Abstract
BACKGROUND
Modafinil is a drug developed and used for the treatment of excessive lethargy. Even though very effective for sleep disorders, it is still controversial whether modafinil can improve performance in high-order cognitive processes such as memory and executive function.
METHODS
This randomized, double-blind, placebo-controlled, crossover trial was designed to evaluate the effect of modafinil (compared to placebo) on the cognitive functions of healthy students. 160 volunteers were recruited and allocated randomly to modafinil or placebo group, and were assessed using the Stroop Test, BCET test and Digit span test.
RESULTS
We found a significant difference in favor of modafinil compared to placebo in the proportion of correct answers of Stroop Test in congruent situation. A significant shorter latency of modafinil group in the incongruent situation of Stroop test was also found. No differences were found in Digit Span, or BCET tests.
CONCLUSIONS
The study demonstrated that modafinil does not enhance the global cognitive performance of healthy non-sleep deprived students, except regarding non-demanding tasks. In particular, this drug does not seem to have positive effects on mental processes that sustain studying tasks in the college population under normal conditions. We expect these findings to demystify the use of this drug and help decision making concerning pharmacological public policies.
Introduction
Modafinil (2-[(diphenylmethyl) sulfinyl] acetamide) is a psychostimulant with wakefulness-promoting properties. It was available for commercial use in France in the 1990s [1]. Modafinil has demonstrated particular effectiveness for treating lethargy and sleep disorders [2],[3],[4],[5],[6],[7].
Some authors state that modafinil increases performance of tasks associated with cognitive functions such as working memory, visuospatial attention, and executive function [8],[9],[10],[11],[12],[13],[14]. For others, modafinil improves cognitive performance in healthy sleep deprived adults, but only in regard to attentional function and alertness [8],[9].
The primary mechanism of action is unknown, [15] although its effects on different neurotransmitter systems have been demonstrated [1],[16],[17],[18],[19],[20],[21].
The use of stimulants to improve academic performance has increased among the young population in the U.S. [22]. More than seven million Americans use over-the-counter stimulants, and approximately 1.6 million of these people are students [23],[24]. An online survey of more than 1,400 people from sixty countries showed that twenty percent of respondents had used a psychoactive drug to enhance their concentration or memory, and forty-four percent of them had used modafinil. The population most often associated with stimulant use to enhance attention and memory are students between eighteen and twenty-five years [25].
No formal studies have been reported on the use of modafinil among students in Chile. However, some media surveys reported an increase in the consumption of stimulants in students between nineteen and twenty-five years old, with higher consumption levels occurring during final examination periods [26],[27].
The effect of modafinil on attention
Most of the research conducted on healthy adults to investigate whether stimulants improve cognitive performance has produced either contradictory or inconclusive results [28]. Although modafinil plays a key role in certain cognitive functions, such as attention, [29],[30] and tasks that demand a certain level of performance, it seems to have little effect when higher exigency is taken into account [31].
Trials that have assessed attention in adults demonstrate advantages among those taking modafinil compared to placebo with regard to attention, using the “stop-signal task,” [29] but this result seems to be inversely correlated with IQ [30],[31],[32].
A study conducted with young volunteers also found evidence of a positive effect on the “Attention Shift Task” (a particularly demanding task) following modafinil intake. Modafinil appears to promote the rapid switching of attention in demanding conditions, although it offers minimal benefit when an unpredictable and infrequent disengagement of attention is required to respond to alternative stimuli in an ongoing task [31].
A clinical trial with forty-five non-sleep-deprived participants did not find conclusive results with regard to attentional performance, possibly due to the small sample size [33]. This finding demonstrates that the evidence in favor of modafinil as an attention enhancer appears to be ambiguous. This is why it is relevant to inspect the role this substance plays with regard to certain attentional features. In particular, selective attention (the inhibition of a response towards irrelevant information), is a key process for proper learning [34]. This dimension of attention has been selected in this research due to the fact that it has been demonstrated that impairments in selective attention among high school and university students are usually accompanied by low information processing and learning capabilites, and therefore, by a deficient academic performance [35].
Memory and executive function
Studies aiming to prove that modafinil can improve performance in high-order cognitive processes such as memory and executive function in healthy participants have been controversial, [29],[30],[33],[36] and no systematic reviews regarding its impact have been made available so far.
The ambiguity of these previous findings motivated the current research. Our goal was to assess the effects of modafinil on the cognitive performance of university students, particularly with regard to short-term memory, executive function, and attention.
Methods
Design
This randomized, double-blind, placebo-controlled, crossover trial was designed to assess the effect of modafinil (compared to placebo) on the cognitive functions of healthy students. It was conducted at the University of Valparaíso, Chile. The protocol was approved by the Institutional Review Board at Faculty of Medicine Universidad de Valparaíso under authorization code 04/2010 CEFM, and was registered on the Clinical Trials webpage (http://clinicaltrials.gov/) under NCT code 01365897.
Participants
Eligible participants included students who were pursuing health sciences degrees, aged eighteen to twenty-nine, and were recruited using open advertisements on a website. Medicine and psychology students (n=180) of both genders were contacted, and those who met the inclusion criteria (n=162) were recruited. The sample size was 155, and was estimated for a two-sample comparison of means considering (a) a two-sided significance level of p=0.05, (b) a power (1-beta) of=0.80 and (c) an expected difference from 19 (SD=0.7) to 19.25 (SD=0.7), and twenty percent possible loss. The inclusion criteria were:
a) Aged between 18 and 29: This age range was chosen because there is a normal physiological cognitive impairment of 1% of total IQ between 25 and 29 years [37]. The purpose of this criterium was working with a sample without cognitive deterioration, which could modify memory performance.
b) Student in health sciences programs.
c) Healthy weight. The rationale behind this criterium comes from the fact that all volunteers received the same dose of modafinil; subjects outside this range might have yielded different plasmatic levels.
The exclusion criteria were:
a) Mental disorders: Assessed by his/her clinical history or by achieving a pathological score in Prime-MD [38].
b) Current use of psychotropic substances of any type.
c) Alcohol intake up to three days before the experiment.
d) History or symptoms of current or chronic physical illness.
e) Pregnant or lactating women.
f) Sleep-deprived.
Outcome
The primary outcome was the attention score measured by the proportion of correct answers on the Stroop Test. Additional analyses were conducted that compared the percentage of correct answers and latencies on the Stroop Test, the Digit Span task, and the Biber Cognitive Estimation Test in both groups.
Procedure
The participants were recruited from various courses of the aforementioned programs and did not receive any financial compensation. They provided informed consent, and the self-administered Prime-MD was used to evaluate their psychiatric conditions. Those who did not meet any exclusion criterion were deemed eligible to participate in this investigation. Subjects were briefly interviewed in order to know if they were in any excluding condition regarding sleep deprivation or substance intake (psychotropic drugs or alcohol use).
Once the final sample was recruited, the volunteers were appointed in randomized groups of twenty participants each, with all twenty participants being tested simultaneously. This was done to ensure that the tests were properly applied, according to the conditions and capacity of the Cognitive Psychology Laboratory of the School of Psychology, and in order to achieve adequate plasmatic levels of modafinil during administration of instruments [39]. The allocation of the participants to the experimental or control groups was performed randomly by a computer-based allocation program, and the sequence was concealed by the principal investigator. On day one, participants received Substance A or B, which corresponded to 200 mg of modafinil or a placebo (placebo pills were made at the Faculty of Pharmacy with same shape and color as the modafinil pills used in this experiment). The volunteers were assessed with the instruments 120 minutes after drug administration to attain the highest plasmatic level [39]. The first experimental phase evaluated attention and executive function using the Stroop Test and the BCET, respectively. Next, we evaluated short-term memory using the Digit Span Test. These tests were administered on computers using MediaLab Software © (New York, USA). After a seven day washout period to allow the modafinil to clear, the participants returned and were assigned to the other arm [39]. Thus, each person received both the modafinil and the placebo during the trial.
Instruments
Stroop Test [40]: The University of Iowa Stroop Test was utilized, in the computerized adaptation via Medialab software. This test requires participants to read color names (blue, green, red, yellow) that are also printed in color (blue, green, red, yellow). Their task is to identify the color in which each word is printed, disregarding the meaning of the word. There were two conditions: a congruent condition, in which the color name and the color of the font are the same, and an incongruent condition, in which the color name and the color of the font are different. The variables recorded were: answer precision (the correct naming of the color) and response latency (in milliseconds). This recording method yields two types of scoring: precision and latency for both the congruent and incongruent condition. The Stroop test primarily assesses selective attention, given that the participant must ignore distractor stimuli on the incongruent condition. According to a systematic review by McLeod, [41] the Stroop Test has been the most widely-used instrument to evaluate this cognitive function.
Forward and Backward Digit Span [37]: The "Digit Span" test is composed of a series of digits shown to the volunteer and measures short-term memory span, attentional skill, and sequencing ability. The test is composed of two sections: forward and backward. In the forward section, the stimuli must be repeated in sequence; in the backward section, the stimuli must be repeated in reverse order. The maximum score for the forward Digit Span is 8 points, and the maximum score for the Backward Digit Span is 7 points, which yields a total maximum score of 15 points. This measure has high split-half reliability (r=0.89),[42] and acceptable test-retest reliability (r=0.80) [43].
The Biber Cognitive Estimation Test (BCET) [44]: The BCET consists of twenty items, five items in each of the following categories: time/duration, quantity, weight, and distance/length. The test requires participants to approximate the answers to questions that do not have readily apparent answers. For example, “What is the average length of a man’s spine?” requires the participant to select an appropriate answer and estimate its plausibility, but does not require complex computation [45]. Each answer that falls within a previously established range scores one point. The maximum score for this test is twenty points.
Design and data analysis
We chose a randomized, crossover study design based on the advantageous characteristic that each participant acts as his or her own control. The allocation sequence was concealed by the principal investigator from volunteers, instructors, and the data analysts.
Based on the methodological design employed, we used paired t-tests to compare means. Specifically, the analysis compared the means obtained for each item in the experimental and placebo condition. The statistical analysis was performed with Stata 12.0 (Statacorp, College Station, Texas, USA). The significance level was considered with p <0.05.
Results
Prior to exclusion, the original sample was composed of 180 medical and psychology students, with ages between eighteen to twenty years old. As shown in the flowchart (figure 1) eighteen participants were not included because of history of either mental disorders, abnormal Prime-MD scores, or both. One hundred sixty-two participants were randomly assigned to the groups; however, thirty-four people did not complete the study. A final sample of 128 volunteers (76 women) completed the trial.
Table 1. Baseline characteristics subjects recruited for modafinil trial with means (SD).
Outcomes
- Stroop Test: A significant difference was found between the experimental and control groups with regard to the proportion of correct answers in the congruent condition (p=0.01); however, no difference was found for the incongruent condition (p=0.81). As shown in Table 2, there is a significantly shorter latency for the modafinil group in the incongruent condition (p<0.05), but no latency difference in the congruent situation (p=0.15).
- BCET: No significant differences were found between the experimental and control groups with regard to the BCET (items 0-20; p=0.26; Table 2).
- Digit Span: The total mean score of correct answers in the Digit Span Test did not reveal significant between-group differences overall (p=0.26), nor were there differences for the forward (p=0.85) and backward (p=0.93) conditions (Table 2).
Discussion
This study showed a positive effect of modafinil on the cognitive performance of healthy non sleep-deprived young university students. Differences were found only with regard to the higher precision of participants using modafinil in the congruent condition and their shorter latencies in the incongruent condition of the Stroop Test. The modafinil group did not show advantages over the placebo group with regard to short-term memory or executive function.
Considering that the major strength of this work is methodological because the crossover design is robust in pharmacological evaluations--as long as drug clearance can be assured--this research addresses a phenomenon that, in our opinion, is highly relevant to university students. Health and education policies should consider the possible abuse of this drug, given the belief that it optimizes studying performance.
We found significant between-group differences with regard to Stroop Test performance in the congruent condition, but not in the incongruent condition. These results are inconsistent with previous findings [30][31],[32][31],[46]. We believe that this discrepancy is due to several differences (i.e., sample size, age range, sleep-deprivation status, experimental protocols, etc) that make it difficult to compare our results with those of other studies.
Given that the congruent condition demands less cognitive resources than the incongruent condition, this result confirms that modafinil enhances selective attentional performance when the task has low cognitive exigency. This result conflicts with those reported by Marchant, who reported that participants using modafinil attained a better specific performance on the Attention Shift Task for both constant and alternating condition (the latter of which has a high level of difficulty). However, this task is not directly comparable to the Stroop Test. Marchant states that the Attention Shift Task mimics an event-based prospective memory (PM), which requires a person to interrupt an ongoing activity to retrieve and act upon a previously formed intention [31]. In a standard PM task, participants exhibit distinct responses when they recognize new targets that are associated with a previously formed intention. These targets appear relatively infrequently and draw attentional resources. Given that modafinil increases arousal and that heightened arousal has been shown to improve sustained attention, attention switching, and PM, [31] this drug may improve performance in PM-like tasks such as Attention Shifting Task, but not regarding selective attention.
In contrast to this result, several studies have demonstrated a lack of physiological or subjective effects of modafinil on arousal. However, they have observed an increase in cognitive function [11],[32],[47],[48],[49]. In principle, attention-shifting requires similar resources to PM. In fact, the literature tends to assume that PM and task-switching capabilities are governed by the same brain regions (i.e. the prefrontal cortex) [50],[51]. However, whereas one requires continuous, rapid shifts of attention, the other requires disengagement from an attention-demanding task to successfully detect and respond to a rarely occurring target at the appropriate time.
This observation is not surprising, and it parallels the inconclusive results of Randall, possibly due to modafinil's best effect when used for disadvantageous conditions (e.g., illness or sleep deprivation), and to restore the basal cognitive level [33]. This means that it allows the nervous system to function and attain full arousal levels, but there is no evidence for cognition improvement in an already awakened individual.
An unexpected finding of our trial was that the Stroop Test latency in the incongruent condition was significantly shorter in the modafinil group compared to the control group. Thus, modafinil does not improve precision but it does shorten reaction time in the incongruent condition.
The participants did not show significant between- group differences with regard to working memory or executive function. Although our results diverge with those of Turner and Randall [29],[32],[46], they agree with those of Baranski, et al. and Müller [30],[36]. This result may be due to the effects of modafinil being mediated by a plethora of variables that have not been fully studied (particularly IQ) [52].
The benefits of modafinil on different memory and executive function for people with sleep disorders or pathologies that involve attention impairment appear irrefutable [1], but our goal to make results more generalizable to healthy populations remains ambiguous and requires further research.
Although participants were told to be well-rested, they may have misreported, or they may have had sleep disorders. Therefore, a potential limitation of this study is that sleep quality was not assessed with the exception of the briefing protocol. It must be considered that our design and the large sample size allowed us to detect very significant statistical differences regarding the Stroop test; whether these statistical differences are clinically relevant may be debatable.
With regard to the ecological validity of this study, it should be noted that modafinil is consumed by students in order to improve academic performance. For this reason, studies like the current trial, which assess the effectiveness of this drug using tasks that assess studying abilities, are pertinent. Nevertheless, it is advisable to complement this research with investigations directed toward other aspects of memory and executive function.
As a conclusion, modafinil does not enhance the global cognitive performance of healthy non-sleep-deprived students, except regarding non-demanding tasks. In particular, this drug does not seem to have positive effects on the basic mental processes that sustain studying tasks in the college population under normal conditions. We expect these findings to demystify the use of this drug and help decision making concerning pharmacological public policies.
Notes
Conflicts of Interests
The authors have completed the conflict of interests declaration form from the ICMJE, and declare not having any conflict of interests with the matter dealt herein. Forms can be requested to the responsible author or the editorial direction of the Journal.
Ethical issues
Participants signed an informed consent form. The protocol was approved by the Institutional Review Board at Faculty of Medicine Universidad de Valparaíso under authorization code 04/2010 CEFM, and was registered on the Clinical Trials website (http://clinicaltrials.gov/) under code NCT01365897.
Institutional support
Dirección de Investigación de la Universidad de Valparaíso - Grant 05/2006.

 Figure 1: CONSORT flow diagram that graphically outlines the design and conduction of the clinical trial.
Figure 1: CONSORT flow diagram that graphically outlines the design and conduction of the clinical trial.

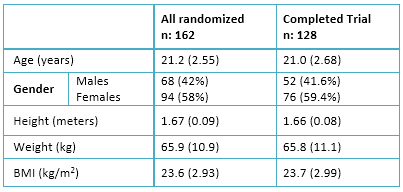 Table 1. Baseline characteristics subjects recruited for modafinil trial with means (SD).
Table 1. Baseline characteristics subjects recruited for modafinil trial with means (SD).

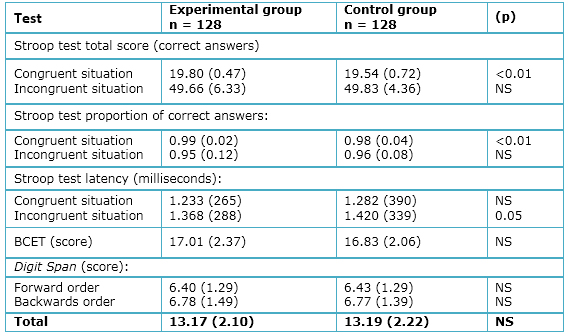 Table 2. Comparison of values of Stroop test, BCET, and Digit Span scores between modafinil and placebo condition, with means (SD) and p values (paired T test).
Table 2. Comparison of values of Stroop test, BCET, and Digit Span scores between modafinil and placebo condition, with means (SD) and p values (paired T test).
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

ANTECEDENTES
El modafinilo es un fármaco desarrollado para el tratamiento del letargo extremo. A pesar de estar probada su efectividad en la intervención de los trastornos del sueño, todavía existe la controversia sobre si es que puede mejorar el desempeño del sujeto en procesos cognitivos superiores, como la memoria y las funciones ejecutivas.
MÉTODOS
Este ensayo clínico aleatorizado, de diseño crossover, doble ciego, controlado por placebo; fue elaborado para evaluar el efecto del modafinilo comparado con un placebo, en las funciones cognitivas de estudiantes universitarios sanos. Se reclutaron 162 voluntarios, asignados aleatoriamente a un grupo de consumo de modafinilo o a un grupo de consumo de placebo. Ambos grupos fueron evaluados usando el Test de Stroop, el Test Biber de Estimación Cognitiva y el Digit Span Test (test de amplitud de memoria de dígitos). Luego de 15 días, fueron asignados a la otra intervención.
RESULTADOS
Se encontró una diferencia significativa en favor del modafinilo comparado con el placebo en la proporción de respuestas correctas del Test de Stroop en la situación congruente. También se encontró una menor latencia de respuesta entre los sujetos que consumieron modafinilo en la situación incongruente de ese mismo test. No se encontraron diferencias en el Digit Span o el Test Biber de Estimación Cognitiva.
CONCLUSIONES
Este estudio demuestra que el modafinilo no mejora el desempeño cognitivo global de estudiantes sanos sin deprivación de sueño, excepto en lo que respecta a tareas que no son demandantes. En particular, este fármaco no parece tener efectos positivos en los procesos mentales que sustentan actividades de estudio en la población universitaria bajo condiciones normales. Se espera que estos hallazgos desmitifiquen el uso de este neuroestimulante y contribuya a la toma de decisiones sobre políticas farmacológicas públicas.
 Authors:
Alejandro Fernández[1], Franco Mascayano[2], Walter Lips[1], Andrés Painel[3], Jonathan Norambuena[3], Eva Madrid[4,5,6]
Authors:
Alejandro Fernández[1], Franco Mascayano[2], Walter Lips[1], Andrés Painel[3], Jonathan Norambuena[3], Eva Madrid[4,5,6]
Affiliation:
[1] Escuela de Psicología, Universidad de Valparaíso, Valparaíso, Chile
[2] Escuela de Salud Pública, Facultad de Medicina, Universidad de Chile, Santiago, Chile
[3] Escuela de Medicina, Universidad de Valparaíso, Valparaíso, Chile
[4] Iberoamerican Cochrane Centre, Barcelona, Spain
[5] Centro de Investigaciones Biomédicas, Escuela de Medicina, Universidad de Valparaíso, Valparaíso, Chile
[6] Departamento de Salud Pública, Escuela de Medicina, Universidad de Valparaíso, Valparaíso, Chile
E-mail: emadrida@hsph.harvard.edu
Author address:
[1] Hontaneda 1653
Of. 401
Valparaíso
Chile.

Citation: Fernández A, Mascayano F, Lips W, Painel A, Norambuena J, Madrid E. Effects of modafinil on attention performance, short-term memory and executive function in university students: a randomized trial. Medwave 2015 Jun;15(5):e6166 doi: 10.5867/medwave.2015.05.6166
Submission date: 31/3/2015
Acceptance date: 5/6/2015
Publication date: 30/6/2015
Type of review: reviewed by two external peer reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008 Jun;33(7):1477-502. | PubMed |
- Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006 Apr;67(4):554-66. | PubMed |
- Black JE, Hull SG, Tiller J, Yang R, Harsh JR. The long-term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open-label extension study. J Clin Sleep Med. 2010 Oct 15;6(5):458-66. | PubMed |
- Czeisler CA, Walsh JK, Roth T, Hughes RJ, Wright KP, Kingsbury L, et al. Modafinil in Shift Work Sleep Disorder Study Group. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. 2005 Aug 4;353(5):476-86. Erratum in: N Engl J Med. 2005 Sep 8;353(10):1078. | PubMed |
- Darwish M, Kirby M, D'Andrea DM, Yang R, Hellriegel ET, Robertson P Jr. Pharmacokinetics of armodafinil and modafinil after single and multiple doses in patients with excessive sleepiness associated with treated obstructive sleep apnea: a randomized, open-label, crossover study. Clin Ther. 2010 Nov;32(12):2074-87. | CrossRef | PubMed |
- Lavault S, Dauvilliers Y, Drouot X, Leu-Semenescu S, Golmard JL, Lecendreux M, et al. Benefit and risk of modafinil in idiopathic hypersomnia vs. narcolepsy with cataplexy. Sleep Med. 2011 Jun;12(6):550-6. | CrossRef | PubMed |
- Bastuji H, Jouvet M. Successful treatment of idiopathic hypersomnia and narcolepsy with modafinil. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(5):695-700. | PubMed |
- Gill M, Haerich P, Westcott K, Godenick KL, Tucker JA. Cognitive performance following modafinil versus placebo in sleep-deprived emergency physicians: a double-blind randomized crossover study. Acad Emerg Med. 2006 Feb;13(2):158-65. Epub 2006 Jan 25. Erratum in: Acad Emerg Med. 2006 Apr;13(4):477. | PubMed |
- Killgore WD, Kahn-Greene ET, Grugle NL, Killgore DB, Balkin TJ. Sustaining executive functions during sleep deprivation: A comparison of caffeine, dextroamphetamine, and modafinil. Sleep. 2009 Feb;32(2):205-16. | PubMed |
- Roth T, Rippon GA, Arora S. Armodafinil improves wakefulness and long-term episodic memory in nCPAP-adherent patients with excessive sleepiness associated with obstructive sleep apnea. Sleep Breath. 2008 Mar;12(1):53-62. | PubMed |
- Saletu M, Anderer P, Semlitsch HV, Saletu-Zyhlarz GM, Mandl M, Zeitlhofer J, et al. Low-resolution brain electromagnetic tomography (LORETA) identifies brain regions linked to psychometric performance under modafinil in narcolepsy. Psychiatry Res. 2007 Jan 15;154(1):69-84. | PubMed |
- Schwartz JR, Hirshkowitz M, Erman MK, Schmidt-Nowara W. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea: a 12-week, open-label study. Chest. 2003 Dec;124(6):2192-9. | PubMed |
- Wesensten NJ, Belenky G, Kautz MA, Thorne DR, Reichardt RM, Balkin TJ. Maintaining alertness and performance during sleep deprivation: modafinil versus caffeine. Psychopharmacology (Berl). 2002 Jan;159(3):238-47. | PubMed |
- Wesensten NJ. Effects of modafinil on cognitive performance and alertness during sleep deprivation. Curr Pharm Des. 2006;12(20):2457-71. | PubMed |
- Gerrard P, Malcolm R. Mechanisms of modafinil: A review of current research. Neuropsychiatr Dis Treat. 2007 Jun;3(3):349-64. | PubMed |
- Ferraro L, Antonelli T, O'Connor WT, Tanganelli S, Rambert FA, Fuxe K. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett. 1998 Sep 4;253(2):135-8. | PubMed |
- Ferraro L, Fuxe K, Agnati L, Tanganelli S, Tomasini MC, Antonelli T. Modafinil enhances the increase of extracellular serotonin levels induced by the antidepressant drugs fluoxetine and imipramine: a dual probe microdialysis study in awake rat. Synapse. 2005 Mar 15;55(4):230-41. | PubMed |
- Ferraro L, Fuxe K, Tanganelli S, Tomasini MC, Rambert FA, Antonelli T. Differential enhancement of dialysate serotonin levels in distinct brain regions of the awake rat by modafinil: possible relevance for wakefulness and depression. J Neurosci Res. 2002 Apr 1;68(1):107-12. | PubMed |
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006 Nov;319(2):561-9. | PubMed |
- Huang Q, Zhang L, Tang H, Wang L, Wang Y. Modafinil modulates GABA-activated currents in rat hippocampal pyramidal neurons. Brain Res. 2008 May 7;1208:74-8. | CrossRef | PubMed |
- Ishizuka T, Murotani T, Yamatodani A. Modanifil activates the histaminergic system through the orexinergic neurons. Neurosci Lett. 2010 Oct 15;483(3):193-6. | CrossRef | PubMed |
- Nixey C. Are 'smart drugs' safe for students? The Guardian 2010.
- Garreau J. Dose of Genius: ‘Smart Pills’ Are on The Rise. But Is Taking Them Wise? The Washington Post. June 11, 2006 [on line] | Link |
- NSDUH. Substance abuse and mental Health Services administration. Results from the 2008 National Survey on Drug Use and Helth: National findings Publication Nº SMA 09-4434. Rockville MD: Office of Applied Studies, NDUH Series H-36, 2009.
- Maher B. Poll results: look who's doping. Nature. 2008 Apr 10;452(7188):674-5. | CrossRef | PubMed |
- Mohor D. Los riesgos del doping univesitario. El Mercurio, 08 de abril de 2009; Tendencias [on line]. | Link |
- Lazcano P. La pastilla del día antes del examen. La Nación. 23 de junio de 2009; Vida y estilo [on line]. | Link |
- Della Marca G, Restuccia D, Rubino M, Maiese T, Tonali P. Influence of modafinil on somatosensory input processing in the human brain-stem. Clin Neurophysiol. 2004 Apr;115(4):919-26. | PubMed |
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl). 2003 Jan;165(3):260-9. | PubMed |
- Baranski JV, Pigeau R, Dinich P, Jacobs I. Effects of modafinil on cognitive and meta-cognitive performance. Hum Psychopharmacol. 2004 Jul;19(5):323-32. | PubMed |
- Marchant NL, Kamel F, Echlin K, Grice J, Lewis M, Rusted JM. Modafinil improves rapid shifts of attention. Psychopharmacology (Berl). 2009 Jan;202(1-3):487-95. | CrossRef | PubMed |
- Randall DC, Viswanath A, Bharania P, Elsabagh SM, Hartley DE, Shneerson JM, et al. Does modafinil enhance cognitive performance in young volunteers who are not sleep-deprived? J Clin Psychopharmacol. 2005 Apr;25(2):175-9. | PubMed |
- Randall DC, Fleck NL, Shneerson JM, File SE. The cognitive-enhancing properties of modafinil are limited in non-sleep-deprived middle-aged volunteers. Pharmacol Biochem Behav. 2004 Mar;77(3):547-55. | PubMed |
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001 Mar;178:200-6. | PubMed |
- Müller U, Steffenhagen N, Regenthal R, Bublak P. Effects of modafinil on working memory processes in humans. Psychopharmacology (Berl). 2004 Dec;177(1-2):161-9. | PubMed |
- Muller U, Steffenhagen N, Regenthal R, et al. Effects of modafinil on working memory processes in humans. Psychopharmacology 2004;177(1-2):161-9.
- Wechsler D. Wechsler adult intelligence scale (3rd ed.). San Antonio, TX: Psychological Corporation; 1997.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999 Nov 10;282(18):1737-44. | PubMed |
- Robertson P Jr, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet. 2003;42(2):123-37. | PubMed |
- Golden CJ. A group version of the Stroop Color and Word Test. J Pers Assess. 1975 Aug;39(4):386-8. | PubMed |
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991 Mar;109(2):163-203. | PubMed |
- Abad F, Colom R, Juan-Espinosa M, García LF. Intelligence differentiation in adult samples. Intelligence 2003;31:157-66. | Link |
- Barr WB. Neuropsychological testing of high school athletes. Preliminary norms and test-retest indices. Arch Clin Neuropsychol. 2003 Jan;18(1):91-101. | PubMed |
- Bullard SE, Fein D, Gleeson MK, Tischer N, Mapou RL, Kaplan E. The biber cognitive estimation test. Arch clin neuropsychol. 2004 Sep;19(6):835-46. Erratum in: Arch Clin Neuropsychol. 2005 Mar;20(2):279. | PubMed |
- Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Third Edition. Oxford: Oxford University Press; 2005.
- Randall DC, Shneerson JM, Plaha KK, File SE. Modafinil affects mood, but not cognitive function, in healthy young volunteers. Hum Psychopharmacol. 2003 Apr;18(3):163-73. | PubMed |
- Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ. Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2004 May 15;55(10):1031-40. | PubMed |
- Turner DC, Clark L, Pomarol-Clotet E, McKenna P, Robbins TW, Sahakian BJ. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology. 2004 Jul;29(7):1363-73. | PubMed |
- Rycroft N, Hutton SB, Clowry O, Groomsbridge C, Sierakowski A, Rusted JM. Non-cholinergic modulation of antisaccade performance: a modafinil-nicotine comparison. Psychopharmacology (Berl). 2007 Dec;195(2):245-53. | PubMed |
- Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41(8):906-18. | PubMed |
- Gilbert SJ, Simons JS, Frith CD, Burgess PW. Performance-related activity in medial rostral prefrontal cortex (area 10) during low-demand tasks. J Exp Psychol Hum Percept Perform. 2006 Feb;32(1):45-58. | PubMed |
- Randall DC, Shneerson JM, File SE. Cognitive effects of modafinil in student volunteers may depend on IQ. Pharmacol Biochem Behav. 2005 Sep;82(1):133-9. | PubMed |
 Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008 Jun;33(7):1477-502. | PubMed |
Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008 Jun;33(7):1477-502. | PubMed | Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006 Apr;67(4):554-66. | PubMed |
Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006 Apr;67(4):554-66. | PubMed | Black JE, Hull SG, Tiller J, Yang R, Harsh JR. The long-term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open-label extension study. J Clin Sleep Med. 2010 Oct 15;6(5):458-66. | PubMed |
Black JE, Hull SG, Tiller J, Yang R, Harsh JR. The long-term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open-label extension study. J Clin Sleep Med. 2010 Oct 15;6(5):458-66. | PubMed | Czeisler CA, Walsh JK, Roth T, Hughes RJ, Wright KP, Kingsbury L, et al. Modafinil in Shift Work Sleep Disorder Study Group. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. 2005 Aug 4;353(5):476-86. Erratum in: N Engl J Med. 2005 Sep 8;353(10):1078. | PubMed |
Czeisler CA, Walsh JK, Roth T, Hughes RJ, Wright KP, Kingsbury L, et al. Modafinil in Shift Work Sleep Disorder Study Group. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. 2005 Aug 4;353(5):476-86. Erratum in: N Engl J Med. 2005 Sep 8;353(10):1078. | PubMed | Darwish M, Kirby M, D'Andrea DM, Yang R, Hellriegel ET, Robertson P Jr. Pharmacokinetics of armodafinil and modafinil after single and multiple doses in patients with excessive sleepiness associated with treated obstructive sleep apnea: a randomized, open-label, crossover study. Clin Ther. 2010 Nov;32(12):2074-87. | CrossRef | PubMed |
Darwish M, Kirby M, D'Andrea DM, Yang R, Hellriegel ET, Robertson P Jr. Pharmacokinetics of armodafinil and modafinil after single and multiple doses in patients with excessive sleepiness associated with treated obstructive sleep apnea: a randomized, open-label, crossover study. Clin Ther. 2010 Nov;32(12):2074-87. | CrossRef | PubMed | Lavault S, Dauvilliers Y, Drouot X, Leu-Semenescu S, Golmard JL, Lecendreux M, et al. Benefit and risk of modafinil in idiopathic hypersomnia vs. narcolepsy with cataplexy. Sleep Med. 2011 Jun;12(6):550-6. | CrossRef | PubMed |
Lavault S, Dauvilliers Y, Drouot X, Leu-Semenescu S, Golmard JL, Lecendreux M, et al. Benefit and risk of modafinil in idiopathic hypersomnia vs. narcolepsy with cataplexy. Sleep Med. 2011 Jun;12(6):550-6. | CrossRef | PubMed | Bastuji H, Jouvet M. Successful treatment of idiopathic hypersomnia and narcolepsy with modafinil. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(5):695-700. | PubMed |
Bastuji H, Jouvet M. Successful treatment of idiopathic hypersomnia and narcolepsy with modafinil. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(5):695-700. | PubMed | Gill M, Haerich P, Westcott K, Godenick KL, Tucker JA. Cognitive performance following modafinil versus placebo in sleep-deprived emergency physicians: a double-blind randomized crossover study. Acad Emerg Med. 2006 Feb;13(2):158-65. Epub 2006 Jan 25. Erratum in: Acad Emerg Med. 2006 Apr;13(4):477. | PubMed |
Gill M, Haerich P, Westcott K, Godenick KL, Tucker JA. Cognitive performance following modafinil versus placebo in sleep-deprived emergency physicians: a double-blind randomized crossover study. Acad Emerg Med. 2006 Feb;13(2):158-65. Epub 2006 Jan 25. Erratum in: Acad Emerg Med. 2006 Apr;13(4):477. | PubMed | Killgore WD, Kahn-Greene ET, Grugle NL, Killgore DB, Balkin TJ. Sustaining executive functions during sleep deprivation: A comparison of caffeine, dextroamphetamine, and modafinil. Sleep. 2009 Feb;32(2):205-16. | PubMed |
Killgore WD, Kahn-Greene ET, Grugle NL, Killgore DB, Balkin TJ. Sustaining executive functions during sleep deprivation: A comparison of caffeine, dextroamphetamine, and modafinil. Sleep. 2009 Feb;32(2):205-16. | PubMed | Roth T, Rippon GA, Arora S. Armodafinil improves wakefulness and long-term episodic memory in nCPAP-adherent patients with excessive sleepiness associated with obstructive sleep apnea. Sleep Breath. 2008 Mar;12(1):53-62. | PubMed |
Roth T, Rippon GA, Arora S. Armodafinil improves wakefulness and long-term episodic memory in nCPAP-adherent patients with excessive sleepiness associated with obstructive sleep apnea. Sleep Breath. 2008 Mar;12(1):53-62. | PubMed | Saletu M, Anderer P, Semlitsch HV, Saletu-Zyhlarz GM, Mandl M, Zeitlhofer J, et al. Low-resolution brain electromagnetic tomography (LORETA) identifies brain regions linked to psychometric performance under modafinil in narcolepsy. Psychiatry Res. 2007 Jan 15;154(1):69-84. | PubMed |
Saletu M, Anderer P, Semlitsch HV, Saletu-Zyhlarz GM, Mandl M, Zeitlhofer J, et al. Low-resolution brain electromagnetic tomography (LORETA) identifies brain regions linked to psychometric performance under modafinil in narcolepsy. Psychiatry Res. 2007 Jan 15;154(1):69-84. | PubMed | Schwartz JR, Hirshkowitz M, Erman MK, Schmidt-Nowara W. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea: a 12-week, open-label study. Chest. 2003 Dec;124(6):2192-9. | PubMed |
Schwartz JR, Hirshkowitz M, Erman MK, Schmidt-Nowara W. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea: a 12-week, open-label study. Chest. 2003 Dec;124(6):2192-9. | PubMed | Wesensten NJ, Belenky G, Kautz MA, Thorne DR, Reichardt RM, Balkin TJ. Maintaining alertness and performance during sleep deprivation: modafinil versus caffeine. Psychopharmacology (Berl). 2002 Jan;159(3):238-47. | PubMed |
Wesensten NJ, Belenky G, Kautz MA, Thorne DR, Reichardt RM, Balkin TJ. Maintaining alertness and performance during sleep deprivation: modafinil versus caffeine. Psychopharmacology (Berl). 2002 Jan;159(3):238-47. | PubMed | Wesensten NJ. Effects of modafinil on cognitive performance and alertness during sleep deprivation. Curr Pharm Des. 2006;12(20):2457-71. | PubMed |
Wesensten NJ. Effects of modafinil on cognitive performance and alertness during sleep deprivation. Curr Pharm Des. 2006;12(20):2457-71. | PubMed | Gerrard P, Malcolm R. Mechanisms of modafinil: A review of current research. Neuropsychiatr Dis Treat. 2007 Jun;3(3):349-64. | PubMed |
Gerrard P, Malcolm R. Mechanisms of modafinil: A review of current research. Neuropsychiatr Dis Treat. 2007 Jun;3(3):349-64. | PubMed | Ferraro L, Antonelli T, O'Connor WT, Tanganelli S, Rambert FA, Fuxe K. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett. 1998 Sep 4;253(2):135-8. | PubMed |
Ferraro L, Antonelli T, O'Connor WT, Tanganelli S, Rambert FA, Fuxe K. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett. 1998 Sep 4;253(2):135-8. | PubMed | Ferraro L, Fuxe K, Agnati L, Tanganelli S, Tomasini MC, Antonelli T. Modafinil enhances the increase of extracellular serotonin levels induced by the antidepressant drugs fluoxetine and imipramine: a dual probe microdialysis study in awake rat. Synapse. 2005 Mar 15;55(4):230-41. | PubMed |
Ferraro L, Fuxe K, Agnati L, Tanganelli S, Tomasini MC, Antonelli T. Modafinil enhances the increase of extracellular serotonin levels induced by the antidepressant drugs fluoxetine and imipramine: a dual probe microdialysis study in awake rat. Synapse. 2005 Mar 15;55(4):230-41. | PubMed | Ferraro L, Fuxe K, Tanganelli S, Tomasini MC, Rambert FA, Antonelli T. Differential enhancement of dialysate serotonin levels in distinct brain regions of the awake rat by modafinil: possible relevance for wakefulness and depression. J Neurosci Res. 2002 Apr 1;68(1):107-12. | PubMed |
Ferraro L, Fuxe K, Tanganelli S, Tomasini MC, Rambert FA, Antonelli T. Differential enhancement of dialysate serotonin levels in distinct brain regions of the awake rat by modafinil: possible relevance for wakefulness and depression. J Neurosci Res. 2002 Apr 1;68(1):107-12. | PubMed | Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006 Nov;319(2):561-9. | PubMed |
Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006 Nov;319(2):561-9. | PubMed | Huang Q, Zhang L, Tang H, Wang L, Wang Y. Modafinil modulates GABA-activated currents in rat hippocampal pyramidal neurons. Brain Res. 2008 May 7;1208:74-8. | CrossRef | PubMed |
Huang Q, Zhang L, Tang H, Wang L, Wang Y. Modafinil modulates GABA-activated currents in rat hippocampal pyramidal neurons. Brain Res. 2008 May 7;1208:74-8. | CrossRef | PubMed | Ishizuka T, Murotani T, Yamatodani A. Modanifil activates the histaminergic system through the orexinergic neurons. Neurosci Lett. 2010 Oct 15;483(3):193-6. | CrossRef | PubMed |
Ishizuka T, Murotani T, Yamatodani A. Modanifil activates the histaminergic system through the orexinergic neurons. Neurosci Lett. 2010 Oct 15;483(3):193-6. | CrossRef | PubMed | Nixey C. Are 'smart drugs' safe for students? The Guardian 2010.
Nixey C. Are 'smart drugs' safe for students? The Guardian 2010.  Garreau J. Dose of Genius: ‘Smart Pills’ Are on The Rise. But Is Taking Them Wise? The Washington Post. June 11, 2006 [on line] | Link |
Garreau J. Dose of Genius: ‘Smart Pills’ Are on The Rise. But Is Taking Them Wise? The Washington Post. June 11, 2006 [on line] | Link | NSDUH. Substance abuse and mental Health Services administration. Results from the 2008 National Survey on Drug Use and Helth: National findings Publication Nº SMA 09-4434. Rockville MD: Office of Applied Studies, NDUH Series H-36, 2009.
NSDUH. Substance abuse and mental Health Services administration. Results from the 2008 National Survey on Drug Use and Helth: National findings Publication Nº SMA 09-4434. Rockville MD: Office of Applied Studies, NDUH Series H-36, 2009.  Maher B. Poll results: look who's doping. Nature. 2008 Apr 10;452(7188):674-5. | CrossRef | PubMed |
Maher B. Poll results: look who's doping. Nature. 2008 Apr 10;452(7188):674-5. | CrossRef | PubMed | Mohor D. Los riesgos del doping univesitario. El Mercurio, 08 de abril de 2009; Tendencias [on line]. | Link |
Mohor D. Los riesgos del doping univesitario. El Mercurio, 08 de abril de 2009; Tendencias [on line]. | Link | Lazcano P. La pastilla del día antes del examen. La Nación. 23 de junio de 2009; Vida y estilo [on line]. | Link |
Lazcano P. La pastilla del día antes del examen. La Nación. 23 de junio de 2009; Vida y estilo [on line]. | Link | Della Marca G, Restuccia D, Rubino M, Maiese T, Tonali P. Influence of modafinil on somatosensory input processing in the human brain-stem. Clin Neurophysiol. 2004 Apr;115(4):919-26. | PubMed |
Della Marca G, Restuccia D, Rubino M, Maiese T, Tonali P. Influence of modafinil on somatosensory input processing in the human brain-stem. Clin Neurophysiol. 2004 Apr;115(4):919-26. | PubMed | Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl). 2003 Jan;165(3):260-9.
| PubMed |
Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl). 2003 Jan;165(3):260-9.
| PubMed | Baranski JV, Pigeau R, Dinich P, Jacobs I. Effects of modafinil on cognitive and meta-cognitive performance. Hum Psychopharmacol. 2004 Jul;19(5):323-32. | PubMed |
Baranski JV, Pigeau R, Dinich P, Jacobs I. Effects of modafinil on cognitive and meta-cognitive performance. Hum Psychopharmacol. 2004 Jul;19(5):323-32. | PubMed | Marchant NL, Kamel F, Echlin K, Grice J, Lewis M, Rusted JM. Modafinil improves rapid shifts of attention. Psychopharmacology (Berl). 2009 Jan;202(1-3):487-95.
| CrossRef | PubMed |
Marchant NL, Kamel F, Echlin K, Grice J, Lewis M, Rusted JM. Modafinil improves rapid shifts of attention. Psychopharmacology (Berl). 2009 Jan;202(1-3):487-95.
| CrossRef | PubMed | Randall DC, Viswanath A, Bharania P, Elsabagh SM, Hartley DE, Shneerson JM, et al. Does modafinil enhance cognitive performance in young volunteers who are not sleep-deprived? J Clin Psychopharmacol. 2005 Apr;25(2):175-9. | PubMed |
Randall DC, Viswanath A, Bharania P, Elsabagh SM, Hartley DE, Shneerson JM, et al. Does modafinil enhance cognitive performance in young volunteers who are not sleep-deprived? J Clin Psychopharmacol. 2005 Apr;25(2):175-9. | PubMed | Randall DC, Fleck NL, Shneerson JM, File SE. The cognitive-enhancing properties of modafinil are limited in non-sleep-deprived middle-aged volunteers. Pharmacol Biochem Behav. 2004 Mar;77(3):547-55. | PubMed |
Randall DC, Fleck NL, Shneerson JM, File SE. The cognitive-enhancing properties of modafinil are limited in non-sleep-deprived middle-aged volunteers. Pharmacol Biochem Behav. 2004 Mar;77(3):547-55. | PubMed | Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001 Mar;178:200-6. | PubMed |
Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001 Mar;178:200-6. | PubMed | Müller U, Steffenhagen N, Regenthal R, Bublak P. Effects of modafinil on working memory processes in humans. Psychopharmacology (Berl). 2004 Dec;177(1-2):161-9. | PubMed |
Müller U, Steffenhagen N, Regenthal R, Bublak P. Effects of modafinil on working memory processes in humans. Psychopharmacology (Berl). 2004 Dec;177(1-2):161-9. | PubMed | Muller U, Steffenhagen N, Regenthal R, et al. Effects of modafinil on working memory processes in humans. Psychopharmacology 2004;177(1-2):161-9.
Muller U, Steffenhagen N, Regenthal R, et al. Effects of modafinil on working memory processes in humans. Psychopharmacology 2004;177(1-2):161-9.  Wechsler D. Wechsler adult intelligence scale (3rd ed.). San Antonio, TX: Psychological Corporation; 1997.
Wechsler D. Wechsler adult intelligence scale (3rd ed.). San Antonio, TX: Psychological Corporation; 1997.  Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999 Nov 10;282(18):1737-44.
| PubMed |
Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999 Nov 10;282(18):1737-44.
| PubMed | Robertson P Jr, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet. 2003;42(2):123-37.
| PubMed |
Robertson P Jr, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet. 2003;42(2):123-37.
| PubMed | Golden CJ. A group version of the Stroop Color and Word Test. J Pers Assess. 1975 Aug;39(4):386-8. | PubMed |
Golden CJ. A group version of the Stroop Color and Word Test. J Pers Assess. 1975 Aug;39(4):386-8. | PubMed | MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991 Mar;109(2):163-203. | PubMed |
MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991 Mar;109(2):163-203. | PubMed | Abad F, Colom R, Juan-Espinosa M, García LF. Intelligence differentiation in adult samples. Intelligence 2003;31:157-66.
| Link |
Abad F, Colom R, Juan-Espinosa M, García LF. Intelligence differentiation in adult samples. Intelligence 2003;31:157-66.
| Link | Barr WB. Neuropsychological testing of high school athletes. Preliminary norms and test-retest indices. Arch Clin Neuropsychol. 2003 Jan;18(1):91-101. | PubMed |
Barr WB. Neuropsychological testing of high school athletes. Preliminary norms and test-retest indices. Arch Clin Neuropsychol. 2003 Jan;18(1):91-101. | PubMed | Bullard SE, Fein D, Gleeson MK, Tischer N, Mapou RL, Kaplan E. The biber cognitive estimation test. Arch clin neuropsychol. 2004 Sep;19(6):835-46. Erratum in: Arch Clin Neuropsychol. 2005 Mar;20(2):279. | PubMed |
Bullard SE, Fein D, Gleeson MK, Tischer N, Mapou RL, Kaplan E. The biber cognitive estimation test. Arch clin neuropsychol. 2004 Sep;19(6):835-46. Erratum in: Arch Clin Neuropsychol. 2005 Mar;20(2):279. | PubMed | Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Third Edition. Oxford: Oxford University Press; 2005.
Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Third Edition. Oxford: Oxford University Press; 2005.  Randall DC, Shneerson JM, Plaha KK, File SE. Modafinil affects mood, but not cognitive function, in healthy young volunteers. Hum Psychopharmacol. 2003 Apr;18(3):163-73. | PubMed |
Randall DC, Shneerson JM, Plaha KK, File SE. Modafinil affects mood, but not cognitive function, in healthy young volunteers. Hum Psychopharmacol. 2003 Apr;18(3):163-73. | PubMed | Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ. Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2004 May 15;55(10):1031-40. | PubMed |
Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ. Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2004 May 15;55(10):1031-40. | PubMed | Turner DC, Clark L, Pomarol-Clotet E, McKenna P, Robbins TW, Sahakian BJ. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology. 2004 Jul;29(7):1363-73. | PubMed |
Turner DC, Clark L, Pomarol-Clotet E, McKenna P, Robbins TW, Sahakian BJ. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology. 2004 Jul;29(7):1363-73. | PubMed | Rycroft N, Hutton SB, Clowry O, Groomsbridge C, Sierakowski A, Rusted JM. Non-cholinergic modulation of antisaccade performance: a modafinil-nicotine comparison. Psychopharmacology (Berl). 2007 Dec;195(2):245-53. | PubMed |
Rycroft N, Hutton SB, Clowry O, Groomsbridge C, Sierakowski A, Rusted JM. Non-cholinergic modulation of antisaccade performance: a modafinil-nicotine comparison. Psychopharmacology (Berl). 2007 Dec;195(2):245-53. | PubMed | Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41(8):906-18. | PubMed |
Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41(8):906-18. | PubMed | Gilbert SJ, Simons JS, Frith CD, Burgess PW. Performance-related activity in medial rostral prefrontal cortex (area 10) during low-demand tasks. J Exp Psychol Hum Percept Perform. 2006 Feb;32(1):45-58.
| PubMed |
Gilbert SJ, Simons JS, Frith CD, Burgess PW. Performance-related activity in medial rostral prefrontal cortex (area 10) during low-demand tasks. J Exp Psychol Hum Percept Perform. 2006 Feb;32(1):45-58.
| PubMed | Randall DC, Shneerson JM, File SE. Cognitive effects of modafinil in student volunteers may depend on IQ. Pharmacol Biochem Behav. 2005 Sep;82(1):133-9. | PubMed |
Randall DC, Shneerson JM, File SE. Cognitive effects of modafinil in student volunteers may depend on IQ. Pharmacol Biochem Behav. 2005 Sep;82(1):133-9. | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








