Key Words: breast cancer, mucinous carcinoma
Abstract
Mucinous carcinoma of the breast is a rare histological type, which represents between 1 and 4% of breast cancers. Treatment does not differ from other histological types, and it occurs more frequently in older adult women. Prognosis is good. We report the case of a 72-year-old patient with a 1-year disease course characterized by the appearance of a slow-growing tumor in the left upper quadrant of the left breast, in which the core biopsy showed mucinous breast carcinoma of a low nuclear grade. The patient underwent quadrantectomy plus a sentinel node biopsy, which confirmed the initial diagnosis.
Introduction
Mucinous carcinoma of the breast, also called colloid or mucous, is a rare histological type, which represents between 1 and 4% of breast cancers, characterized by a marked production of mucin [1],[2],[3]. There are two histological subtypes according to tumor cellularity: pure and mixed [4]. Epidemiological characteristics, pathogenesis as well as clinical presentation differ from patients with other histological types of breast cancer [3]: it usually occurs in postmenopausal women with a mean age of 70 years and is associated with a good prognosis [2],[4]. We report the case of a 72-year-old woman with the diagnosis of mucinous carcinoma of the breast.
Case presentation
A 72-year-old female patient from Junín (Peru), no medical history of importance, with 1 year disease time characterized by the appearance of a slow-growing tumor in the upper outer quadrant of the left breast. The physical examination showed a patient in good condition, at the preferential exam: symmetrical breasts of soft consistency, at the left breast tail a tumor of 3x3 cm in diameter was felt, with irregular borders, associated with slight retraction of the surrounding skin. No axillary or supraclavicular lymph nodes were felt; bilateral mammography and ultrasound guided biopsy were required.
Ancillary examinations
In mammograms, the breasts were composed of scattered areas of fibroglandular tissue (B-type mammary pattern). In the left upper outer quadrant, a rounded nodule of high density was identified, with no circumscribed contours, of 32 by 21 mm diameter. Bilateral diffuse round calcifications were reported, in addition the skin and nipple had normal configuration.
Figure 1. Bilateral mammography
In bilateral breast ultrasound, both breasts had heterogeneous ecotexture (type C). In the left breast, a mass of 3 cm by 2 cm in greater diameters was found, located in Radio 2, at 6.5 cm from the nipple, with irregular borders, imprecise margins, tenuous posterior reinforcement, heterogeneous internal echoes, non-parallel orientation, non-circumscribed, spiculated edges. The areola-nipple complex, the retroareolar region, and the skin of both breasts without significant alterations, there was no evidence of ductal ectasia, there was no evidence of lymphadenopathy in both axillary regions. With these findings it was classified as ultrasound BI-RADS 5.
The left breast core biopsy revealed a myxoid background with groups of neoplastic epithelial cells with regular and hyperchromatic central nuclei and abundant eosinophil cytoplasm, It was reported as mucinous breast carcinoma with low nuclear grade. Neoplastic cells showed nuclear immunoreactivity for estrogen receptor in a ratio of 100% and intensity 3 + (Allred Score 8), progesterone receptor in a ratio of 90% and intensity of 3 + (Allred Score 8), the expression of CerbB2 was negative (0) and a proliferative index measured with Ki-67 about 4%.
Figure 3. Expression of estrogen receptor in mucinous carcinoma.
Figure 4. Expression of progesterone receptor in mucinous carcinoma.
Figure 5. Negative circumferential membrane staining and cell proliferation.
With these results, the patient is classified as a carrier of Luminal T2 N0 Mx breast cancer. She underwent a breast Cuadrantectomy + margin expansion + sentinel node biopsy, whose freezing biopsies were reported as negative for macrometastasis. Macroscopy revealed solid whitish brown color tumor with areas of mucinous aspect with partially delimited edges of 3.0 x 2.5 cm. Microscopy showed intermediate nuclear grade tumor cells in amorphous mucin lakes surrounded by scarce connective tissue bands. Tumor cells showed eosinophilic cytoplasm, hyperchromatic oval nucleus and small nucleolus, with very few tubules. Lymphovascular and perineural invasion were absent, associated ductal carcinoma in situ with cribiform pattern of intermediate nuclear grade was described in 2%.
The patient had a favorable evolution in the postoperative period and was discharged. Subsequently it was evaluated in an outpatient clinic, where an operative wound in appropriate conditions was evidenced. She was sent to the radiotherapy department for complementary treatment.
Discussion
Mucinous carcinoma of the breast is a rare histological type of breast cancer and differs both by its molecular characteristics as clinical epidemiological among these [2]. It is a disease of older women, it has been described that only 1% of patients with mucinous carcinoma of the breast are younger than 35 years [2]. In the reported case, the patient belonged to the age group corresponding to the maximum incidence of coloid carcinoma of the breast. Regarding the localization of the tumor presented by the patient in the upper outer quadrant, it correlates with other studies such as that found in a retrospective review where the majority of the tumors were located in the upper outer quadrant (44%) and the other 56% Were distributed between the other quadrants [5].
Unlike conventional invasive carcinoma, there are few data regarding precursor lesions of mucinous carcinoma with various patterns of mucinous ductal carcinoma in situ (DCIS) as a precursor lesion. Among the transitional patterns described are: cribiform/solid, cribiform/papillary, papillary, micropapillary and flat, with distinctive features that link DCIS with aggressive mucinous carcinoma phenotypes [6]. This is in agreement with some authors, who propose that the mucinous carcinoma of the breast would be mucinous variants of the papillary or lobular carcinoma with extracellular mucin, since 86% of colloid carcinoma have a micropapillary pattern [7].
Although mucinous mammary carcinoma is classified as an invasive tumor, Rosai [8] has proposed that this neoplasm is a form of in situ carcinoma whose production of mucin, which by means of a polarity inversion mechanism, produces secretion towards the stroma, rather than towards the luminous surface, which causes the detachment of the epithelium from its underlying stroma. In this way mucin invades the stroma and separates the cells from its basal membrane, with two histological types: pure and mixed mucinous carcinoma, with different implications in diagnosis and prognosis [9]. Capella et al. divided the pure forms into two main groups A and B, group A (hypocellular variant) characterized by abundant extracellular mucin with few tumor cells and less intracellular mucin without presence of granules, group B (hypercellular variant) characterized by more cellular tumors, with greater amount of intracellular mucin [6].
Mammographically pure mucinous carcinomas present as lesions of well-defined margins with high percentages of mucin [10], in the presented case a nodule with increased density was evidenced in agreement to the descriptions reported in the literature. Ecographically, mixed-type carcinomas are hypoechogenic [10] and pure-type carcinomas present as an isoecogenic lesion which tends to go unnoticed in some cases, for this reason can sometimes go unnoticed or reported as a benign lesion, delaying The diagnosis of this pathology [2]. Therefore, the correlation between clinical and imaging characteristics with the histological findings is important for the differential diagnosis of this type of mammary carcinomas [10],[11],[12].
Tumor size is usually smaller in comparison to other types of breast cancer, due to the slow growth of this type of tumors it is possible to diagnose them with a smaller tumor size [2]. Komenaka et al. reported that tumor size has no impact on the prognosis of these patients [13]; However, other studies have reported tumor size as an independent prognostic factor, less important than nodal involvement and the magnitude of the latter is directly proportional to tumor size [1],[2],[4]. In addition, the increased production of mucin has been associated with a better prognosis [6], because this surrounding component behaves as a barrier to limit the invasive capacity of these tumors [14].
Nodal involvement, the most important prognostic factor, is rare; being able to vary according to the histological type: between 2 to 14% in pure mucinous carcinoma and 46 to 64% in the mixed type [2]. This gives value to the sentinel node biopsy. There is a slight tendency to predilection for the left breast according to the literature, being more frequent in the upper outer quadrant. Clinical stage II is the most frequent at diagnosis, in addition it has been described that the Luminal type is the most frequent phenotype among the mucinous carcinomas of the breast [4],[15].
The treatment of this type of cancer does not differ from that of the other histological types of breast cancer, being the mastectomy, quadrantectomy associated with adjuvant chemotherapy, radiotherapy and hormone valid therapy indications [16].
The colloid carcinoma of the breast has a favorable prognosis, with a cancer-specific survival of 94% at five years and 81% at twenty years [2],[4],[7]. It has been shown that the survival of these patients does not differ from the general population; however, it has been demonstrated that the micropapillary variety subgroup of pure coloid carcinoma of the breast presents a worse prognosis evidenced by lymphovascular and nodal involvement [17],[18],[19].
Conclusions
Mucinous carcinoma is a rare variant of invasive ductal breast carcinoma that tends to occur among older women. At diagnosis, it tends to be smaller, less nuclear grade, less lymph node involvement. It is possible to have a diagnosis and timely treatment by mammography, histopathological confirmation, surgical and adjuvant treatment, which gives a favorable prognosis.
Notes
Ethical aspects
The informed consent requested by Medwave, has been signed by the patient; A copy of this was sent to the editorial board of the magazine. The authors state that the privacy of the patient was respected according to the CIOMS standards, of privacy of the data collected.
Declaration of conflicts of interest
The authors have completed the ICMJE declaration of conflicts of interest form, and declare that they have not received funding for the report; Not having financial relationships with organizations that might have interests in the published article in the past three years; And not having other relationships or activities that could influence the published article. Forms can be requested by contacting the responsible author or the editorial board of the Journal.
Financing
The authors state that there were no external sources of funding.
Acknowledgments
To Alejandro Dagnino Varas MD and to the Department of Radiodiagnosis of the Instituto Nacional de Enfermedades Neoplásicas for the access to the films of pathology and imaging studies respectively.

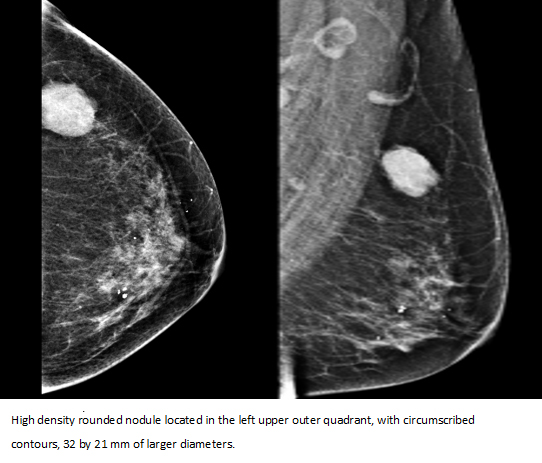 Figure 1. Bilateral mammography
Figure 1. Bilateral mammography

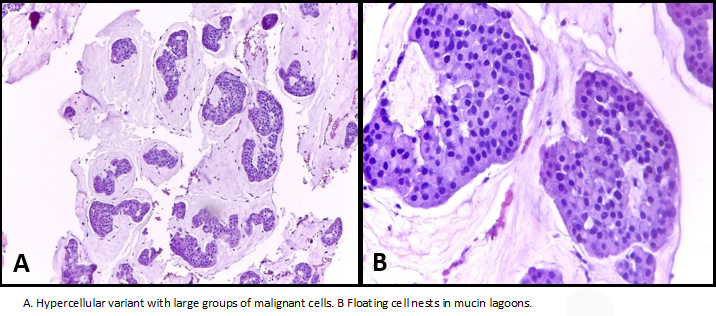 Figure 2. Mucinous carcinoma.
Figure 2. Mucinous carcinoma.

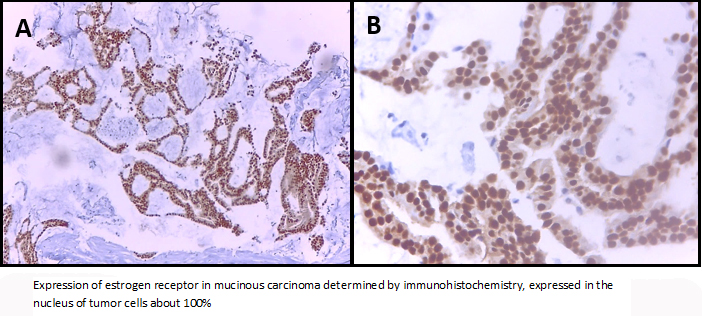 Figure 3. Expression of estrogen receptor in mucinous carcinoma.
Figure 3. Expression of estrogen receptor in mucinous carcinoma.

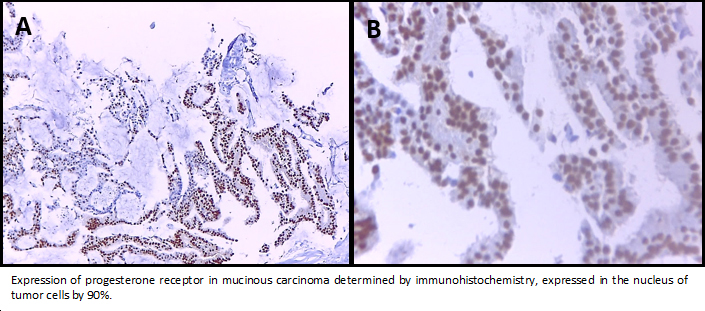 Figure 4. Expression of progesterone receptor in mucinous carcinoma.
Figure 4. Expression of progesterone receptor in mucinous carcinoma.

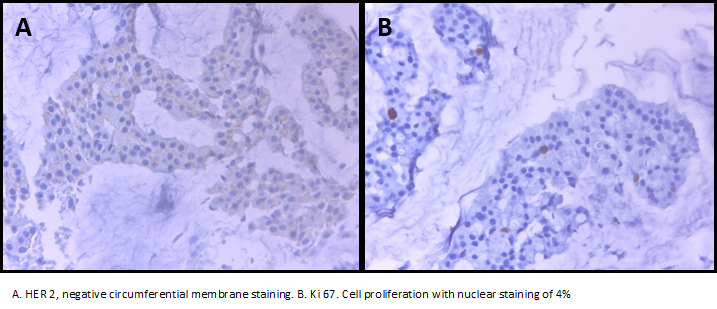 Figure 5. Negative circumferential membrane staining and cell proliferation.
Figure 5. Negative circumferential membrane staining and cell proliferation.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

El carcinoma mucinoso de la mama es un tipo histológico raro, que representa entre el 1 y el 4% de los cánceres de mama. Su tratamiento no difiere de los demás tipos histológicos. Se presenta con mayor frecuencia en mujeres adultas mayores y se distingue por un buen pronóstico. En este artículo, se reporta el caso de una paciente de 72 años, con tiempo de enfermedad de un año, caracterizado por la aparición de una tumoración de crecimiento lento y progresivo en el cuadrante superior externo de la mama izquierda, a la cual se realizó biopsia core. La biopsia arrojó un resultado de carcinoma mucinoso de mama, con grado nuclear bajo. La paciente fue sometida a cuadrantectomía más biopsia de ganglio centinela, lo cual confirmó el diagnóstico inicial.
 Authors:
Jorge Luna-Abanto[1,2], Grivette Mendoza Tisoc[3,4]
Authors:
Jorge Luna-Abanto[1,2], Grivette Mendoza Tisoc[3,4]
Affiliation:
[1] Departamento de Cirugía Oncológica, Instituto Nacional de Enfermedades Neoplásicas, Lima, Perú
[2] Escuela de Posgrado, Universidad Peruana Cayetano Heredia, Lima, Perú
[3] Departamento de Patología Quirúrgica, Instituto Nacional de Enfermedades Neoplásicas, Lima, Perú
[4] Escuela de Posgrado, Universidad Nacional Mayor de San Marcos. Lima, Perú
E-mail: jorgelunaabanto@gmail.com
Author address:
[1] Avenida Angamos Este 2520
Surquillo
Lima
Perú

Citation: Luna-Abanto J, Mendoza Tisoc G. Mucinous carcinoma of the breast: a case report and review of the literature. Medwave 2017 Jul;17(6):7003 doi: 10.5867/medwave.2017.06.7003
Submission date: 27/4/2017
Acceptance date: 8/7/2017
Publication date: 24/7/2017
Origin: not requested
Type of review: reviewed by four external peer reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011 Dec;14(4):308-13. | CrossRef | PubMed |
- Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008 Oct;111(3):541-7. | PubMed |
- Tseng HS, Lin C, Chan SE, Chien SY, Kuo SJ, Chen ST, et al. Pure mucinous carcinoma of the breast: clinicopathologic characteristics and long-term outcome among Taiwanese women. World J Surg Oncol. 2013 Jun 14;11:139. | CrossRef | PubMed |
- Zhang L, Jia N, Han L, Yang L, Xu W, Chen W. Comparative analysis of imaging and pathology features of mucinous carcinoma of the breast. Clin Breast Cancer. 2015 Apr;15(2):e147-54. | CrossRef | PubMed |
- Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008 Oct;111(3):541-7. | PubMed |
- Kryvenko ON, Chitale DA, Yoon J, Arias-Stella J 3rd, Meier FA, Lee MW. Precursor lesions of mucinous carcinoma of the breast: analysis of 130 cases. Am J Surg Pathol. 2013 Jul;37(7):1076-84. | CrossRef | PubMed |
- Shet T. Time to change the way we diagnose mucinous carcinomas of the breast. Hum Pathol. 2014 Feb;45(2):434-5. | CrossRef | PubMed |
- Rosai J. Ackerman's Surgical Pathology 10th Edition. USA: Mosby; 2011: 1699-1701.
- Ornia Rodriguez M, López Secades MA, Domínguez Iglesias F. Tumor mucinoso de mama: diagnóstico radiológico. SERAM 2014. posterng.netkey.at [on line]. | Link |
- Anan K, Mitsuyama S, Tamae K, Nishihara K, Iwashita T, Abe Y, et al. Pathological features of mucinous carcinoma of the breast are favourable for breast-conserving therapy. Eur J Surg Oncol. 2001 Aug;27(5):459-63. | PubMed |
- Barbashina V, Corben AD, Akram M, Vallejo C, Tan LK. Mucinous micropapillary carcinoma of the breast: an aggressive counterpart to conventional pure mucinous tumors. Hum Pathol. 2013 Aug;44(8):1577-85. | CrossRef | PubMed |
- Bitencourt AG, Graziano L, Osório CA, Guatelli CS, Souza JA, Mendonça MH, et al. MRI Features of Mucinous Cancer of the Breast: Correlation With Pathologic Findings and Other Imaging Methods. AJR Am J Roentgenol. 2016 Feb;206(2):238-46. | CrossRef | PubMed |
- Komenaka IK, El-Tamer MB, Troxel A, Hamele-Bena D, Joseph KA, Horowitz E, et al. Pure mucinous carcinoma of the breast. Am J Surg. 2004 Apr;187(4):528-32. | PubMed |
- Barkley CR, Ligibel JA, Wong JS, Lipsitz S, Smith BL, Golshan M. Mucinous breast carcinoma: a large contemporary series. Am J Surg. 2008 Oct;196(4):549-51. | CrossRef | PubMed |
 Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011 Dec;14(4):308-13. | CrossRef | PubMed |
Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011 Dec;14(4):308-13. | CrossRef | PubMed | Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008 Oct;111(3):541-7. | PubMed |
Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008 Oct;111(3):541-7. | PubMed | Tseng HS, Lin C, Chan SE, Chien SY, Kuo SJ, Chen ST, et al. Pure mucinous carcinoma of the breast: clinicopathologic characteristics and long-term outcome among Taiwanese women. World J Surg Oncol. 2013 Jun 14;11:139. | CrossRef | PubMed |
Tseng HS, Lin C, Chan SE, Chien SY, Kuo SJ, Chen ST, et al. Pure mucinous carcinoma of the breast: clinicopathologic characteristics and long-term outcome among Taiwanese women. World J Surg Oncol. 2013 Jun 14;11:139. | CrossRef | PubMed | Zhang L, Jia N, Han L, Yang L, Xu W, Chen W. Comparative analysis of imaging and pathology features of mucinous carcinoma of the breast. Clin Breast Cancer. 2015 Apr;15(2):e147-54. | CrossRef | PubMed |
Zhang L, Jia N, Han L, Yang L, Xu W, Chen W. Comparative analysis of imaging and pathology features of mucinous carcinoma of the breast. Clin Breast Cancer. 2015 Apr;15(2):e147-54. | CrossRef | PubMed | Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008 Oct;111(3):541-7. | PubMed |
Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008 Oct;111(3):541-7. | PubMed | Kryvenko ON, Chitale DA, Yoon J, Arias-Stella J 3rd, Meier FA, Lee MW. Precursor lesions of mucinous carcinoma of the breast: analysis of 130 cases. Am J Surg Pathol. 2013 Jul;37(7):1076-84. | CrossRef | PubMed |
Kryvenko ON, Chitale DA, Yoon J, Arias-Stella J 3rd, Meier FA, Lee MW. Precursor lesions of mucinous carcinoma of the breast: analysis of 130 cases. Am J Surg Pathol. 2013 Jul;37(7):1076-84. | CrossRef | PubMed | Shet T. Time to change the way we diagnose mucinous carcinomas of the breast. Hum Pathol. 2014 Feb;45(2):434-5. | CrossRef | PubMed |
Shet T. Time to change the way we diagnose mucinous carcinomas of the breast. Hum Pathol. 2014 Feb;45(2):434-5. | CrossRef | PubMed | Rosai J. Ackerman's Surgical Pathology 10th Edition. USA: Mosby; 2011: 1699-1701.
Rosai J. Ackerman's Surgical Pathology 10th Edition. USA: Mosby; 2011: 1699-1701.  Ornia Rodriguez M, López Secades MA, Domínguez Iglesias F. Tumor mucinoso de mama: diagnóstico radiológico. SERAM 2014. posterng.netkey.at [on line]. | Link |
Ornia Rodriguez M, López Secades MA, Domínguez Iglesias F. Tumor mucinoso de mama: diagnóstico radiológico. SERAM 2014. posterng.netkey.at [on line]. | Link | Anan K, Mitsuyama S, Tamae K, Nishihara K, Iwashita T, Abe Y, et al. Pathological features of mucinous carcinoma of the breast are favourable for breast-conserving therapy. Eur J Surg Oncol. 2001 Aug;27(5):459-63. | PubMed |
Anan K, Mitsuyama S, Tamae K, Nishihara K, Iwashita T, Abe Y, et al. Pathological features of mucinous carcinoma of the breast are favourable for breast-conserving therapy. Eur J Surg Oncol. 2001 Aug;27(5):459-63. | PubMed | Barbashina V, Corben AD, Akram M, Vallejo C, Tan LK. Mucinous micropapillary carcinoma of the breast: an aggressive counterpart to conventional pure mucinous tumors. Hum Pathol. 2013 Aug;44(8):1577-85. | CrossRef | PubMed |
Barbashina V, Corben AD, Akram M, Vallejo C, Tan LK. Mucinous micropapillary carcinoma of the breast: an aggressive counterpart to conventional pure mucinous tumors. Hum Pathol. 2013 Aug;44(8):1577-85. | CrossRef | PubMed | Bitencourt AG, Graziano L, Osório CA, Guatelli CS, Souza JA, Mendonça MH, et al. MRI Features of Mucinous Cancer of the Breast: Correlation With Pathologic Findings and Other Imaging Methods. AJR Am J Roentgenol. 2016 Feb;206(2):238-46. | CrossRef | PubMed |
Bitencourt AG, Graziano L, Osório CA, Guatelli CS, Souza JA, Mendonça MH, et al. MRI Features of Mucinous Cancer of the Breast: Correlation With Pathologic Findings and Other Imaging Methods. AJR Am J Roentgenol. 2016 Feb;206(2):238-46. | CrossRef | PubMed | Komenaka IK, El-Tamer MB, Troxel A, Hamele-Bena D, Joseph KA, Horowitz E, et al. Pure mucinous carcinoma of the breast. Am J Surg. 2004 Apr;187(4):528-32. | PubMed |
Komenaka IK, El-Tamer MB, Troxel A, Hamele-Bena D, Joseph KA, Horowitz E, et al. Pure mucinous carcinoma of the breast. Am J Surg. 2004 Apr;187(4):528-32. | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








