Clinical practice guidelines: Concepts, limitations and challenges
Juan Víctor Ariel Franco, Marcelo Arancibia, Nicolás Meza, Eva Madrid, Karin Kopitowski
Abstract
Clinical practice guidelines are the most important documents for the incorporation of scientific evidence in health decision making through the formulation of recommendations. There is a variable terminology used to refer to the documents that guide health professionals in decision making. When clinical practice guidelines are of high quality, they appraise contextual aspects such as the use of resources, applicability, and patients’ values and preferences. Even so, they are not recipe books, since they may have limitations. In this review, we propose to clarify the different denominations across the various types of documents available to guide the health professional when making clinical decisions. We discuss the main characteristics of clinical practice guidelines, quality assessment, challenges, and limitations.
|
Main messages
|
Introduction
There are numerous ways to synthesize available biomedical information[1], so health professionals make decisions using diverse sources[2]. In certain scenarios, documents are continuously prepared for the standardization of clinical practice, but their denomination and objectives are sometimes unclear[3],[4], using terms such as “protocols”, “algorithms”, “consensus” and “guides” to refer to the same type of instrument[3], but with a non-standardized methodological development. The purpose of this article is to discuss these conceptual differences and facilitate the understanding around the various types of documents available to guide health professional in evidence-based practice; at the same time, the main characteristics of clinical practice guidelines and some considerations regarding their quality and limitations are disclosed.
Guidelines, protocols, consensuses, and others: What is the role of each?
For the purposes of this review, we will restrict the use of the concept “clinical practice guideline” to the “statements that include recommendations to optimize patient care based on a systematic review of evidence and an assessment of the benefits and harms of alternative care options (sic)”[5].
Because there are other documents that include the word “guideline” in their description (“guideline”, “clinical guideline”, “practical guideline”, “abbreviated guideline”, among others), a clarification is necessary[3],[4], especially considering that such documents often do not meet the criteria that define a clinical practice guideline that, as we will see later, is the most comprehensive document for the formulation of healthcare recommendations. Also, in many cases these documents do not contain a systematic search of scientific evidence to support their recommendations[3]. Feliciano and colleagues[4] specified the need for a concept analysis to study the linguistic and formal attributes of these terms. They reported that the terms “clinical practice guideline,” “clinical route” and “protocol” overlap and constitute a hypernym they called “protocol-based care,” which in turn would be part of a larger set they called “integrative studies”[4]. However, the authors suggest that the emergence of these concepts is circumstantial and regional in nature, and that what they have in common is their inclination to achieve evidence-based medicine-related objectives[4]. We will review these concepts and their definitions below.
Some documents named as “protocols” in the biomedical literature establish a series of procedural steps or consensual rules for the clinical management of certain health situations[3]. Similarly, the term “algorithm” is often used to signal the flow of decision-making in these protocols and may be included in two other documents such as “consensus” and “guidelines”[3]. There are other “protocols” or “norms,” which, in addition to adjusting clinical practice, seek to adapt current legal regulations to clinical practice (e.g. protocols for the assistance of victims of sexual abuse), considering that health professionals may not be familiar with some local standards and/or procedures[3]. On the other hand, “standards” or “strategies” are documents that synthesize the articulation of health policies in the care or organizational practice of health systems. Other terms such as “approaches” or “recommendations” may be included in a “guideline” and are more difficult to define independently. Finally, in biomedical bibliography, certain “manuals” can be found with therapeutic indications that usually point to a compendium of procedures or “protocols” for health care, with a detailed description of the process involved[3].
At the same time, there are documents called “consensus,” which seek to draft recommendations arising from the agreement between individuals, usually expert professionals on a given subject, and are usually endorsed by scientific societies[6],[7]. In this case, the search for evidence to support the statements is not necessarily systematic, while the recommendations are not necessarily based on an adequate level of certainty of the evidence. Even so, it would be desirable for different stakeholders to have participated broadly in these consensuses, considering the values and preferences of patients and the perspective of the funding agents. Where such discussions are constituted exclusively by professionals of the respective discipline, significant financial interest or other types of conflicts of interest could be found[7]. It should be noted that consensus can be important when the input of human thought, both in ethical matters and in axiomatic definitions, for example, contributes more to the document. Table 1 exemplifies each of the designated documents.

|
| Table 1. Examples of guidance documents for health professionals. |
Development of clinical practice guidelines: From evidence to recommendation
Once the body of evidence is identified for a given clinical question, such information should be integrated and adapted to the circumstance that is the subject of consensual health decision-making. Generally, these scenarios of daily clinical practice are beyond the scope of literature reviews (systematic and non-systematic), so it is expected that reviews (systematic or not) do not include recommendations for practice[12]. Conversely, clinical practice guidelines are documents that usually cover the context-specific information needed to make explicit and, ideally, transparent recommendations[13].
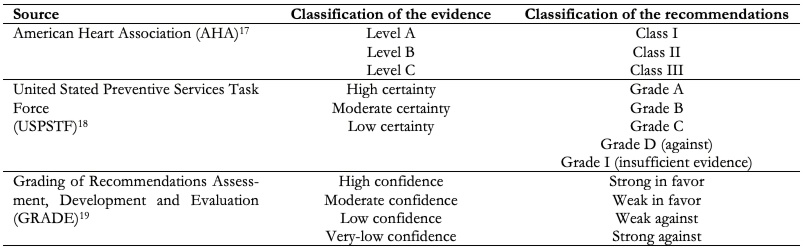
There are different methodological frameworks used for the development of clinical practice guidelines (Table 2), each with a different system of gradation of evidence and strength to recommendations, which can confuse health professionals. Along this line, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to the assessment of evidence and health recommendations is presented as a unifying, simple and accessible alternative that, through the use of simple language, seeks to promote access and use of evidence[14],[15] (Table 2).
The Grading of Recommendations Assessment, Development, and Evaluation approach uses criteria to categorize the certainty (or quality) of scientific evidence, namely: The source of bias of primary studies, their consistency and precision in estimates, aspects related to external validity (indirectness, or how direct the evidence is in relation to the question from the clinical practice guideline), the possibility of publication bias, the dose-response effect of interventions, the overall magnitude of the effect and the paradox effect of the unmeasured confounders in the estimates. In a second instance, the Grading of Recommendations Assessment, Development, and Evaluation approach provides the Evidence to Decision Framework[16], which allows to guide the developer panels of clinical practice guidelines in the contextualization of findings based on patient values and preferences, the acceptability and feasibility of the evaluated interventions, their applicability, cost-related aspects, implications for equity, among others. According to the Grading of Recommendations Assessment, Development, and Evaluation approach, there is not necessarily a direct association between the classification of evidence and recommendations (e.g. a high quality of evidence can support a weak recommendation), but other classification systems recognize a more direct association.
|
| Table 2. Coding of evidence and recommendations according to different methodological approaches. |
How to rely on clinical practice guidelines?
In the same way that there are guidelines for the design, conduct and reporting of clinical trials, observational studies and other methodological approaches to information, regulations have been developed to evaluate the quality of clinical practice guidelines. The Appraisal of Guidelines for Research & Evaluation II (AGREE II[20] is the most widely used and recommended instrument for quality evaluation. It consists of six domains and 23 items, which must be graded from 1 to 7 by two to four evaluators. At its conclusion, a quantitative and qualitative global assessment is required, so as to evaluate how recommended are the clinical practice guidelines. Table 3 summarizes the main domains that this tool evaluates.
![<b> Table 3. </b> Characteristics of the instrument AGREE II[20].](/medios/medwave/Abril2020/7887/TB03_e7887.jpg)
|
| Table 3. Characteristics of the instrument AGREE II[20]. |
The development of clinical practice guideline in low- and medium-income countries has been slowly and progressively adjusting to the standards of developed countries, replacing the adaptation of high-quality foreign clinical practice guidelines, or the development of other kinds of documents, by the generation of clinical practice guidelines that include recommendations that respond to the challenges of each region[21],[22],[23]. Therefore, the implementation of the Appraisal of Guidelines for Research & Evaluation II has made it possible to identify the main limitations in the development of clinical practice guidelines in different regions of the world. In the case of Latin America and the Caribbean, deficiencies identified in processing local clinical practice guidelines are as follows: non-inclusion of patient values and preferences, non-systematic treatment of evidence, and lack of thoroughness in the construction of recommendations, among others[24],[25],[26],[27],[28],[29],[30],[31],[32]. For example, some evaluations of Chile’s clinical practice guidelines conducted in 2009[24] and 2014[28] showed that, while the overall quality of the documents was acceptable, there were deficits in the domains of “participation of those involved,” “rigor in elaboration,” and “applicability.” Similarly, in clinical practice guidelines developed in Peru[26], Argentina[32], and Brazil[29],[33],[34], their overall quality was low. On the other hand, some healthcare systems consider that the local adaptation of high quality foreign clinical practice guidelines is more feasible, due to the high costs and resources required for the complex process of synthesis and assessment of evidence. In this way, different groups have proposed rules and regulations for the adaptation of high-quality clinical practice guidelines[23],[35].
Limitations and challenges
Although the development of clinical practice guidelines has been widespread and there are now multiple working groups that successfully develop and implement them, some challenges remain.
1. Multiplicity of clinical practice guidelines
A healthcare professional who uses recommendations for the management of a clinical or health situation usually encounters various sources of information: clinical practice guidelines developed by government agencies, by local scientific societies, or by recognized international institutions (e.g. the American Heart Association or the National Institute for Health and Care Excellence (NICE), among others). However, recommendations may differ among clinical practice guidelines, as well as the professional may doubt which guideline to use for his or her clinical decision. Generally, the professional can choose those of more methodological rigor, but these regulations do not necessarily incorporate information applicable to the local context. This problem will be resolved to the extent to which high quality clinical practice guidelines are created to adapt to the local environment of healthcare professionals and their patients[13].
2. Multimorbidity
Most clinical practice guidelines are oriented to a single condition (e.g. diabetes mellitus, coronary heart disease, depression). However, patients often have several conditions at the same time, i.e. multimorbidity, a fact that is not usually covered in clinical practice guidelines[36] and could lead to the need to apply recommendations from different guidelines in parallel. Recent results show that the simultaneous application of independent clinical practice guidelines in patients with multimorbidity may be associated with serious adverse reactions related to drug-drug or drug-disease interactions[37]. Because of this complication, the field of developing clinical practice guidelines allow to address multimorbidity when considering the treatment burden[38], the identification of interventions with maximum benefit and their possible interactions, among others[39],[40].
3. Conflicts of interest
High-quality clinical practice guidelines collect the best available evidence and incorporate additional criteria for formulating recommendations[19]. However, the latter part is often consensual by a multidisciplinary “expert panel,” composed of experts who have excelled as opinion leaders in the clinical world and patient representatives. Expert panels risk incorporating members with conflicts of interest that may vitiate the referral process. For example, half of the members of clinical practice guidelines panels in Canada and the United States have been found to have conflicts of interest[41]. Fortunately, the agencies responsible have worked on conflicts of interest policies to try to mitigate their potential impact[42].
4. Patient involvement
Both the Evidence to Decision Framework of the Grading of Recommendations Assessment, Development and Evaluation approach and the Appraisal of Guidelines for Research & Evaluation II instrument in its domain “Stakeholder involvement” stress the importance of gathering information related to the values and preferences of patients receiving a given clinical practice guideline[43]. The involvement of patient values and preferences can be done by incorporating patient representatives into the expert group, bibliographic review on patient values and preferences, external review of the patient clinical practice guideline document, among others. However, the inclusion of patient values and preferences is still scarce. That is why proposals have emerged for the systematic incorporation of the patient perspective into the formulation of recommendations and to implement shared decision-making[44],[45] as a technical-communicative approach to add the perspective of patients when weighing the different therapeutic alternatives.
5. Quality indicators
There are health systems that audit the activity of clinical professionals using quality indicators, such as the percentage of people receiving statins for reducing cardiovascular risk for a certain risk threshold[46]. These systems formulate “good practice indicators” based on the most recognized clinical practice guidelines that often do not include patient values and preferences[47]. Therefore, if patient preferences are not aligned with the recommendation and, consequently, the indicator (for example, people have variable preferences regarding receiving statins based on the cardiovascular risk threshold[48]), certain “tyrannies”[49],[50] could occur, in which both health professionals and patients end up “adhering” to indicators based on clinical practice guidelines rather than their perspectives, even more so if these indicators involve financial incentives for health professionals[51]. Some approaches that would mitigate this phenomenon would be the implementation of shared decision-making and the systematic incorporation of patient values and preferences into the formulation of recommendations[43].
Currently, many of these limitations persist in the development of high-quality clinical practice guidelines. However, it is these gaps that constitute the challenges and opportunities necessary for the development of new regulations for evaluating clinical practice guidelines, leading to the consideration of new quality criteria.
Conclusions
Clinical practice guidelines are the most important documents for incorporating scientific evidence into healthcare decision-making by making recommendations. When these guidelines are of high quality, they evaluate, in addition to the aspects of the intervention, contextual aspects such as the use of resources and the values and preferences of patients. However, clinical practice guidelines are not recipe books, as they may have limitations in their availability and applicability in the local context.
It is therefore of great importance that health agencies promote the development and/or adaptation of clinical practice guidelines tailored to the practice of the health professional and the local reality of patients in terms of their values, preferences, and the presence of multimorbidity.
Notes
Authorship contributions
All authors contributed to the planning and writing of the original manuscript. JVAF and KK developed the introduction sections, roles of the clinical practice guidelines, development of a clinical practice guideline and provided Tables 1, 2, and 3. MA, NM, and EM developed the paragraph limitations, outstanding challenges, and conclusions.
Funding
The authors state that there were no external sources of funding.
Competing interests
The authors completed the ICMJE statement of conflicts of interest and stated that they received no funds for the completion of this article; they do not have financial relationships with organizations that may have an interest in the article published in the last three years and have no other relationships or activities that may influence the publication of the article. Forms can be requested by contacting the author responsible or the Editorial Committee of the Journal.
Ethics
This study did not require evaluation by an ethics-scientific committee as it was based on secondary sources.
From the editors
The original version of this article was submitted in Spanish. This English version is the translated article as submitted by the authors, lightly edited by the journal.
Referencias
- Franco JVA, Arancibia M, Simancas-Racines D, Madrid E. Syntheses of biomedical information: narrative reviews, systematic reviews and emerging formats. Medwave. 2018 Nov 27;18(7):e7354. | CrossRef | PubMed |
- Urrea G, Carvajal-Juliá N, Arcos CP-BJ. How do physicians fill gaps in their medical knowledge? An overview of systematic reviews. Cochrane Database Syst Rev Abstr 26th Cochrane Colloq. Santiago, Chile: 2019.
- Román A. Clinical guidelines, clinical pathways and protocols of care. Medwave 2012;12:e5436–e5436. | CrossRef |
- Feliciano Alfonso JE, Sebastián Castillo J. Guías de práctica clínica, vías clínicas y protocolos de manejo: similitudes, diferencias y alcances. Rev Colomb Cancerol 2013;17:182. | CrossRef |
- Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines; Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E, editors. Clinical Practice Guidelines We Can Trust. Washington (DC): National Academies Press (US); 2011. | CrossRef | PubMed |
- De Boeck K, Castellani C, Elborn JS; ECFS Board. Medical consensus, guidelines, and position papers: a policy for the ECFS. J Cyst Fibros. 2014 Sep;13(5):495-8. | CrossRef | PubMed |
- Ingravallo F, Dietrich CF, Gilja OH, Piscaglia F. Guidelines, clinical practice recommendations, position papers and consensus statements: definition, preparation, role and application. Ultraschall Med. 2014 Oct;35(5):395-9. | CrossRef | PubMed |
- Ministerio de Salud y Desarrollo Social. Abordaje de la Salud Mental en Hospitales Generales. Dirección Nacional de Salud Mental y Adicciones. Buenos Aires, Argentina: 2018.
- Ministerio de Salud Pública. Protocolos de atención prehospitalaria para emergencias médicas. Quito, Ecuador: 2011.
- Valderrama Beltrán SL, Gualtero SM, Quiroga C, Garzón JR, de Mendivelson EL, Tamara JR, y colaboradores. Evaluation and management of cardiovascualr risk in VIH infection: Expert consensus of ACIN (Colombian Association of Infectious Diseases). Infectio 2019;23:73–91. | CrossRef |
- Gutiérrez CM, Beroiza WT, Borzone TG, Caviedes SI, Céspedes GJ, Gutiérrez NM. Espirometría: Manual de procedimientos. SERChile. Rev Chil Enfermedades Respir 2018;34:171–88. | CrossRef |
- Chandler J, Churchill R, Higgins J. Methodological Expectations of Cochrane Intervention Reviews (MECIR): Methodological standards for the conduct of new Cochrane Intervention. 2012.
- Kredo T, Bernhardsson S, Machingaidze S, Young T, Louw Q, Ochodo E, et al. Guide to clinical practice guidelines: the current state of play. Int J Qual Health Care. 2016 Feb;28(1):122-8. | CrossRef | PubMed |
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011 Apr;64(4):383-94. | CrossRef | PubMed |
- Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009 May;64(5):669-77. | CrossRef | PubMed |
- Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. | CrossRef | PubMed |
- American College of Cardiology Foundation, American Heart Association. Methodology Manual and Policies. ACCF / AHA Task Force on Practice Guidelines 2010.
- Agency for Healthcare Research and Quality MD. R. U.S. Preventive Services Task Force Grade Definitions 2008;2009. [Internet] | Link |
- Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. [GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines]. Gac Sanit. 2018 Mar - Apr;32(2):167.e1-167.e10. | CrossRef | PubMed |
- Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. J Clin Epidemiol. 2010 Dec;63(12):1308-11. | CrossRef | PubMed |
- English M, Irimu G, Nyamai R, Were F, Garner P, Opiyo N. Developing guidelines in low-income and middle-income countries: lessons from Kenya. Arch Dis Child. 2017 Sep;102(9):846-851. | CrossRef | PubMed |
- Cabrera PA, Pardo R. Review of evidence based clinical practice guidelines developed in Latin America and Caribbean during the last decade: an analysis of the methods for grading quality of evidence and topic prioritization. Global Health. 2019 Feb 19;15(1):14. | CrossRef | PubMed |
- Haycox A. Should Low- and Middle-Income Countries Adopt Clinical Guidelines Developed in 'Rich' Countries? Pharmacoeconomics. 2018 Jul;36(7):731-732. | CrossRef | PubMed |
- Pantoja T, Valenzuela L, Léniz J, Castañón C. [Clinical practice guidelines in the Chilean health sector reform: a critical assessment of their quality]. Rev Med Chil. 2012 Nov;140(11):1391-1400. | CrossRef | PubMed |
- Ronsoni Rde M, Pereira CC, Stein AT, Osanai MH, Machado CJ. [Evaluation of eight Clinical Protocols and Therapeutic Guidelines under the Brazilian Ministry of Health using the AGREE II instrument: a pilot study]. Cad Saude Publica. 2015 Jun;31(6):1157-62. | CrossRef | PubMed |
- Canelo-Aybar C, Balbin G, Perez-Gomez Á, Florez ID. Guías de práctica clínica en el Perú: evaluación de su calidad usando el instrumento AGREE II. Rev Peru Med Exp Salud Publica 2016;33:732–8.
- Toledo-Fernández A, Cabrera-Cruz N, Arteaga-García A, Mejías-Sánchez Y. Quality of the Cuban clinical practice guidelines. Rev Cub Salud Pública 2011; 37:349-58.
- Rodríguez MF, Pineda I, Rozas MF. [Quality assessment of clinical practice guidelines of the Chilean explicit guarantees in healthcare program]. Rev Med Chil. 2016 Jul;144(7):862-9. | CrossRef | PubMed |
- Molino CG, Romano-Lieber NS, Ribeiro E, de Melo DO. Non-Communicable Disease Clinical Practice Guidelines in Brazil: A Systematic Assessment of Methodological Quality and Transparency. PLoS One. 2016 Nov 15;11(11):e0166367. | CrossRef | PubMed |
- Delgado-Noguera MF, Merchán-Galvis ÁM, Mera-Mamián AY, Muñoz-Manquillo DM, Calvache JA. Evaluación de la calidad metodológica de las Guías Colombianas de Práctica Clínica en Pediatría. Pediatria 2015;48:87–93. | CrossRef |
- Pantoja T, Strain H, Valenzuela L. [Clinical practice guidelines in primary health care: a critical appraisal]. Rev Med Chil. 2007 Oct;135(10):1282-90. | CrossRef | PubMed |
- Esandi ME, Ortiz Z, Chapman E, Dieguez MG, Mejía R, Bernztein R. Production and quality of clinical practice guidelines in Argentina (1994-2004): a cross-sectional study. Implement Sci. 2008 Oct 13;3:43. | CrossRef | PubMed |
- Santana RS, de Oliveira Lupatini E, Zanghelini F, de March Ronsoni R, Rech N, Leite SN. The different clinical guideline standards in Brazil: High cost treatment diseases versus poverty-related diseases. PLoS One. 2018 Oct 17;13(10):e0204723. | CrossRef | PubMed |
- Ronsoni Rde M, Pereira CC, Stein AT, Osanai MH, Machado CJ. [Evaluation of eight Clinical Protocols and Therapeutic Guidelines under the Brazilian Ministry of Health using the AGREE II instrument: a pilot study]. Cad Saude Publica. 2015 Jun;31(6):1157-62. | CrossRef | PubMed |
- Organizacion Mundial de la Salud, Organizacion Panamericana de la Salud. Directriz para el fortalecimiento de los programas nacionales de guías informadas por la evidencia: Una herramienta para la adaptación e implementación de guías en las Américas. Washington, D.C: OPS; 2018.
- Hughes LD, McMurdo ME, Guthrie B. Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Ageing. 2013 Jan;42(1):62-9. | CrossRef | PubMed |
- Dumbreck S, Flynn A, Nairn M, Wilson M, Treweek S, Mercer SW, et al. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ. 2015 Mar 11;350:h949. | CrossRef | PubMed |
- Dobler CC, Harb N, Maguire CA, Armour CL, Coleman C, Murad MH. Treatment burden should be included in clinical practice guidelines. BMJ. 2018 Oct 12;363:k4065. | CrossRef | PubMed |
- Farmer C, Fenu E, O'Flynn N, Guthrie B. Clinical assessment and management of multimorbidity: summary of NICE guidance. BMJ. 2016 Sep 21;354:i4843. | CrossRef | PubMed |
- Kernick D, Chew-Graham CA, O'Flynn N. Clinical assessment and management of multimorbidity: NICE guideline. Br J Gen Pract. 2017 May;67(658):235-236. | CrossRef | PubMed |
- Neuman J, Korenstein D, Ross JS, Keyhani S. Prevalence of financial conflicts of interest among panel members producing clinical practice guidelines in Canada and United States: cross sectional study. BMJ. 2011 Oct 11;343:d5621. | CrossRef | PubMed |
- American Heart Association. Disclosure – Conflict of Interest. Policy and Procedure Manual 2011.
- Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: Incorporating patient preferences in practice guidelines. JAMA. 2013 Dec 18;310(23):2503-4. | CrossRef | PubMed |
- Barani M, Kopitowski K. Toma de decisiones compartidas: centrando los cuidados médicos realmente en nuestros pacientes. Rev Hosp Ital B Aires 2012;33:60–4.
- Yudkin JS, Kavanagh J, McCormack JP. Guidelines for treating risk factors should include tools for shared decision making. BMJ. 2016 Jun 14;353:i3147. | CrossRef | PubMed |
- NICE. Quality and Outcomes Framework Indicators, Standards & Indicators n.d. [Internet] | Link |
- Chong CA, Chen IJ, Naglie G, Krahn MD. How well do guidelines incorporate evidence on patient preferences? J Gen Intern Med. 2009 Aug;24(8):977-82. doi: 10.1007/s11606-009-0987-8. | CrossRef | PubMed |
- Albarqouni L, Doust J, Glasziou P. Patient preferences for cardiovascular preventive medication: a systematic review. Heart. 2017 Oct;103(20):1578-1586. | CrossRef | PubMed |
- McCartney M. Margaret McCartney: Have we given guidelines too much power? BMJ. 2014 Oct 6;349:g6027. doi: 10.1136/bmj.g6027. Erratum in: BMJ. 2015;351:h5550. | CrossRef | PubMed |
- Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014 Oct 1;312(13):1295-6. | CrossRef | PubMed |
- Sicsic J, Krucien N, Franc C. What are GPs' preferences for financial and non-financial incentives in cancer screening? Evidence for breast, cervical, and colorectal cancers. Soc Sci Med. 2016 Oct;167:116-27. | CrossRef | PubMed |
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Phone: 56-2-22743013
ISSN 0717-6384