguroSoc 2006; 44 (2): 3-6. | Link | Moyer VA; U.S. Preventive Services Task Force.. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013 Sep 3;159(5):349-57. | CrossRef | PubMed |
Moyer VA; U.S. Preventive Services Task Force.. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013 Sep 3;159(5):349-57. | CrossRef | PubMed | Blejer JL, Carreras Vescio LA, Salamone HJ. [Risk of transfusional-transmitted infections]. Medicina (B Aires). 2002;62(3):259-78. | PubMed |
Blejer JL, Carreras Vescio LA, Salamone HJ. [Risk of transfusional-transmitted infections]. Medicina (B Aires). 2002;62(3):259-78. | PubMed | Panduro A, Roman S, Khan A, Tanaka Y, Kurbanov F, Martinez-Lopez E,et al. Molecular epidemiology of hepatitis C virus genotypes in west Mexico. Virus Res. 2010 Jul;151(1):19-25. | CrossRef | PubMed |
Panduro A, Roman S, Khan A, Tanaka Y, Kurbanov F, Martinez-Lopez E,et al. Molecular epidemiology of hepatitis C virus genotypes in west Mexico. Virus Res. 2010 Jul;151(1):19-25. | CrossRef | PubMed | Contreras AM, Sotelo M, Celis A, Villalobos DB, Ancona-Piste O, Ochoa-Jiménez RJ, López-Ramírez K. [Nosocomial transmission of hepatitis C associated with anesthesia procedures: a case-control study]. Salud Publica Mex. 2011;53 Suppl1:S19-25. | PubMed |
Contreras AM, Sotelo M, Celis A, Villalobos DB, Ancona-Piste O, Ochoa-Jiménez RJ, López-Ramírez K. [Nosocomial transmission of hepatitis C associated with anesthesia procedures: a case-control study]. Salud Publica Mex. 2011;53 Suppl1:S19-25. | PubMed | Coughlin SS, Benichou J, Weed DL. Estimación del riesgo atribuible en los estudios de casos y controles. Bol Oficina Sanit Panam. 1996;121(2):143-58. | Link |
Coughlin SS, Benichou J, Weed DL. Estimación del riesgo atribuible en los estudios de casos y controles. Bol Oficina Sanit Panam. 1996;121(2):143-58. | Link | Takeuchi LC, Pham TK, Katz AR. Hepatitis C virus antibody prevalence, demographics and associated factors among persons screened at Hawai'i community-based health settings, 2010-2013. Hawaii J Med Public Health. 2015 Jan;74(1):9-15. | PubMed |
Takeuchi LC, Pham TK, Katz AR. Hepatitis C virus antibody prevalence, demographics and associated factors among persons screened at Hawai'i community-based health settings, 2010-2013. Hawaii J Med Public Health. 2015 Jan;74(1):9-15. | PubMed | Zamorano, F. Donación Voluntaria de Sangre: Análisis de Estrategias de Articulación entre los Servicios de Salud y la Sociedad. Documento Técnico Área Temática IV: Políticas de Salud Pública y Control de Riesgos. EUROPEAID 2007.
Zamorano, F. Donación Voluntaria de Sangre: Análisis de Estrategias de Articulación entre los Servicios de Salud y la Sociedad. Documento Técnico Área Temática IV: Políticas de Salud Pública y Control de Riesgos. EUROPEAID 2007.  Lopez-Colombo A, Melendez-Mena A, Sedeño-Monge V, Camacho-Hernandez JR, Vazquez-Cruz E, Morales-Hernandez ER, et al. Hepatitis C virus infection in patients and family members attending two primary care clinics in Puebla, Mexico. Annals of hepatology, 2014: 13(6): 746-752. | Link |
Lopez-Colombo A, Melendez-Mena A, Sedeño-Monge V, Camacho-Hernandez JR, Vazquez-Cruz E, Morales-Hernandez ER, et al. Hepatitis C virus infection in patients and family members attending two primary care clinics in Puebla, Mexico. Annals of hepatology, 2014: 13(6): 746-752. | Link | Santos-López G, Sosa-Jurado F, Vallejo-Ruiz V, Meléndez-Mena D, Reyes-Leyva J. Prevalence of hepatitis C virus in the Mexican population: a systematic review. J Infect. 2008 Apr;56(4):281-90. | CrossRef | PubMed |
Santos-López G, Sosa-Jurado F, Vallejo-Ruiz V, Meléndez-Mena D, Reyes-Leyva J. Prevalence of hepatitis C virus in the Mexican population: a systematic review. J Infect. 2008 Apr;56(4):281-90. | CrossRef | PubMed | Valdespino JL, Conde-González CJ, Olaiz-Fernández G, Oswaldo-Palma O, Kershenobich D, Sepúlveda J. Seroprevalencia de la hepatitis C en adultos de México: ¿un problema de salud pública emergente? Salud Publica Mex. 2007;49:(suppl 3):S395-S-403. | Link |
Valdespino JL, Conde-González CJ, Olaiz-Fernández G, Oswaldo-Palma O, Kershenobich D, Sepúlveda J. Seroprevalencia de la hepatitis C en adultos de México: ¿un problema de salud pública emergente? Salud Publica Mex. 2007;49:(suppl 3):S395-S-403. | Link | Chiquete E, Panduro A. Low prevalence of anti-hepatitis C virus antibodies in Mexico: A systematic review. Intervirology. 2007;50(1):1-8. Review. | PubMed |
Chiquete E, Panduro A. Low prevalence of anti-hepatitis C virus antibodies in Mexico: A systematic review. Intervirology. 2007;50(1):1-8. Review. | PubMed | Ministério da Saúde. Estudo de prevalência de base populacional das infecções pelos vírus das hepatites A, B e C nascapitais do Brasil.Boletim Epidemiológico HepatitesVirais. Brasília: Ministério da Saúde; 2010:11-15.
Ministério da Saúde. Estudo de prevalência de base populacional das infecções pelos vírus das hepatites A, B e C nascapitais do Brasil.Boletim Epidemiológico HepatitesVirais. Brasília: Ministério da Saúde; 2010:11-15.  Rosa RS, Martinelli Ade L, Passos AD. Risk factors for hepatitis C virus transmission in the municipality of Catanduva, State of São Paulo: a case-control study. Rev Soc Bras Med Trop. 2014 May-Jun;47(3):295-301. | PubMed |
Rosa RS, Martinelli Ade L, Passos AD. Risk factors for hepatitis C virus transmission in the municipality of Catanduva, State of São Paulo: a case-control study. Rev Soc Bras Med Trop. 2014 May-Jun;47(3):295-301. | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








