Severe diabetic ketoacidosis precipitated by COVID-19 in pediatric patients: Two case reports
Jesús Ángel Domínguez Rojas, Mariela Violeta Tello Pezo, Jaime Tasayco Muñoz, Álvaro Coronado Muñoz
Abstract
Introduction
The association of COVID-19 with diabetes mellitus is bidirectional. In one direction, diabetes mellitus is associated with an increased risk of severe COVID-19. In the opposite direction, in patients with COVID-19 new-onset diabetes mellitus, severe diabetic ketoacidosis and severe metabolic complications have been described.
Clinical case
This report describes two patients with diabetes mellitus who came to our hospital with ketoacidosis resulting from new-onset diabetes mellitus. We describe the clinical course and the management approach during the COVID-19 pandemic.
Conclusion
COVID-19 is associated with metabolic complications such as severe diabetic ketoacidosis.
|
Main messages
|
Introduction
Since it was classified as a pandemic, COVID-19 infection by the new SARS-CoV-2 coronavirus has behaved as a disease that predominantly affects the respiratory tract, with higher case fatality rates in adults (mostly men) in the sixth decade of life[1].
Country health systems have had to prioritize their human and economic resources in the COVID-19 containment. Consequently, other chronic diseases have been exacerbated due to poor control[2].
In the case of children, COVID-19 infection mostly follows an asymptomatic course and is associated with lower death rates. However, extrapulmonary presentations different from the adult stage have been described, representing a challenge for those who assist these patients[3]. Case series and cross-sectional studies have identified a higher proportion of complications in children with comorbidities, leading to these children developing new diseases.
Diabetes mellitus usually debuts with ketoacidosis in 43% of cases, and the coexistence of infectious diseases has been identified in 59% of cases[4],[5]. This pathology and its complications have been associated with a higher probability of complications in adults infected by the new coronavirus. However, this has not yet been demonstrated in the pediatric population. The purpose of this article is to report the possible exacerbation of diabetes mellitus during the COVID-19 pandemic.
Method
Following the methodology of the CARE (CAse REports) guidelines[6], we present two cases of patients under 18 years of age with a diagnosis of SARS-CoV-2 infection (detected by polymerase chain reaction test for this virus) and diabetic ketoacidosis (acidemia with pH less than 7.31, glucose greater than 350 milligrams per deciliter, ketones in urine), from a tertiary care hospital.
Case 1
11-year-old male with obesity according to body mass index greater than 95% according to the World Health Organization charts.
Positive family history for type 2 diabetes mellitus in father and paternal grandfather. He was a contact of a confirmed COVID-19 close relative (father).
Case report
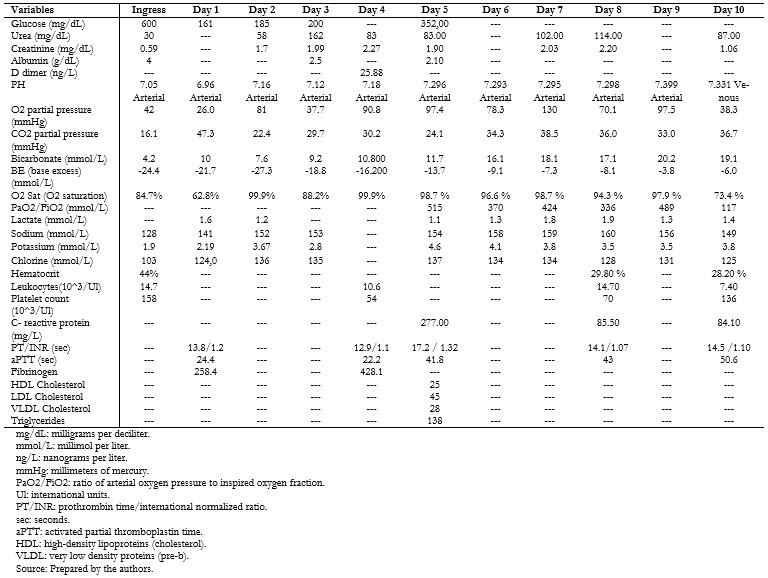
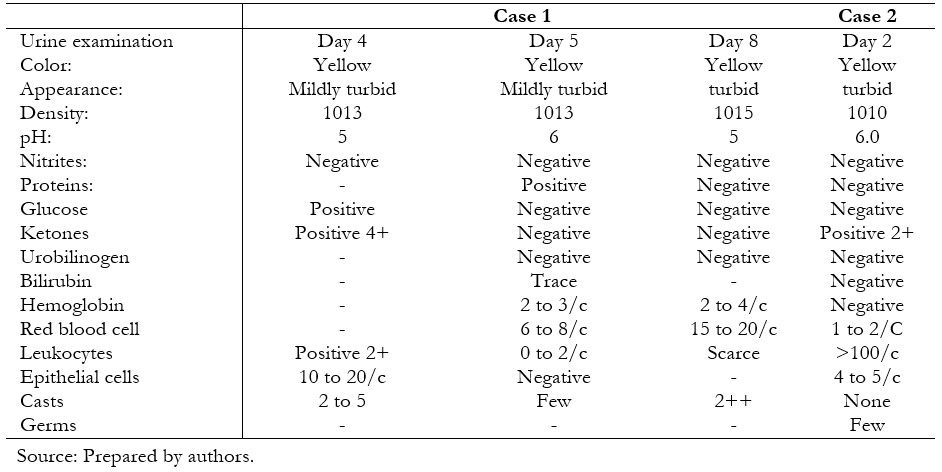
The patient reported respiratory symptoms initially and then presented polyuria, polydipsia, polyphagia, and a 10-kilogram weight loss fifteen days prior to admission. On admission, the antibody test for COVID-19 (immunoglobulins G and M) was positive, and SARS-CoV-2 infection was documented by a positive molecular test taken from a nasopharyngeal swab. The patient presented respiratory distress and alterations in consciousness, receiving oxygen therapy, followed by an asystole event that required resuscitation maneuvers and ventilatory support. Baseline laboratory tests were: serum glucose 600 milligrams per deciliter, blood gas with decompensated metabolic acidemia, bicarbonate concentration less than 10 millimoles per liter, and ketonuria. Inflammatory markers were: quantitative C-reactive protein greater than 200 milligrams per liter, leukocytosis, anemia and thrombocytopenia, hypoalbuminemia, d-dimer greater than 20 nanograms per liter (Tables 1 and 3).
|
| Table 1. Ancillary examinations during the hospital stay. |
Initial management was based on parenteral rehydration of losses and rapid insulin infusion of 0.05 to 0.1 units per kilo per hour for seven days. After clinical improvement and improvement of the acid-base status, intermediate and rapid-acting human insulin was prescribed on a sliding scale according to glycemia levels.
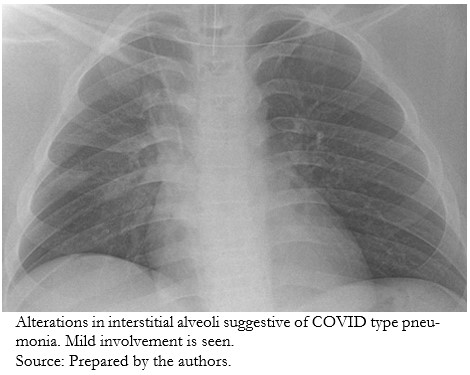
The respiratory condition evaluated by chest X-ray showed interstitial alveolar alterations indicative of COVID-19 type pneumonia, mild grade (Figure 1).
|
| Figure 1. Chest X-ray. |
Encephalopathy secondary to mild acute hypoxic damage and cerebral edema occurred, and the patient was kept under sedation and analgesia with propofol infusion, plus fentanyl infusion. He was treated with noradrenaline for hemodynamic status in the range of 0.05 to 0.2 micrograms per kilogram per minute for six days. He presented uremic retention and oligoanuria that evolved favorably. After six days of mechanical ventilation, sedation, pain control, and mechanical ventilation were withdrawn. No neurological deficit was documented. After eight days, COVID-19 extubation protocol was followed, and the patient progressed with Venturi mask to an inspired oxygen fraction of 0.6%. Empirical antibiotic coverage for nosocomial infection and atypical bacteria was indicated with ceftriaxone at 100 milligrams per kilo per day and clindamycin at 30 milligrams per kilo per day, for seven days. It was decided to add enoxaparin at an anticoagulant dose of 1 milligram per kilo per dose.
According to serological serial glycemia, the patient improved progressively and was able to go home with treatment based on intermediate-acting human insulin and rapid-acting insulin on a sliding scale.
Case 2
An 8-year-old girl with a positive history of insulin-dependent controlled diabetes mellitus since age 5. The patient was a contact (neighbor) of a confirmed case of COVID-19. She manifested clinical symptoms compatible with urinary symptoms (dysuria, pyuria, fever), and a nasopharyngeal swab molecular test documented SARS-CoV-2 infection.
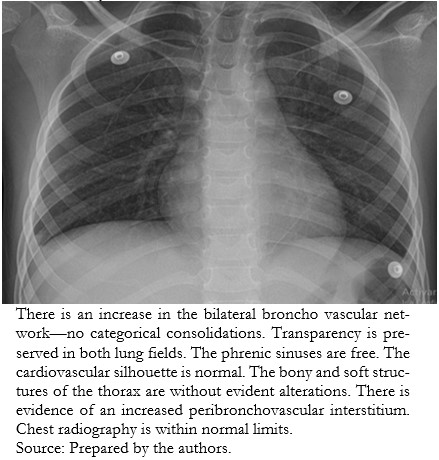
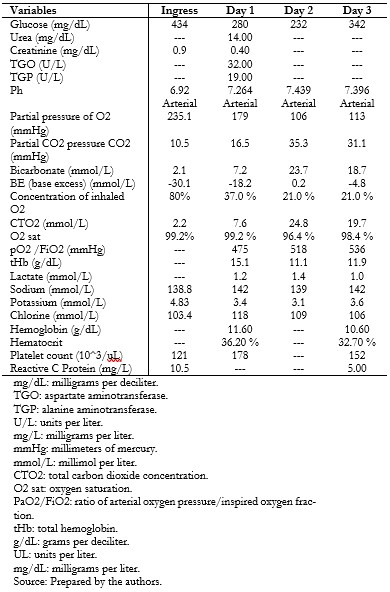
During her course, the patient presented vomiting, dyspnea, Kussmaul’s respiration, and hypoactivity lasting 18 hours. For this reason, she was seen in the emergency department, where the diagnosis of diabetic ketoacidosis was corroborated (Tables 2 and 3), and treatment was started with fluid resuscitation, rapid insulin infusion 0.05 to 0.1 units per kilogram per hour, and empirical antimicrobial coverage. Chest radiography did not show images compatible with a ground-glass appearance (Figure 2).
|
| Figure 2. Chest X-ray. |
During her evolution, she was hospitalized in an intensive care unit, where fluid therapy and empirical antimicrobial control (amikacin) were provided for a probable urinary tract infection. Immunoglobulins G and M for the SARS-CoV-2 virus were documented. She had a favorable course and was discharged with insulin glargine and rapid insulin on a sliding scale according to serum glycemia controls.
|
| Table 2. Ancillary tests during the hospital stay. |
|
| Table 3. Characteristics of the urine test. |
Discussion
In the two cases presented, a coexistence of diabetic ketoacidosis and infection by the new coronavirus was demonstrated.
The first case corresponds to a patient with the comorbidity of obesity who debuted with the complication of diabetic ketoacidosis coexisting with pulmonary manifestations of COVID-19 infection. The second case is a pediatric patient with preexisting diabetes mellitus who developed diabetic ketoacidosis and COVID-19 without pulmonary manifestations.
These two cases underscore the importance of continuing adequate control of metabolic conditions, which could be worsened by SARS-CoV-2 infection. It is important to recognize this factor in physicians responsible for patients’ health with chronic conditions, such as obesity and diabetes mellitus, since they are at risk of worse morbidities. This was described by Karmath et al. in a study conducted in Germany, where they described a significant increase in cases of severe diabetic ketoacidosis during the pandemic[7]. The presentation with diabetic ketoacidosis could be lethal if not compensated and treated in time. The first case had cerebral edema and cardiorespiratory arrest. Both patients required intensive care management with a prolonged hospital stay. Without previous routine controls or metabolic follow-up, these two cases were affected by the pandemic before being diagnosed with diabetic ketoacidosis. For this reason, the interest arises to correlate decompensation in a serious condition such as severe diabetic ketoacidosis due to COVID-19 and the need for management in pediatric intensive care. These cases suggest that children with metabolic disorders without endocrinological follow-up are among the populations most vulnerable to COVID-19 and may develop severe decompensation, which increases hospital stay and mortality.
Regarding the relationship between diabetes mellitus and COVID-19, Samir et al. described 86 children with diabetes mellitus and COVID-19 who developed mild symptoms, five of them requiring hospitalization in the intensive care unit. All the cases studied maintained therapeutic control of glucose levels during the infection[8].
On the other hand, a questionnaire-based Italian study reported fewer new cases of diabetes mellitus in the pediatric population but a higher number of cases with severe ketoacidosis[9]. A similar study in the United States based on a quality registry of new cases of diabetes mellitus found no difference in diabetic ketoacidosis in cases with or without COVID-19. However, the authors argue that the number of cases of ketoacidosis is higher than other years in the same registry[10]. A database study in Australia described an increased frequency of severe diabetic ketoacidosis during the pandemic[11].
One question these studies raise is whether this increased frequency of ketoacidosis is part of the pathophysiology of the virus or whether it is related to the social changes imposed to control the pandemic, such as reduced access to health check-ups or postponed emergency visits, increasing the severity of presentation.
Juyi Li studied 658 adult patients hospitalized with confirmed COVID-19; 42 (6.4%) of them presented diabetic ketoacidosis at admission as the first manifestation, the same who presented a more extended in-hospital stay and mortality[12].
Causality studies are needed to determine whether pancreatic cellular involvement accelerates the lesion in patients who debut with diabetes mellitus[13]. There are reports of COVID-19 patients with pediatric inflammatory multisystemic syndrome who also present with pancreatitis[14]. Amylase and lipase studies were not obtained in our patients since the evolution was favorable.
An important public health aspect is to avoid hospitalization for preventable and treatable conditions. The increase in seroprevalence for SARS-CoV-2 and the reduction in the supply of health services during the pandemic make it relevant to analyze and reorient the flow of patients in the intrahospital setting to optimize the resources available to us. This information must be known by physicians caring for pediatric patients in the emergency context in order to take appropriate precautions. Patients with COVID-19 may present ketoacidosis, and this should be considered in the management.
Conclusions
The cases reported in this article show that COVID-19 can precipitate severe ketoacidosis. These patients may present with more significant complications than other milder forms of presentation.
Notes
Contributor roles
JD, MT, JT: drafting and data collection of the manuscript. JD, ACM: corrections, drafting and final editing.
Competing interests
The authors completed the ICMJE conflict of interest statement and declared that they received no funding for the completion of this article; have no financial relationships with organizations that may have an interest in the published article in the last three years; and have no other relationships or activities that may influence the publication of the article. Forms can be requested by contacting the responsible author or the Editorial Committee of the Journal.
Funding
The authors have not received any funding.
Ethics
The present work was approved by the Teaching and Research and Ethics Unit of the Emergency Hospital of Villa el Salvador, and no objections were found in this project. Acceptance of informed consent was also received from the parents of the cases presented.
Language of submission
Spanish
Referencias
- Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020 May 25;11(1):29. | CrossRef | PubMed |
- Juang SF, Chiang HC, Tsai MJ, Huang MK. Integrated Hospital Quarantine System against COVID-19. Kaohsiung J Med Sci. 2020 May;36(5):380-381. | CrossRef | PubMed |
- Song W, Li J, Zou N, Guan W, Pan J, Xu W. Clinical features of pediatric patients with coronavirus disease (COVID-19). J Clin Virol. 2020 Jun;127:104377. | CrossRef | PubMed |
- Kanikarla-Marie P, Jain SK. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic Biol Med. 2016 Jun;95:268-77. | CrossRef | PubMed |
- Maletkovic J, Drexler A. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinol Metab Clin North Am. 2013 Dec;42(4):677-95. | CrossRef | PubMed |
- Rison RA, Kidd MR, Koch CA. The CARE (CAse REport) guidelines and the standardization of case reports. J Med Case Rep. 2013 Nov 27;7:261. | CrossRef | PubMed |
- Kamrath C, Mönkemöller K, Biester T, Rohrer TR, Warncke K, Hammersen J, et al. Ketoacidosis in Children and Adolescents With Newly Diagnosed Type 1 Diabetes During the COVID-19 Pandemic in Germany. JAMA. 2020 Aug 25;324(8):801-804. | CrossRef | PubMed |
- Elbarbary NS, Dos Santos TJ, de Beaufort C, Agwu JC, Calliari LE, Scaramuzza AE. COVID-19 outbreak and pediatric diabetes: Perceptions of health care professionals worldwide. Pediatr Diabetes. 2020 Nov;21(7):1083-1092. | CrossRef | PubMed |
- Rabbone I, Schiaffini R, Cherubini V, Maffeis C, Scaramuzza A; Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetes. Has COVID-19 Delayed the Diagnosis and Worsened the Presentation of Type 1 Diabetes in Children? Diabetes Care. 2020 Nov;43(11):2870-2872. | CrossRef | PubMed |
- Beliard K, Ebekozien O, Demeterco-Berggren C, Alonso GT, Gallagher MP, Clements M, Rapaport R. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021 Mar;13(3):270-272. | CrossRef | PubMed |
- Lawrence C, Seckold R, Smart C, King BR, Howley P, Feltrin R, et al. Increased paediatric presentations of severe diabetic ketoacidosis in an Australian tertiary centre during the COVID-19 pandemic. Diabet Med. 2021 Jan;38(1):e14417. | CrossRef | PubMed |
- Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020 Oct;22(10):1935-1941. | CrossRef | PubMed |
- Reddy PK, Kuchay MS, Mehta Y, Mishra SK. Diabetic ketoacidosis precipitated by COVID-19: A report of two cases and review of literature. Diabetes Metab Syndr. 2020 Sep-Oct;14(5):1459-1462. | CrossRef | PubMed |
- Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children (Basel). 2020 Jul 1;7(7):69. | CrossRef | PubMed |
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Phone: 56-2-22743013
ISSN 0717-6384